Volume 8 (2025) Issue 1 No.4 Pages 167-169
Abstract
Graft rejection is an important concern following haploidentical transplant with a reported incidence of 10-15%. The number of human leukocyte antigen (HLA) mismatches and the vector of mismatch have not been found to be associated with the risk of graft rejection in haploidentical transplants. Patients with HLA homozygosity at all loci (HLA-A, B, C, DRB1, and DQB1) undergoing haploidentical transplant is a rare scenario that results in zero mismatches in the graft-versus-host (GvH) direction and 2-5 mismatches in the host-versus-graft (HvG) direction depending on the donor haplotype. This results in a heavily skewed vector of HLA mismatch with unopposed allo-reactivity in the HvG direction. We reviewed our haploidentical transplant database for patients who were homozygous at all five loci and studied their outcomes. Seventy-one patients underwent haploidentical transplant at our center for malignant indications between July 1, 2010, and June 30, 2022. All but one patient received PTCy-based graft-versus-host disease (GvHD) prophylaxis. Of these 71 patients, two were homozygous at all five loci, and both patients developed graft rejection (100%). This was significantly higher than the risk of rejection in the remaining 69 patients where 5/69 (7.2%) had rejection (p=0.0085). Herein, we describe these two cases and review the literature on the impact of patient HLA homozygosity on graft rejection in patients undergoing haploidentical transplant.
Introduction
Haploidentical stem cell transplant using post-transplant cyclophosphamide (PTCy) is being increasingly used for both malignant and benign hematological disorders. Graft rejection is an important concern following haploidentical transplant with a reported incidence of 10-15%1. Presence of donor specific antibodies (DSA) is the most important risk factor for graft rejection2. Other risk factors include T-cell-depleted (TCD) grafts and the use of reduced intensity conditioning regimens. The number of human leukocyte antigen (HLA) mismatches and the vector of mismatch have not been found to be associated with the risk of graft rejection in haploidentical transplants3. Patients with HLA homozygosity at all loci (HLA-A, B, C, DRB1, and DQB1) undergoing haploidentical transplant is a rare scenario that results in zero mismatches in the graft-versus-host (GvH) direction and 2-5 mismatches in the host-versus-graft (HvG) direction depending on the donor haplotype. This results in a heavily skewed vector of HLA mismatch with unopposed allo-reactivity in the HvG direction. There is limited literature on the impact of patient HLA homozygosity on transplant outcomes in patients undergoing haploidentical transplant.
We reviewed our haploidentical transplant database for patients with HLA homozygosity at all loci. Seventy-one patients underwent haploidentical transplant for malignant indications at our center between July 1, 2010, and June 30, 2022. All but one patient received PTCy-based graft-versus-host disease (GvHD) prophylaxis. Of these 71 patients, two were homozygous at all five loci and both patients developed graft rejection within three months of transplant (100%). This was significantly higher than the risk of rejection in the remaining 69 patients where 5/69 (7.2%) had rejection (p=0.0085). In this report, we describe these two cases and review the literature on the impact of patient HLA homozygosity on graft rejection in patients undergoing haploidentical transplant.
Case Presentation
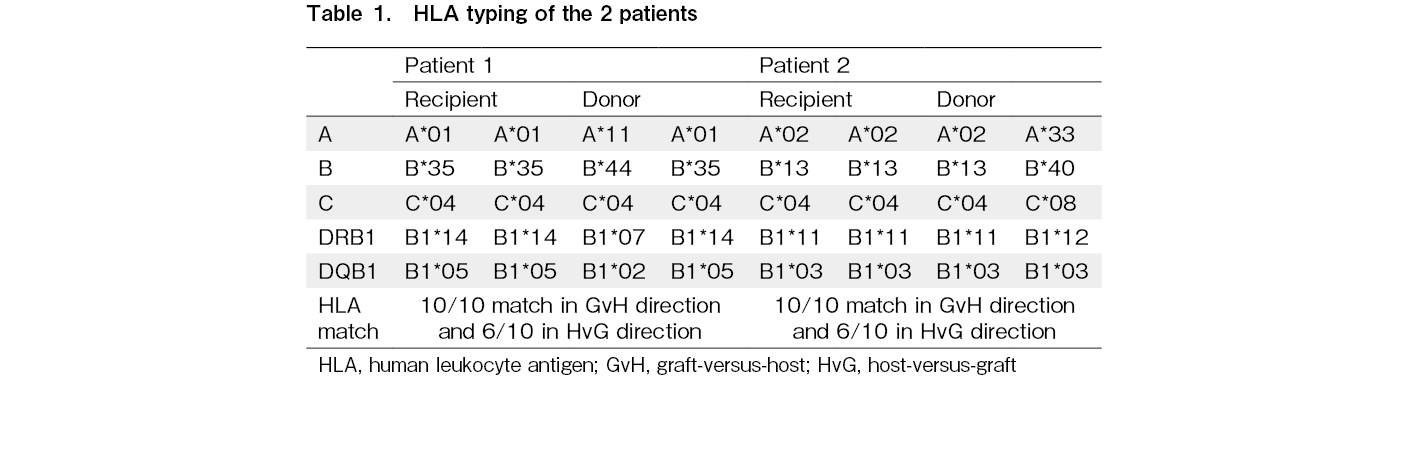
Patient 1 was a 17-year-old male who underwent haploidentical transplant for Ph-positive AML in first complete remission. Pre-transplant bone marrow flow MRD was negative and BCR-ABL PCR was 0.178%. The patient was homozygous at HLA-A, B, C, DQB1, and DRB1. The donor was his father who was heterozygous at four of these five loci except HLA-C (Table 1). There were no mismatches in the GvH direction and four mismatches in the HvG direction. DSA was negative. He received Flu-Mel (melphalan 140 mg/m2) conditioning followed by peripheral blood stem cell (PBSC) graft (5.32 million CD34 cells/kg patient weight). PTCy (50 mg/kg) on day 3 and day 4 followed by CSA and MMF from day 5 onwards was used for GvHD prophylaxis. He achieved neutrophil and platelet engraftment on days 14 and 17, respectively. On day 30 and day 60, peripheral blood chimerism was 100% donor by the variable nucleotide tandem repeat (VNTR) method. He did not develop acute GvHD. On day 90, bone marrow was MRD negative with 83% donor chimerism. BCR-ABL was 0.168%. In view of mixed chimerism, CSA was rapidly tapered and discontinued on day 112. A repeat marrow on day 111 showed a further decline in chimerism to 52% and PCR remained stable at 0.160%. In view of persistent molecular MRD and the drop in chimerism, he was started on dasatinib from day 121. He also received a donor lymphocyte infusion (DLI) on day 132 with a CD3 dose of 4.39 million cells/kg patient body weight. Peripheral blood chimerism further declined to < 3% on day 150 post-transplant. He was continued on dasatinib and remains disease-free seven years post-transplant.
