Volume 8 (2025) Issue 2 No.3 Pages 200-209
Abstract
This study aimed to evaluate the efficacy and safety of programmed death receptor 1 (PD-1) antibody in patients with acute myeloid leukaemia (AML) or myelodysplastic syndrome (MDS) with minimal residual disease (MRD) after allogeneic haematopoietic stem cell transplantation (allo-HSCT). Six patients were retrospectively reviewed in this study, and all had failed prior treatment (donor lymphocyte infusion or interferon) before PD-1 antibody administration. Among these 6 patients, two received PD-1 alone while four received PD-1 plus azacitidine. The median treatment with the PD-1 antibody was four doses (range, 1-7 doses). Three patients developed > grade 3 toxicity, including 2 deaths. Among the five evaluable patients, four achieved negative MRD with a median time to response of 2 months (range: 1-3 months); and the median duration of response was 105 days (range: 26-211 days). The median survival time of the five patients was 320 days (range: 107-350 days). Our data suggest that anti-PD-1 antibody in AML/MDS patients with positive MRD following allo-HSCT may be a treatment option.
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is the major curative treatment option for patients with hematologic malignancies1. However, relapse is one of the most important causes of HSCT failure. Approximately 20-40% of patients with acute myeloid leukaemia (AML) and myelodysplastic syndrome (MDS) relapse after HSCT. Once relapsed, salvage treatment options are limited, and the efficacy and survival rates are very low2–5.
Minimal residual disease (MRD) is an excellent early warning biomarker of haematological relapse after HSCT6,7. MRD-based pre-emptive interventions can effectively reduce relapse rates and improve patient survival after HSCT. At present, pre-emptive intervention strategies for patients who are MRD-positive after transplantation mainly include donor lymphocyte infusion (DLI), interferons, targeted drugs, and alternative strategies8–10. However, for more than 22-39% of patients the above treatment methods are ineffective11–14, and there are serious toxicity problems caused by the treatment, including graft-versus-host disease (GVHD), lung injury, and serious infection. Therefore, there is still an unmet need to find new MRD intervention strategies in clinical practice15–17. Clinical treatment is even more difficult in patients who have failed treatment or become MRD-positive again after the aforementioned interventions. Therefore, a novel treatment method is urgently needed.
It has been shown that immune escape is one of the possible mechanisms for relapse (either haematological or MRD relapse) after HSCT. The mechanisms of immune escape include downregulation of HLA gene expression or abnormal regulation of immune checkpoints18. Programmed death receptor 1 (PD-1) is an essential immune checkpoint inhibitory molecule on the surface of T cells, and its increased expression is a crucial mechanism leading to tumour immune escape19,20. Studies have shown that increased PD-1 expression is one of the mechanisms underlying the persistence of MRD and relapse after HSCT21. Theoretically, treatment with anti-PD-1 monoclonal antibodies (mAb) may be effective; however, data on MRD positivity after transplantation are lacking. In this study, we summarised the safety profile and preliminary efficacy of anti-PD-1 antibodies in patients with MRD after transplantation who failed DLI and/or interferon therapy.
Patients and Methods
Study design
This retrospective analysis included patients with AML or MDS who were MRD-positive after allo-HSCT and who received anti-PD-1 antibodies at Peking University People's Hospital between 1 January, 2022 and 31 March, 2022. According to expert consensus on ethical review exemption practices in medical institutions in China, it can be exempted from ethical review. All participants signed an informed consent document prior to enrolment in the study. The study was conducted in accordance with the principles of the Declaration of Helsinki.
Transplantation protocols
Patients who underwent haploidentical transplantation were pre-treated with a modified busulfan-based conditioning regimen, including cytarabine (4g/m2/d) on days -10 to -9, busulfan (3.2 mg/kg/d) on days -8 to -6, cyclophosphamide (1.8 g/m2/d) on days -5 to -4, oral semustine (250 mg/m2) on day -3, and anti-thymocyte globulin (2.5 mg/kg/d) on days -5 to -2. Patients who received a human leukocyte antigen-matched sibling donor transplant received the same regimen as described above but without anti-thymocyte globulin. Granulocyte colony-stimulating factor (5 ug/kg/d for 5 days) was used to mobilise donor bone marrow and/or peripheral blood. Prophylaxis against GVHD consisted of the immunosuppressive agents, cyclosporin A, mycophenolate mofetil, and short-term methotrexate. The detailed method has been described in our previous publication22.
