Volume 7 (2024) Issue 4 No.7 Pages 129-137
Abstract
COVID-19 became a global pandemic in 2020 and significantly affected the activity of hematopoietic cell transplants (HCT) worldwide. Despite these challenges, a total of 28,793 transplants, including 18,518 allogeneic and 10,275 autologous transplants, were performed in 719 facilities in 2020 in the Asia-Pacific (AP) region. This represented a 5.1% increase in allogeneic transplants and a 3.1% increase in autologous transplants, an overall increase of 4.4% compared to the numbers in 2019. With respect to the donor source, haploidentical transplants increased significantly by 18.6%, related transplants by 8.8%, and cord blood transplants (CBT) by 9.2%. However, the number of unrelated transplants, excluding CBT, decreased for the first time by 8.2%. As a result, COVID-19 facilitated the growth of haploidentical transplants due to cross-border restrictions. Regarding the changes in the total number of transplants by country/region in 2020, it increased by 2,048 transplants in China, followed by Japan (210 transplants) and Korea (230 transplants); however, 14 of the 22 countries and regions decreased their number of transplants in 2020 compared to the previous year. There was no correlation between the increase or decrease in the number of transplants in 2020 and the Gross National Income (GNI) per capita of each country/region in 2020, as well as Domestic General Government Health Expenditure as a percentage of General Government Expenditure (GGHE-D/GGE). In 2021, the total number of transplants in this region was 34,754. With the exception of a few countries/regions that decreased the number of transplants in 2020, most countries/regions have started to see a recovery in 2021. The COVID-19 pandemic significantly affected the supply chain and logistics involved in HCT rather than its numbers; however, we have found ways to overcome logistical challenges to carry out transplant medicine without delay, even under these circumstances.
Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has significantly affected the activity of hematopoietic cell transplantation (HCT) in both developed and emerging countries/regions worldwide. Since the first documented case of COVID-19 was reported in December 2019, the infection spread globally with a high mortality rate from 2020 and 20211. As COVID-19 spread worldwide, the global medical community had to prioritize its treatments, so routine medical care could no longer be maintained. In particular, as COVID-19 infection is likely to become severe in immunocompromised hosts, HCT recipients needed to take strict precautions against infection for an extended period before, during, and after treatment. On the other hand, allogeneic HCT requires unique preparations before the treatment, such as securing donors and stem cell collection and transportation, and this process requires in-person work by many stakeholders. However, social restrictions for