Patient 2 was a 22-year-old male with CML in chronic phase with T315I mutation and failure of tyrosine kinase inhibitors. He underwent haploidentical transplant in the first chronic phase. Pre-transplant PCR was 252% in peripheral blood. The patient was homozygous and the donor, his brother, was heterozygous at four of the five loci except HLA DQB1 (Table 1). The patient tested negative for antibodies against donor HLA. He received conditioning with Flu-Treo (treosulfan dose 36 mg/m2) and 2 Gy TBI followed by infusion of PBSC graft (6.33 million CD34 cells/kg patient weight). Standard PTCy with tacrolimus and MMF was used as GvHD prophylaxis. He achieved platelet engraftment on day 11 and neutrophil engraftment on day 14 with 85% donor chimerism by VNTR. Day 27 peripheral blood chimerism was 90% donor and BCR-ABL was 40.96%. A repeat chimerism after a week was 51% donor. For mixed chimerism and residual disease, tacrolimus was stopped without taper on day 48. Bone marrow on day 69 showed CML in chronic phase with no excess blasts and 15% donor chimerism. He was given DLI with a CD3 dose of 8.85 million cells/kg patient weight on day 69. Chimerism dropped further to < 3% by day 83. A second transplant was not feasible due to financial constraints. He had progressive disease and succumbed to his illness 18 months post-transplant.
Discussion
In our cohort, we found a high incidence of graft rejection in patients with HLA homozygosity who underwent haploidentical transplant using PTCy. Homozygosity at all loci is rare. In Japan, the incidence of homozygosity is higher due to highly prevalent haplotypes. In a retrospective analysis of nine patients with HLA homozygosity who underwent haploidentical transplant, two (22%) had graft rejection4. In a larger analysis of Japanese registry data, the authors reported outcomes of 78 patients with HLA homozygosity who underwent haploidentical transplant and compared it with HLA-matched transplants5. CSA with MTX/MMF was used for GvHD prophylaxis. No ex vivo T-cell depletion or post-transplant cyclophosphamide was used as the risk of GvHD was deemed low due to absent mismatches in the GvH direction. They found the outcomes to be comparable with a trend toward lesser engraftment in haploidentical transplants as compared to HLA-identical transplants (not statistically significant). The incidence of graft rejection was not reported. The notable difference between our patients and the Japanese cohort is the use of PTCy in our patients. PTCy depletes the donor alloreactive T cells, and this may further increase the risk of rejection in patients where the risk is already high due to unopposed HLA mismatches in the HvG direction. We could not find studies on outcomes of patients with HLA homozygosity who underwent haploidentical transplant using PTCy. In a study by Raiola et al. that included 318 patients who underwent haploidentical transplant using PTCy, the authors compared outcomes based on the degree and vector of HLA mismatches. They grouped patients as low (0-2) or high (3-4) HLA mismatches but did not specifically examine the outcomes of patients who were homozygous at all loci3.
From our experience, we believe haploidentical transplant using PTCy may not be the best strategy for patients with HLA homozygosity. In such patients, it may be best to use a non-PTCy-based GvHD prophylaxis if unrelated donor/cord transplant is not feasible. A larger, multicenter dataset is needed to confirm our findings.
Author Contributions
NJ and NK conceived the study. NJ, KP, MG, SM, AC, LN, AG, SP, BB, and LJM collected the clinical data. SDS and MS collected the HLA data. NJ and MG analyzed the data. NJ and NK wrote the paper with input from all authors.
Conflicts of Interest
NK is one of the editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Informed Consent
An informed consent was obtained from all patients at the time of transplant.
References
1.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplantation. 2008; 14: 641-50.
2.Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009; 88: 1019-24.
3.Raiola AM, Risitano A, Sacchi N, Giannoni L, Signori A, Aquino S, et al. Impact of HLA Disparity in Haploidentical Bone Marrow Transplantation Followed by High-Dose Cyclophosphamide. Biol Blood Marrow Transplant. 2018; 24: 119-26.
4.Ikegame K, Kaida K, Yoshihara S, Fujiwara M, Taniguchi K, Kato R, et al. Feasibility of unmanipulated haploidentical stem cell transplantation using standard GVHD prophylaxis for HLA-homozygous patients. Int J Hematol. 2012; 96: 101-8.
5.Kanda J, Ikegame K, Fuji S, Kurokawa M, Kanamori H, Fukuda T, et al. Haploidentical and Matched Sibling Donor Hematopoietic Cell Transplantation for Patients with HLA-Homozygous Haplotypes. Biol Blood Marrow Transplant. 2016; 22: 2031-7.
Search
News