MRD monitoring and intervention
MRD was monitored at 1, 2, 3, 4.5, 6, 9, and 12 months after allogeneic transplantation and at 6-month intervals thereafter. Both polymerase chain reaction (PCR) and multiparameter flow cytometry (FCM) were used to ensure the sensitivity and specificity of MRD monitoring. The expression of Wilms' tumour gene 1 or
The management of anti-PD-1 antibodies
Treatment with anti-PD-1 antibodies was considered in AML/MDS patients who met the following criteria: (1) MRD-positive status was confirmed after transplantation, (2) patients had refractory and recurrent MRD positivity after interferon or DLI therapy, and (3) there was no acute or chronic GVHD and no active infection before entering the study. Anti-PD-1 mAb (Tislelizumab, 240 mg every 2-3 weeks) administered alone or in combination with AZA (Azacitidine, 100 mg qd for 5-7 days) until disease progression or grade ≥ 3 nonhematologic toxicity occurred. Peripheral blood samples from healthy donors and from patients before and 2-3 weeks after treatment with the PD-1 mAb were collected, and the PD-1 expression levels on T cell subsets were measured by FCM. The monoclonal antibodies used included antihuman PD-1-PE-Cy7 (clone EH12.1) (BD Biosciences). T cells were divided into four subsets using the CD45RA and CCR7 expression: Naive T cells, CCR7+CD45RA+, central memory T cells CCR7+CD45RA-, effector memory T cells, CCR7-CD45RA- and effector T cells, CCR7-CD45RA+. The details have been described previously26.
Efficacy assessment
(1) The treatment response was defined as at least a 1-log decrease in MRD results detected by multiplex PCR or FCM after treatment compared to pre-treatment. (2) MRD negative was defined as the negative detection of MRD using PCR or FCM. (3) Overall survival was defined as the time from the date of anti-PD-1 mAb administration to the date of death due to any cause or the last follow-up date.
Safety assessment
The severity of treatment-related adverse events was assessed using the National Cancer Institute's Common Terminology Criteria for Adverse Events version 5.0. The diagnosis and severity of chronic GVHD (cGVHD) were graded according to the Chinese Expert Consensus Guidelines (2021 edition)27.
Follow-up
Follow-up was primarily conducted through outpatient visits and phone calls and ended on 8 February, 2023.
Statistical analysis
Safety was evaluated in all patients receiving anti-PD-1 mAb, and efficacy was evaluated in patients who underwent bone marrow aspiration at > 2 weeks after treatment. SPSS software (version 26.0 IBM Corp Armonk, NY USA) was used for the statistical analysis. Descriptive statistics were used to analyse the demographic and clinical characteristics of the patients.
Results
