Volume 8 (2025) Issue 1 No.7 Pages 181-185
Abstract
Acute myeloid leukemia (AML) with inv(3)(q21q26.2) or t(3;3)(q21;q26.2) has a dismal prognosis and poor response to conventional chemotherapy. Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative treatment for adult AML with inv(3)/t(3;3) during complete remission (CR). Nevertheless, because fewer than half of patients achieve a CR with induction conventional chemotherapy, allogeneic HCT is frequently performed for AML with inv(3)/t(3;3) in non-remission. Here, we report six patients with adult AML with inv(3)/t(3;3) in non-remission who underwent allogeneic HCT at our institute between 2010 and 2024. The median age at the time of HCT was 43.5 years (range, 28-53 years). The median proportion of blasts in the bone marrow at HCT was 47.5% (range, 0.7-75.0%). The median duration from diagnosis to HCT was 65.5 days (range, 41-123 days). A total of five patients received single-unit cord blood transplantation, and one received bone marrow transplantation from an HLA-matched sibling donor. All patients received a myeloablative conditioning regimen, including 12 Gy total body irradiation and granulocyte colony-stimulating factor (G-CSF) combined with high-dose cytarabine, as well as standard cyclosporine and methotrexate for graft-versus-host disease prophylaxis. With a median follow-up of 41 months for survivors, three patients experienced relapse at 18, 5, and 2 months, whereas the remaining three patients were alive and disease-free at 173, 110, and 30 months after HCT. Our data demonstrate that G-CSF-combined myeloablative conditioning following allogeneic HCT could lead to favorable long-term remission for adult AML with inv(3)/t(3;3) in non-remission at HCT.
Introduction
Chromosome 3 inversion, including inv(3)(q21q26.2) or t(3;3)(q21;q26.2), represents a distinct genetic abnormality that accounts for approximately 1% of acute myeloid leukemia (AML). This abnormality has been recognized as a unique subtype of AML with recurrent genetic abnormalities in the World Health Organization (WHO) classification since 2008. AML with inv(3)/t(3;3) is characterized by adverse outcomes, including poor response to conventional chemotherapy1,2 and a higher risk of relapse even after allogeneic hematopoietic cell transplantation (HCT)3–6. Currently, allogeneic HCT is the only potential long-term curative approach for patients who have achieved first complete remission (
Priming with granulocyte colony-stimulating factor (G-CSF) enhances the susceptibility of cytarabine (Ara-C), a cell-cycle-specific drug, in AML cells. Using this concept, we administered G-CSF-combined high-dose Ara-C in myeloablative conditioning for allogeneic HCT and reported that a G-CSF-combined conditioning regimen provided better overall survival in allogeneic HCT for myeloid malignancies, even in non-remission7,8. Here, we report the clinical outcomes of allogeneic HCT after G-CSF-combined myeloablative conditioning in adult non-remission AML patients with inv(3)/t(3;3) at our institute.
Materials and Methods
We retrospectively reviewed cases of adult AML with inv(3)/t(3;3) that underwent allogeneic HCT at our institute between 2010 and 2024. Donor and graft types, conditioning regimen, graft-versus-host disease (GVHD) prophylaxis, and supportive care were determined by the treating physicians. This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The Institutional Review Board of the Institute of Medical Science, the University of Tokyo, approved this retrospective study (2024-35-0828) and the use of an opt-out consent mechanism.