infection control significantly interfered with this series of tasks. Under these severe circumstances, organizations involved in HCT have made efforts to maintain transplant medicine as usual, coping with the pandemic, by reframing the entire medical system, adjusting HCT programs, and formulating HCT guidelines under COVID-19, both in international societies2 and in individual countries/regions3. Here, we report the changes in the number of transplants in the Asia-Pacific (AP) region during the two years from 2020 to 2021, at the beginning of the epidemic, compared with the previous period.
Materials and Methods
Data collection
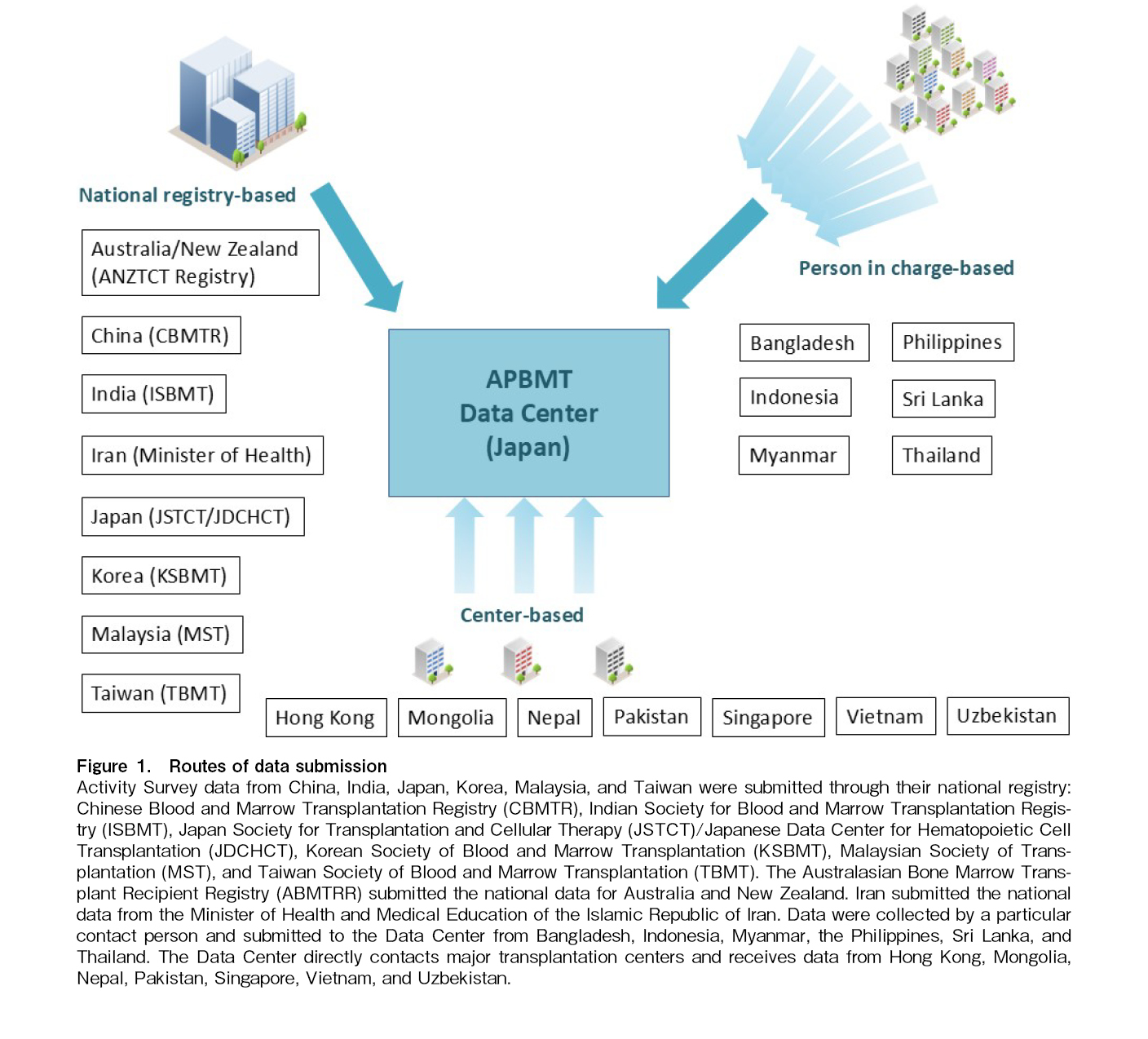
In 2023, 23 countries and regions are participating in the Asia-Pacific Blood and Marrow Transplantation Group (APBMT). Those include Australia, Bangladesh, Cambodia, China, Hong Kong, India, Indonesia, Iran, Japan, Korea (the Republic of Korea will be referred to as Korea in this paper), Malaysia, Mongolia, Myanmar, Nepal, New Zealand, Pakistan, Philippines, Singapore, Sri Lanka, Taiwan, Thailand, Uzbekistan, and Vietnam. As shown in Figure 1, excluding Cambodia where HCT has not yet started, 22 out of 23 countries/regions submitted the activity survey reports and reporting facility lists to the APBMT Data Center by email through different routes owing to the differences in the data collection systems in each country/region.
Statistical analyses and ethical approval
The APBMT Data Center performed all necessary analyses for this survey using Excel functions. To clarify the change of stem cell source selection in this period, unrelated transplants excluded cord blood transplants (CBT). As a result, unrelated transplants included only bone marrow (BM) and peripheral blood stem cells (PBSC) transplants. To avoid overestimating the increasing rate of the HCT activity in 2021, the increasing/decreasing rates of the year 2021 were calculated with the number of 2019 as the reference. We evaluated the increase or decrease in the number of transplants in each country/region in 2019 and 2020, the delta value was calculated using the following formula:
The total population of each country/region was extracted from World Bank4. The Gross National Income (GNI) indicates the total income calculated by adding the gross domestic product (GDP), which represents the total amount of value generated in the country, to income from abroad, and was extracted from World Bank Group5. GNI per capita in each country/region was calculated and expressed in US dollars for 2020. Domestic General Government Health Expenditure as a percentage of General Government Expenditure (GGHE-D/GGE) is defined as the ratio of general government health expenditure to general government expenditure in each country/region and was extracted from the World Health Organization6. The correlation between GNI/capita or GGHE-D/GGE and the delta value was evaluated by a coefficient of determination (R2) using Excel functions.