Patient characteristics
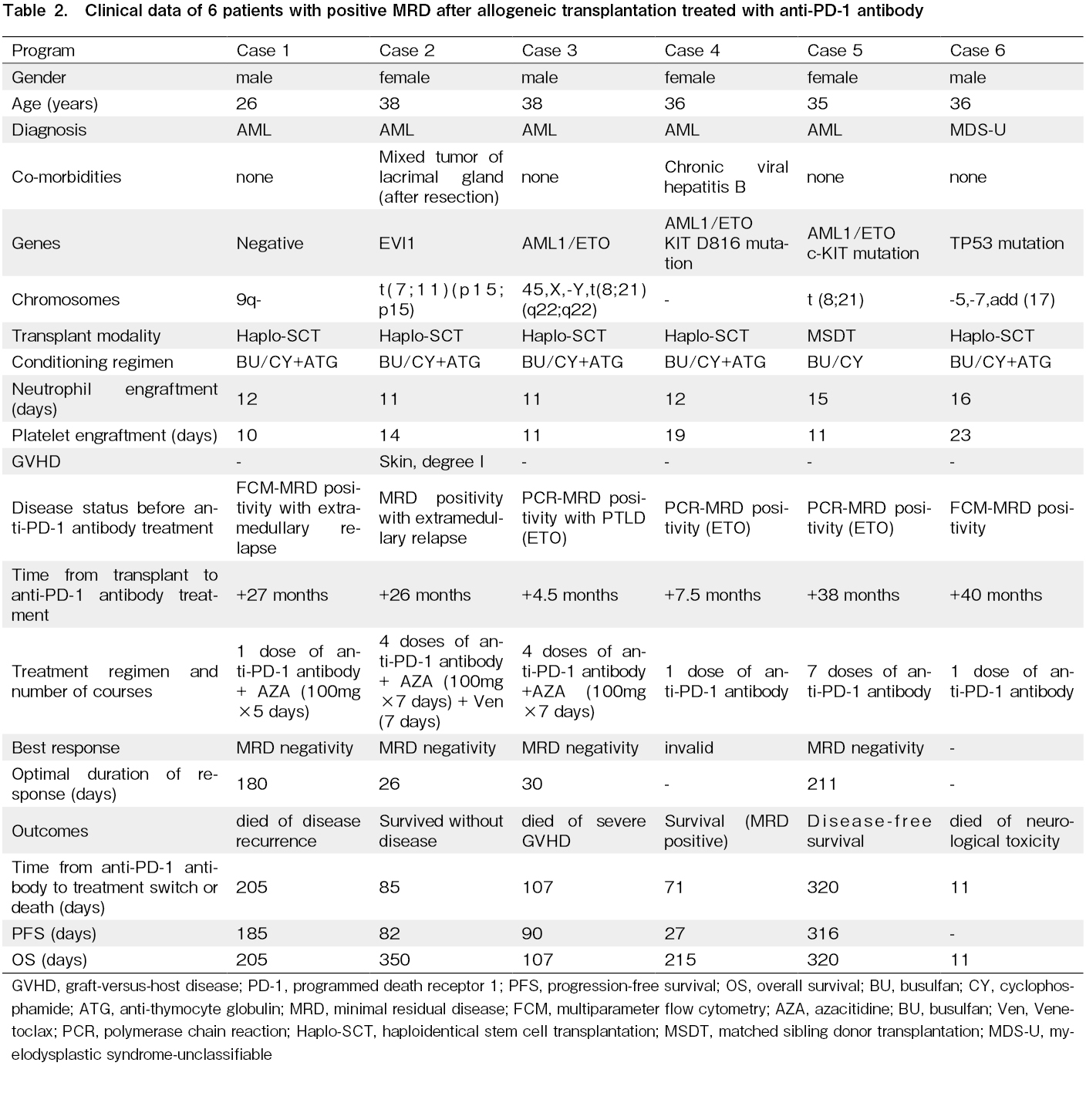
The patients' characteristics are summarized in Table 1. The median age of the six patients was 36 years (range: 26-38 years). Five patients were diagnosed with AML and one with MDS. Five patients underwent haploidentical transplantation, and one underwent HLA-matched sibling transplantation. Three patients were
MRD positive status and previous interventions prior to treatment with anti-PD-1 mAb
Three patients were positive for AML1/ETO PCR, and the level of AML1/ETO was above 0.1% and showed an upward trend before the administration of anti-PD-1 treatment. One patient was also positive by multiparameter flow cytometry. Of the six patients, the median interval from transplantation to the first MRD positivity was 135 days (range: 72-831 days). Four patients (66.7%) discontinued immunosuppressive agents after the first positive MRD, four patients (66.7%) received interferon therapy, and six patients (100%) received chemotherapy plus DLI. All patients had received two or more post-transplantation interventions prior to the anti-PD-1 mAb treatment.
Efficacy analysis
The median time from transplantation to the first dose of anti-PD-1 mAb was 796 days (range: 146-1,214 days) and the median time from the first MRD positivity to the first dose of PD-1 mAb was 430 (74-1,087) days (Table 1). Three patients were administered only one dose, two had four doses, and one had 7 doses. Two patients discontinued treatment due to severe GVHD, two due to disease progression, one due to a fatal side effect, and the remaining case due to coronavirus disease 2019 (COVID-19). One patient developed red maculopapular rash, pulmonary infection, and seizures after anti-PD-1 antibody treatment. Anti-infection and sedative treatment were given, but the patient died of sudden respiratory and cardiac arrest 5 days after anti-PD-1 antibody treatment and was excluded from the evaluation. Overall, four of the five evaluated patients (80%) achieved MRD negativity at a median of 2 months (range: 1-3 months) after a median of 3 doses (range: 1-5 doses), and the median duration of MRD negativity was 105 days (range: 26-211 days). Of the four patients with negative MRD, three tested positive again at 82, 90, and 316 days after treatment. One patient remained MRD-negative but had an extramedullary relapse. Of the five patients, one died of relapse, one died of cGVHD, and three survived. The patient with severe cGVHD of skin and liver died of progressive liver failure even after the administration of methylprednisolone, anti-CD25 monoclonal antibody and plasma exchange. At the last follow-up, the median survival time after anti-PD-1 mAb intervention was 320 days (range: 107-350 days).