Results and Discussion
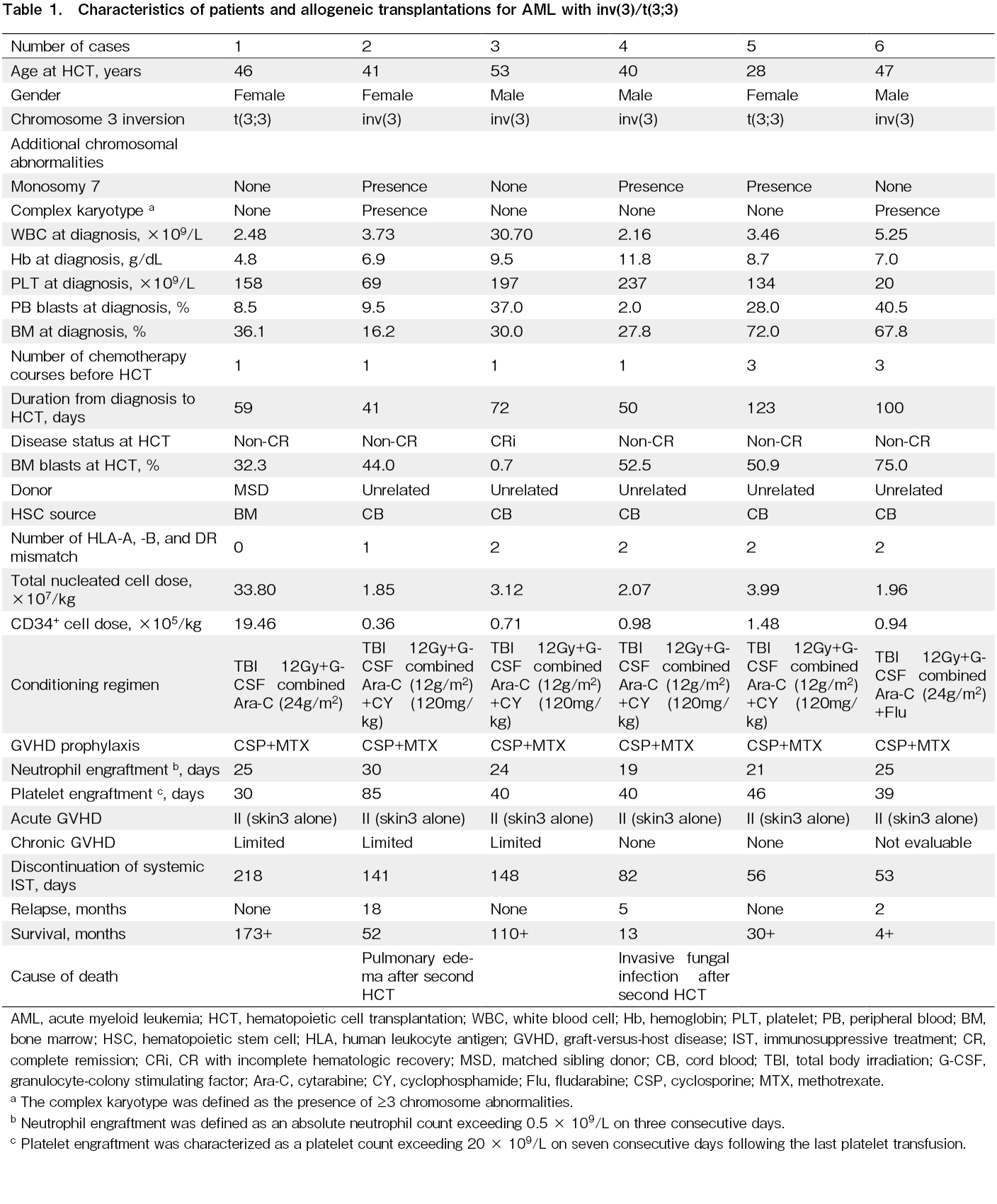
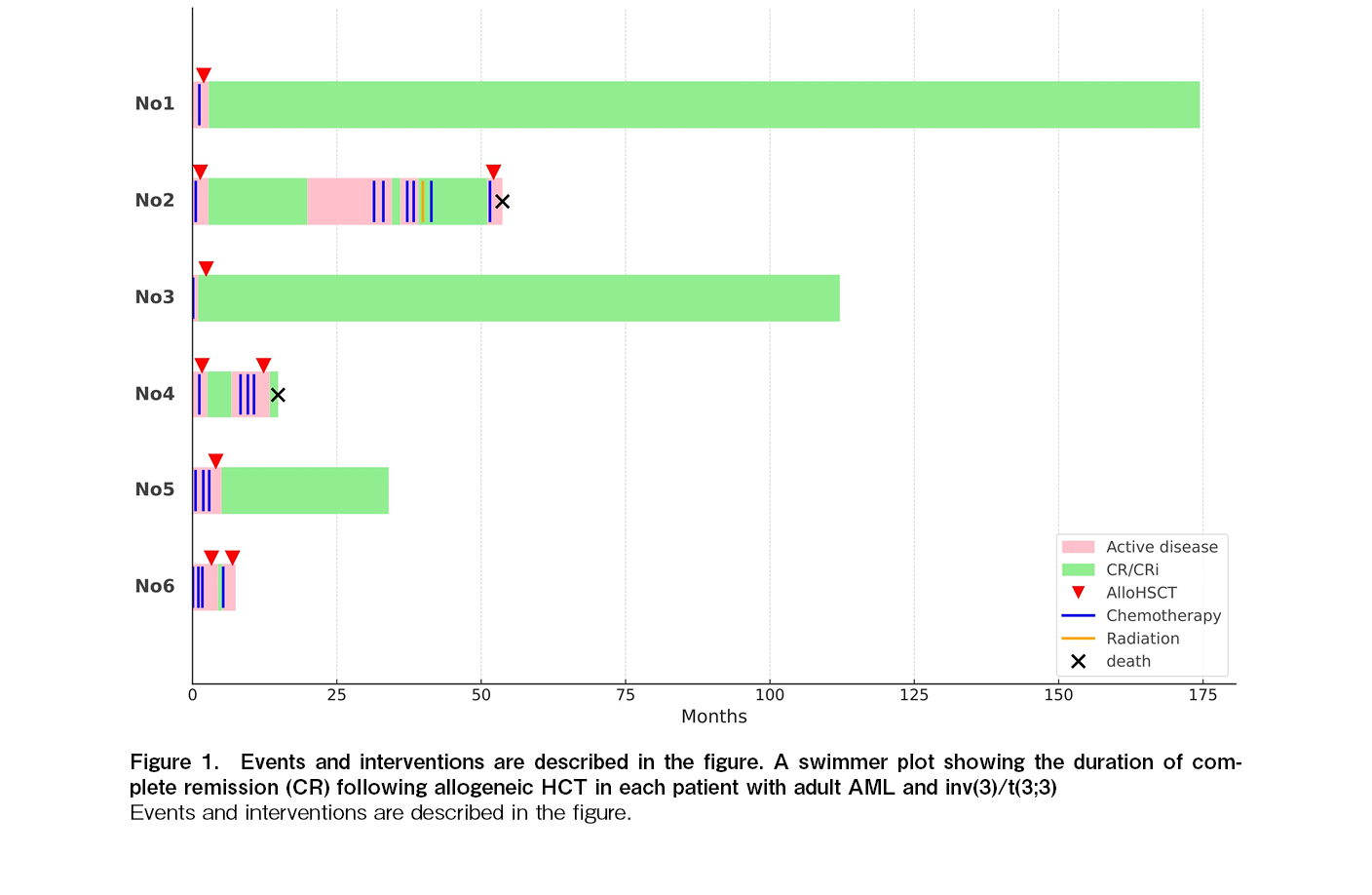
Between April 2010 and June 2024, six patients with AML and inv(3)/t(3;3) underwent allogeneic HCT at our institution. The characteristics of the patients and the allogeneic HCT procedures are summarized in Table 1. The median age at the time of HCT was 43.5 years (range, 28-53 years). One patient (Case 2) had a prior history of myelodysplastic syndrome (MDS). Chromosomal analysis revealed inv(3)(q21q26.2) in four patients and t(3;3)(q21;q26.2) in two patients. Additional chromosomal abnormalities included monosomy 7 in three patients and a complex karyotype in two patients. Three patients (Cases 3, 5, and 6) received conventional induction chemotherapy (Idarubicin and Ara-C), whereas the remaining three (Cases 1, 2, and 4) received low-dose Ara-C-based chemotherapy. No patient achieved complete remission (CR), except for one patient who achieved CR with incomplete hematologic recovery after receiving Idarubicin and Ara-C. The median proportion of blasts in the bone marrow at HCT was 47.5% (range, 0.7-75.0%). A total of five patients received single-unit cord blood transplantation (CBT), and one received bone marrow transplantation from an HLA-matched sibling donor. All patients received a myeloablative conditioning regimen, including four fractionated doses of 12 Gy total body irradiation (TBI), G-CSF combined with high-dose Ara-C, and standard cyclosporine and methotrexate for GVHD prophylaxis7,8. The median duration from diagnosis to HCT was 65.5 days (range, 41-123 days). Neutrophil engraftment, defined as an absolute neutrophil count >0.5×109/L, was achieved in all patients at a median time of 24.5 days (range, 19-30 days). All patients developed grade II acute GVHD, and limited chronic GVHD occurred in three of five evaluable patients. All patients discontinued systemic immunosuppressive treatment (IST), with a median time to discontinuation of 111.5 days (range, 53-218 days) after HCT. The median follow-up for survivors was 41 months (range, 4-173 months). Three patients experienced relapse at 18, 5, and 2 months, whereas the remaining three patients were alive and disease-free at 173, 110, and 30 months after HCT (Figure 1). Of the three patients who relapsed after the first HCT, two received a second allogeneic HCT. However, two of these patients died, one from pulmonary edema on day 25 and the other from invasive fungal infection on day 79 after the second HCT (Figure 1).
AML with inv(3)/t(3;3) has a dismal prognosis due to a low response rate and high mortality when conventional chemotherapy is used alone. Several studies have shown the beneficial effect of allogeneic HCT over conventional chemotherapy alone in AML with inv(3)/t(3;3)3,4,6. In allogeneic HCT for AML with inv(3)/t(3;3), CR at the time of HCT is the only prognostic factor associated with better survival5. Therefore, allogeneic HCT should be considered in AML with inv(3)/t(3;3), especially in CR1. However, Lugthart et al. reported that CR rates were significantly lower in AML with inv(3)/t(3;3) compared with AML without 3q abnormalities (31% versus 70%, p < 0.001)1. Indeed, in our cohort, five of six patients did not achieve CR1 and were not in remission at HCT, but three patients maintained long-term remission following allogeneic HCT. This case series suggests that allogeneic HCT may be a potentially curative treatment for adult AML with inv(3)/t(3;3) even when patients are not in remission, which is consistent with the observations from the ASAP trial9.
In our cases, two of three patients without monosomy 7 and three of four patients without a complex karyotype were alive and disease-free in the long term after allogeneic HCT. This indicates that monosomy 7 or a complex karyotype may be prognostic factors for disease-free survival after allogeneic HCT in adult AML with inv(3)/t(3;3). Several studies have evaluated the prognostic impact of additional chromosomal abnormalities in AML with inv(3)/t(3;3), regardless of the type of treatment4–6. Rogers et al. showed that complex karyotype and monosomal karyotype, but not bone marrow blast percentage, were unfavorable factors for survival in AML and MDS with inv(3)/t(3;3)4. In contrast, a recent study by Richard-Carpentier et al. demonstrated that the presence of monosomy 7 or a complex karyotype was not associated with poor survival in AML with inv(3)/t(3;3)6. Among patients with AML and inv(3)/t(3;3) who received allogeneic HCT, monosomy 7 was associated with higher relapse rates, but complex karyotype was not associated with it5. These conflicting results may be partly due to the small sample size in studies of AML with inv(3)/t(3;3). A recent study demonstrated that mutations in KRAS, ASXL1, and DNMT3A were associated with poor survival in AML with inv(3)/t(3;3), regardless of the treatment type6, but the mutation profiles were insufficiently detailed in our cohort. Therefore, further studies are needed to assess the prognostic impact of additional chromosomal abnormalities and gene mutations in adult AML with inv(3)/t(3;3) following allogeneic HCT.
The long-term disease-free survival following allogeneic HCT for non-remission AML with inv(3)/t(3;3) in our cohort is better than in previous reports, which showed overall survival rates of less than 20%5,6. This may be partly due to the greater proportion of patients receiving CBT. According to our previous study, CBT can produce a stronger graft-versus-leukemia (GVL) effect, which lowers the risk of relapse after HCT10. Given the quick availability and stronger GVL effects of CBT, it may be advantageous to choose CBT for AML with inv(3)/t(3;3) in non-remission status.
Furthermore, all six patients in our cohort received an intensified myeloablative conditioning regimen, involving the addition of G-CSF-combined high-dose Ara-C to 12 Gy TBI7,8. Based on the concept of a G-CSF priming effect, G-CSF may increase the vulnerability of leukemia cells to the cell-cycle-specific drug Ara-C, both in vitro and in vivo, as well as in AML treatment. When Ara-C is combined with G-CSF, quiescent AML stem cells in the bone marrow endosteal region undergo apoptosis and enter the cell cycle in patient-derived xenograft models of AML11. This suggests that the priming effect of G-CSF may help eradicate leukemia stem cells, which could otherwise contribute to relapse following treatment. Indeed, the ecotropic viral integration (EVI1) gene, located on chromosome 3q26.2, is aberrantly upregulated in almost all cases of AML with inv(3)/t(3;3). Overexpression of EVI1 maintains the quiescence of both normal hematopoietic stem cells and leukemia stem cells12, which may contribute to chemotherapy resistance. Therefore, G-CSF-combined myeloablative conditioning may help overcome the poor prognosis of non-remission AML with inv(3)/t(3;3) following allogeneic HCT.