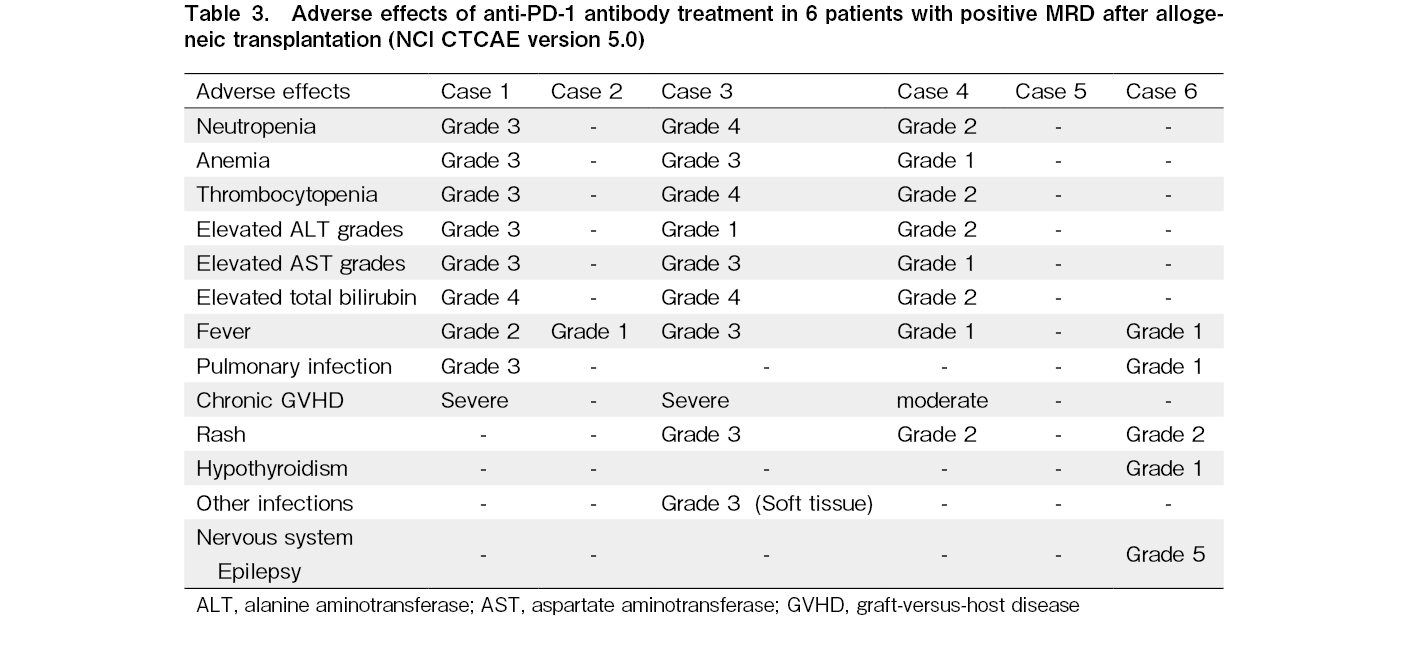
Safety analysis
In this study, five of six patients experienced adverse events, with 50% grade 3-4 and one grade 5 adverse event. Common toxicities were myelosuppression, fever, and GVHD. Three patients developed grade 3 or 4 myelosuppression and five patients had fever, but all recovered spontaneously after withdrawal. Three cases were considered as cGVHD based on the typical manifestations of multiple organ involvement, liver involvement mainly with elevated bilirubin, and treatment response, one had moderate cGVHD and two had severe cGVHD. Two of the three patients showed improvement after treatment. The occurrence of immune-related adverse events (irAEs) after drug administration was 83%, mainly fever, including one case of immune-related thyroiditis. Clinical data for the six patients are presented in Table 2 and adverse reactions to the anti-PD-1 mAb therapy are presented in Table 3.
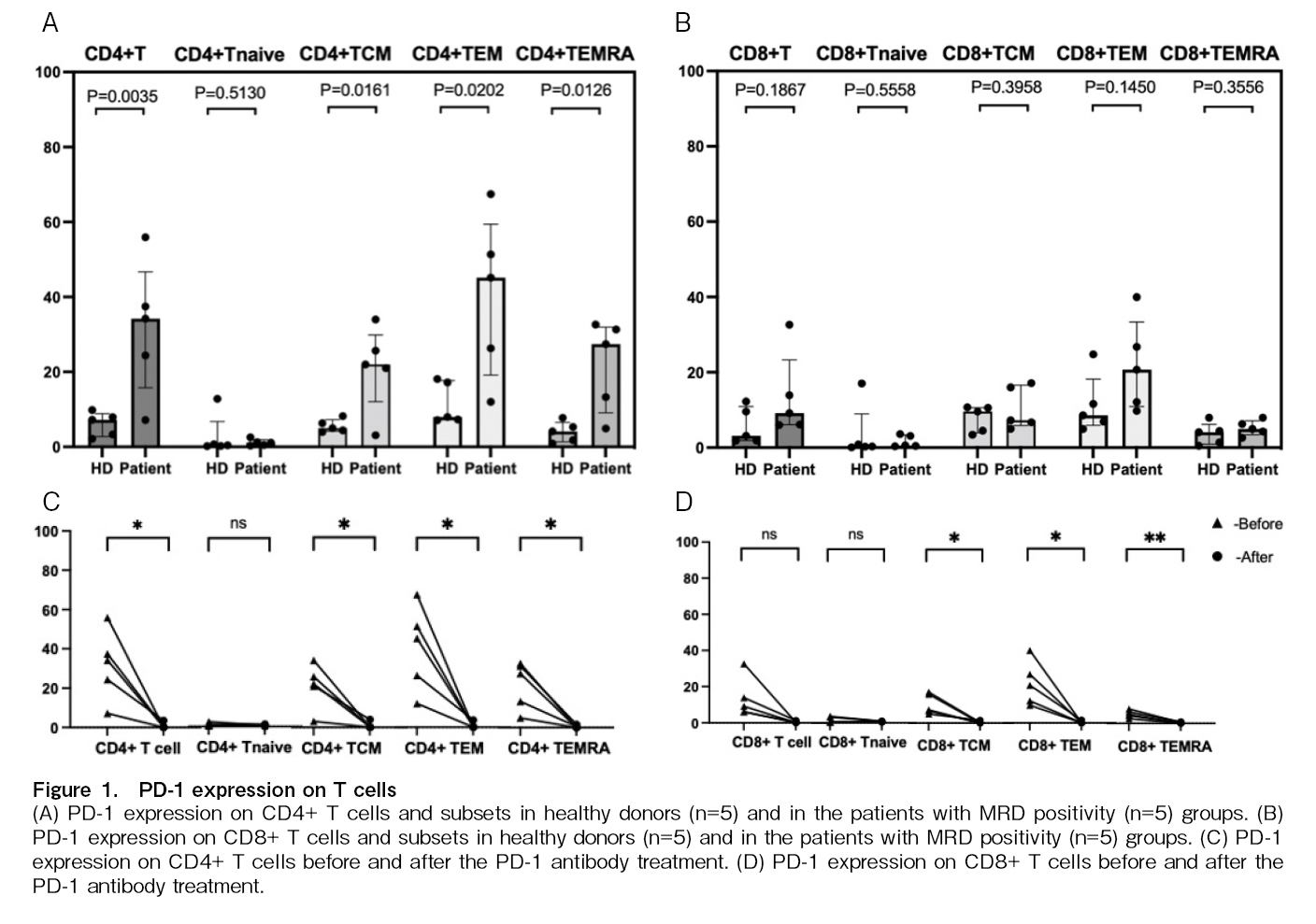
Monitoring of PD-1 levels before and after administration of anti-PD-1 mAb
PD-1 expression on T cells in five patients before and 2-3 weeks after treatment with PD-1 mAb and in five healthy donors was measured by FCM. Higher expression of PD-1 on subsets of CD4+ T cells and
Discussion
We report the preliminary data of anti-PD-1 mAb in six AML/MDS patients with recurrent positive MRD following allo-HSCT and multiple lines of treatment, including interferon, chemotherapy, and DLI intervention. These results demonstrate the safety and efficacy of anti-PD-1 mAb alone or in combination with AZA in AML/MDS patients with positive MRD following allo-HSCT.
Several studies19,20 have shown that the PD-1 pathway is an immune escape mechanism of cancer stem cells after allogeneic transplantation. However, there are limited data on the safety and efficacy of immune checkpoint inhibitors in patients with myeloid neoplasms after transplantation. In animal models, PD-1 pathway blockade therapy has shown potent anti-leukaemic effects but is also associated with enhanced GVHD in xenografted nude mice28. Two retrospective cohort studies found that anti-PD-1 mAb also showed anti-tumour activity in Hodgkin's lymphoma that relapsed after transplantation but was also associated with severe and refractory GVHD29,30. The use of anti-PD-1 mAb in AML/MDS patients after transplantation has rarely been reported. Recently, a multicenter phase 1 study31 of nivolumab for relapsed hematologic malignancies after allo-HSCT showed an objective response rate of 21% in nine patients with myeloid malignancies. Another prospective study32 showed no objective response to pembrolizumab in nine patients with relapsed myeloid malignancies after allo-HSCT. Overall, the effect of monotherapy was poor. Qian et al.33 reported two cases of anti-PD-1 mAb combined with AZA and low-dose DLI in the treatment of AML with haematological relapse after transplantation; both cases achieved complete remission, which lasted for 101 days and 257 days, respectively. Tang et al.34 reported that anti-PD-1 mAb combined with AZA were effective for the treatment of AML (AML1/ETO), with the first molecular relapse occurring after transplantation. In our study, six patients who remained refractory with recurrent positive MRD after transplantation, which was converted to positive after previous treatment with interferon, chemotherapy, and DLI while some had a haematologic relapse. Of the five evaluated patients, four responded and eventually achieved MRD negativity.