In summary, our data suggest that G-CSF-combined myeloablative conditioning following allogeneic HCT may result in favorable long-term remission in adult AML with inv(3)/t(3;3), even in cases where the patient is in the absence of remission at the time of transplantation.
Acknowledgments
The authors thank all of the physicians and staff at our hospital.
Author Contributions
YO collected the data, analyzed the data, and wrote the manuscript. TK designed the research, collected the data, analyzed the data, and wrote the manuscript. All the other authors contributed to data collection. All authors approved the final version.
Conflicts of Interest
ST is one of the editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
The authors thank all of the physicians and staff at our hospital.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
1.Lugthart S, Gröschel S, Beverloo HB, Kayser S, Valk PJ, van Zelderen-Bhola SL, et al. Clinical, molecular, and prognostic significance of WHO type inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J Clin Oncol. 2010; 28: 3890-8.
2.Wanquet A, Prebet T, Berthon C, Sebert M, Roux C, Kulasekararaj A, et al. Azacitidine treatment for patients with myelodysplastic syndrome and acute myeloid leukemia with chromosome 3q abnormalities. Am J Hematol. 2015; 90: 859-63.
3.Sun J, Konoplev SN, Wang X, Cui W, Chen SS, Medeiros LJ, et al. De novo acute myeloid leukemia with inv(3)(q21q26.2) or t(3;3)(q21;q26.2): a clinicopathologic and cytogenetic study of an entity recently added to the WHO classification. Mod Pathol. 2011; 24: 384-9.
4.Rogers HJ, Vardiman JW, Anastasi J, Raca G, Savage NM, Cherry AM, et al. Complex or monosomal karyotype and not blast percentage is associated with poor survival in acute myeloid leukemia and myelodysplastic syndrome patients with inv(3)(q21q26.2)/t(3;3)(q21;q26.2): a Bone Marrow Pathology Group study. Haematologica. 2014; 99: 821-9.
5.Halaburda K, Labopin M, Houhou M, Niederwieser D, Finke J, Volin L, et al. AlloHSCT for inv(3)(q21;q26)/t(3;3)(q21;q26) AML: a report from the acute leukemia working party of the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018; 53: 683-91.
6.Richard-Carpentier G, Rausch CR, Sasaki K, Hammond D, Morita K, Takahashi K, et al. Characteristics and clinical outcomes of patients with acute myeloid leukemia with inv(3)(q21q26.2) or t(3;3)(q21;q26.2). Haematologica. 2023; 108: 2331-42.
7.Konuma T, Kato S, Ishii H, Oiwa-Monna M, Asano S, Tojo A, et al. Long-term outcomes of granulocyte colony-stimulating factor-combined conditioning in allogeneic hematopoietic stem cell transplantation from HLA-identical family donors for myeloid malignancies. Leuk Res. 2015; 39: 625-31.
8.Konuma T, Kato S, Ooi J, Oiwa-Monna M, Ebihara Y, Mochizuki S, et al. Single-unit cord blood transplantation after granulocyte colony-stimulating factor-combined myeloablative conditioning for myeloid malignancies not in remission. Biol Blood Marrow Transplant. 2014; 20: 396-401.
9.Stelljes M, Middeke JM, Bug G, Wagner-Drouet EM, Müller LP, Schmid C, et al. Remission induction versus immediate allogeneic haematopoietic stem cell transplantation for patients with relapsed or poor responsive acute myeloid leukaemia (ASAP): a randomised, open-label, phase 3, non-inferiority trial. Lancet Haematol. 2024; 11: e324-35.
10.Konuma T, Kanda J, Kuwatsuka Y, Yanada M, Kondo T, Hirabayashi S, et al. Differential Effect of Graft-versus-Host Disease on Survival in Acute Leukemia according to Donor Type. Clin Cancer Res. 2021; 27: 4825-35.
11.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nat Biotechnol. 2010; 28: 275-80.
12.Glass C, Wilson M, Gonzalez R, Zhang Y, Perkins AS. The role of EVI1 in myeloid malignancies. Blood Cells Mol Dis. 2014; 53: 67-76.
Search
News