However, the duration of these responses was insufficient (28-294 days). In clinical trials of relapsed and refractory AML, the clinical effect of a PD-1 inhibitor as a single agent was poor; eight patients received a single dose of PD-1 inhibitor, and only one patient responded35. The escape mechanism of PD-1 inhibitor therapy includes the reduction of tumour antigen expression level, downregulation of the major histocompatibility complex, and loss of costimulatory ligand expression36, while demethylation drugs can inhibit the immune response by upregulating the expression of PD-1, PD-L1, PD-L2, and CTLA-437, which is related to the emergence of drug resistance. Thus, the combined application of hypomethylating agents (HMA) and PD-1/PD-L1 inhibitors may exert stronger antitumour effects. In a phase II clinical trial38, nivolumab (a PD-1 inhibitor) combined with AZA was evaluated in 70 AML patients with previous treatment failure (including HMA treatment). The overall response rate was 33%, which was 20% higher than that of relapsed/refractory-AML patients at centres that had previously participated in single-agent and combination HMA trials. The results showed that patients without previous HMA treatment exhibited a better response rate than those with previous HMA treatment (overall response rate, 58% vs. 22%). The frequency of bone marrow aspirate CD3+T cells prior to AZA + nivolumab treatment was significantly higher than that in non-responders, and there was a trend toward higher frequencies of T effector and CD8+T cells. Several studies on the use of PD-1 inhibitors in combination with HMA for the treatment of AML/MDS are currently ongoing39.
Studies have shown that patients with lymphoma who receive early PD-1 blockade therapy after allogeneic transplantation are more likely to develop GVHD29,30; however, the definition of
Previous studies26,42,43 have shown T cell exhaustion in patients with relapsed AML after transplantation, which can be reversed by DLI, and is associated with sustained complete remission26. This study showed that patients with MRD after transplantation also had T cell exhaustion, and treatment with a PD-1 mAb reduced the expression of PD-1 on the surface of T cells; however, no correlation was found between PD-1 expression and MRD negativity.
The limitations of this study included the limited number of cases and inadequate monitoring of adverse events, including non-specific reactions, such as fatigue and loss of appetite. In addition, we did not confirm that there was no competition between the therapeutic PD-1 antibody and the PD-1 antibody for expression evaluation, it was possible that the epitope was masked by the therapeutic PD-1 antibody when evaluating PD-1 expression after treatment.
Overall, the initial efficacy of anti-PD-1 monoclonal antibodies in the treatment of post-transplant MRD-positive myeloid neoplasms was positive, but their durability was modest. Additional studies are needed to determine whether unsatisfactory efficacy is associated with late intervention and the number of prior lines of treatment. The safety profile of anti-PD-1 mAb in the treatment of MRD-positive patients after allo-HSCT is acceptable, and its efficacy merits further exploration.
Acknowledgments
We thank all the faculty members who participated in this study. The authors would also like to thank Editage (www.editage.cn) for assistance in editing this manuscript.
Author Contributions
YQS and XJH designed the study, YQS revised the paper; LM collected the data, analyzed the data, and drafted the manuscript; all authors contributed to the data interpretation, manuscript preparation, and approval of the final version.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website. YQS is one of the editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
Acknowledgments
We thank all the faculty members who participated in this study. The authors would also like to thank Editage (www.editage.cn) for assistance in editing this manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant number: 8227010768) and the National Key Research and Development Program of China (grant number: 2021YFC2500300).
Acknowledgments
We thank all the faculty members who participated in this study. The authors would also like to thank Editage (www.editage.cn) for assistance in editing this manuscript.
Data Availability
The dataset supporting the conclusions of this article is available at the clinical data repository of Peking University People's Hospital and Peking University Institute of Hematology, National Clinical Research Center for Hematologic Disease, Beijing Key Laboratory of Hematopoietic Stem Cell Transplantation, No.11 South Street of Xizhimen, Xicheng District, Beijing, 100044, China.
References
1.Cagnetta A, Adamia S, Acharya C, Patrone F, Miglino M, Nencioni A, et al. Role of genotype-based approach in the clinical management of adult acute myeloid leukemia with normal cytogenetics. Leuk Res. 2014; 38: 649-59.
2.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016; 127: 62-70.
3.Oran B, Jorgensen JL, Marin D, Wang S, Ahmed S, Alousi AM, et al. Pre-transplantation minimal residual disease with cytogenetic and molecular diagnostic features improves risk stratification in acute myeloid leukemia. Haematologica. 2017; 102: 110-7.
4.Barrett AJ, Locatelli F, Treleaven JG, Gratwohl A, Szydlo R, Zwaan FE. Second transplants for leukaemic relapse after bone marrow transplantation: high early mortality but favourable effect of chronic GVHD on continued remission. A report by the EBMT Leukaemia Working Party. Br J Haematol. 1991; 79: 567-74.
5.Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhäuser M, et al. Second allograft for hematologic relapse of acute leukemia after first allogeneic stem-cell transplantation from related and unrelated donors: the role of donor change. J Clin Oncol. 2013; 31: 3259-71.
6.Schuurhuis GJ, Heuser M, Freeman S, Béné MC, Buccisano F, Cloos J, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018; 131: 1275-91.
7.Chinese Society of Immunology, Clinical Flow Cytometry Group. Expert consensus on minimal residual disease detection of acute leukemia and plasma cell neoplasms by multi-parameter flow cytometry. Chin J Hematol. 2017; 38: 1001-11. in Chinese.
8.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012; 119: 3256-62.
9.Shen MZ, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Preemptive Interferon-alpha Therapy Could Protect Against Relapse and Improve Survival of Acute Myeloid Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation: Long-Term Results of Two Registry Studies. Front Immunol. 2022; 13: 757002.
10.Zhou Q, Munger ME, Highfill SL, Tolar J, Weigel BJ, Riddle M, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010; 116: 2484-93.
11.Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Comparison of outcomes after donor lymphocyte infusion with or without prior chemotherapy for minimal residual disease in acute leukemia/myelodysplastic syndrome after allogeneic hematopoietic stem cell transplantation. Ann Hematol. 2017; 96: 829-38.
12.Platzbecker U, Middeke JM, Sockel K, Herbst R, Wolf D, Baldus CD, et al. Measurable residual disease-guided treatment with azacitidine to prevent haematological relapse in patients with myelodysplastic syndrome and acute myeloid leukaemia (RELAZA2): an open-label, multicentre, phase 2 trial. Lancet Oncol. 2018; 19: 1668-79.
13.Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. IFN-alpha Is Effective for Treatment of Minimal Residual Disease in Patients with Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant. 2017; 23: 1303-10.
14.Mo XD, Wang Y, Zhang XH, Xu LP, Yan CH, Chen H, et al. Interferon-alpha Is Effective for Treatment of Minimal Residual Disease in Patients with t(8;21) Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Prospective Registry Study. Oncologist. 2018; 23: 1349-57.
15.Williams P, Basu S, Garcia-Manero G, Hourigan CS, Oetjen KA, Cortes JE, et al. The distribution of T-cell subsets and the expression of immune checkpoint receptors and ligands in patients with newly diagnosed and relapsed acute myeloid leukemia. Cancer. 2019; 125: 1470-81.
16.Chen C, Liang C, Wang S, Chio CL, Zhang Y, Zeng C, et al. Expression patterns of immune checkpoints in acute myeloid leukemia. J Hematol Oncol. 2020; 13: 28.
17.Duan J, Cui L, Zhao X, Bai H, Cai S, Wang G, et al. Use of Immunotherapy With Programmed Cell Death 1 vs Programmed Cell Death Ligand 1 Inhibitors in Patients With Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2020; 6: 375-84.
18.Daver N, Alotaibi AS, Bücklein V, Subklewe M. T-cell-based immunotherapy of acute myeloid leukemia: current concepts and future developments. Leukemia. 2021; 35: 1843-63.
19.Norde WJ, Maas F, Hobo W, Korman A, Quigley M, Kester MG, et al. PD-1/PD-L1 interactions contribute to functional T-cell impairment in patients who relapse with cancer after allogeneic stem cell transplantation. Cancer Res. 2011; 71: 5111-22.
20.Vago L. Clonal evolution and immune evasion in posttransplantation relapses. Hematology Am Soc Hematol Educ Program. 2019; 2019: 610-6.
21.Saudemont A, Quesnel B. In a model of tumor dormancy, long-term persistent leukemic cells have increased B7-H1 and B7.1 expression and resist CTL-mediated lysis. Blood. 2004; 104: 2124-33.
22.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006; 107: 3065-73.
23.Zhao XS, Jin S, Zhu HH, Xu LP, Liu DH, Chen H, et al. Wilms' tumor gene 1 expression: an independent acute leukemia prognostic indicator following allogeneic hematopoietic SCT. Bone Marrow Transplant. 2012; 47: 499-507.
24.Qin YZ, Wang Y, Xu LP, Zhang XH, Chen H, Han W, et al. The dynamics of RUNX1-RUNX1T1 transcript levels after allogeneic hematopoietic stem cell transplantation predict relapse in patients with t(8;21) acute myeloid leukemia. J Hematol & Oncol. 2017; 10: 44.
25.Wang Y, Chen H, Chen J, Han M, Hu J, Jiong H, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett. 2018; 438: 63-75.
26.Liu L, Chang YJ, Xu LP, Zhang XH, Wang Y, Liu KY, et al. Reversal of T Cell Exhaustion by the First Donor Lymphocyte Infusion Is Associated with the Persistently Effective Antileukemic Responses in Patients with Relapsed AML after Allo-HSCT. Biol Blood Marrow Transplant. 2018; 7: 1350-9.
27.Hematopoietic Stem Cell Application Group, Chinese Society of Hematology, Chinese Medical Association; China Association for the Prevention of Hematology Diseases. Chinese consensus on the diagnosis and management of chronic graft-versus-host disease (2021). Chin J Hematol. 2021; 42: 265-75. in Chinese.
28.Sanmamed MF, Rodriguez I, Schalper KA, Oñate C, Azpilikueta A, Rodriguez-Ruiz ME, et al. Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2-/-IL2Rγnull Immunodeficient Mice. Cancer Res. 2015; 75: 3466-78.
29.Herbaux C, Gauthier J, Brice P, Drumez E, Ysebaert L, Doyen H, et al. Efficacy and tolerability of nivolumab after allogeneic transplantation for relapsed Hodgkin lymphoma. Blood. 2017; 129: 2471-8.
30.Haverkos BM, Abbott D, Hamadani M, Armand P, Flowers ME, Merryman R, et al. PD-1 blockade for relapsed lymphoma post-allogeneic hematopoietic cell transplant: high response rate but frequent GVHD. Blood. 2017; 130: 221-8.
31.Davids MS, Kim HT, Costello C, Herrera AF, Locke FL, Maegawa RO, et al. A multicenter phase 1 study of nivolumab for relapsed hematologic malignancies after allogeneic transplantation. Blood. 2020; 135: 2182-91.
32.Godfrey J, Liu H, Yu J, Tallarico M, Curran E, Artz AS, et al. Pembrolizumab for the treatment of disease relapse after allogeneic hematopoietic stem cell transplantation. Blood Adv. 2023; 7: 963-70.
33.Qian CS, Ma X, Wang J, Wang TJ, Bai L, Zhou HX, et al. PD1 inhibitor in combination with 5-azacytidine and low-dose DLI for the successful treatment of AML patients who relapsed after transplantation. Bone Marrow Transplant. 2021; 56: 1003-5.
34.Tang Y, Zhou Z, Yan H, You Y. Case Report: Preemptive Treatment With Low-Dose PD-1 Blockade and Azacitidine for Molecular Relapsed Acute Myeloid Leukemia With RUNX1-RUNX1T1 After Allogeneic Hematopoietic Stem Cell Transplantation. Front Immunol. 2022; 13: 810284.
35.Berger R, Rotem-Yehudar R, Slama G, Landes S, Kneller A, Leiba M, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res. 2008; 14: 3044-51.
36.Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating agents in combination with immune checkpoint inhibitors in acute myeloid leukemia and myelodysplastic syndromes. Leukemia. 2018; 32: 1094-105.
37.Yang H, Bueso-Ramos C, DiNardo C, Estecio MR, Davanlou M, Geng QR, et al. Expression of PD-L1, PD-L2, PD-1 and CTLA4 in myelodysplastic syndromes is enhanced by treatment with hypomethylating agents. Leukemia. 2014; 28: 1280-8.
38.Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov. 2019; 9: 370-83.
39.Jimbu L, Mesaros O, Popescu C, Neaga A, Berceanu I, Dima D, et al. Is There a Place for PD-1-PD-L Blockade in Acute Myeloid Leukemia?. Pharmaceuticals (Basel). 2021; 14: 288.
40.Leotta S, Condorelli A, Sciortino R, Milone GA, Bellofiore C, Garibaldi B, et al. Prevention and Treatment of Acute Myeloid Leukemia Relapse after Hematopoietic Stem Cell Transplantation: The State of the Art and Future Perspectives. J Clin Med. 2022; 11: 253.
41.Holderried TAW, Fraccaroli A, Schumacher M, Heine A, Brossart P, Stelljes M, et al. The role of checkpoint blockade after allogeneic stem cell transplantation in diseases other than Hodgkin's Lymphoma. Bone Marrow Transplant. 2019; 54: 1662-7.
42.Noviello M, Manfredi F, Ruggiero E, Perini T, Oliveira G, Cortesi F, et al. Bone marrow central memory and memory stem T-cell exhaustion in AML patients relapsing after HSCT. Nat Commun. 2019; 10: 1065.
43.Kong Y, Zhu L, Schell TD, Zhang J, Zheng H, Claxton DF, et al. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016; 22: 3057-66.
Search
News