As we collected only the annual number of all types of HCTs, the Data Center and registries/facilities did not obtain informed consent from each patient. The APBMT Activity Survey was approved by the Institutional Review Board of the Aichi Medical University School of Medicine (2016-M029) and the APBMT Registry Committee.
Results
Number of reported facilities and all transplants
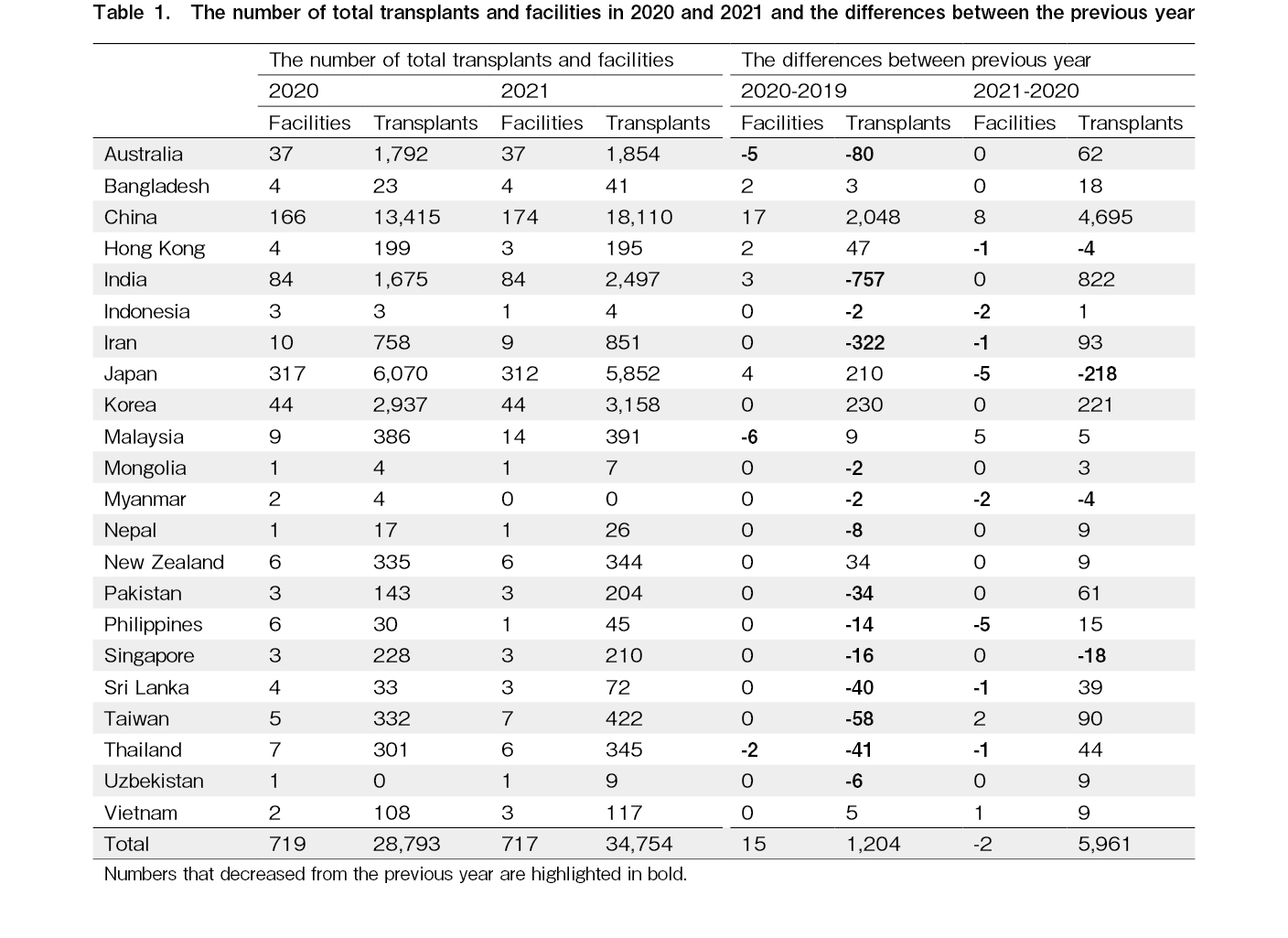
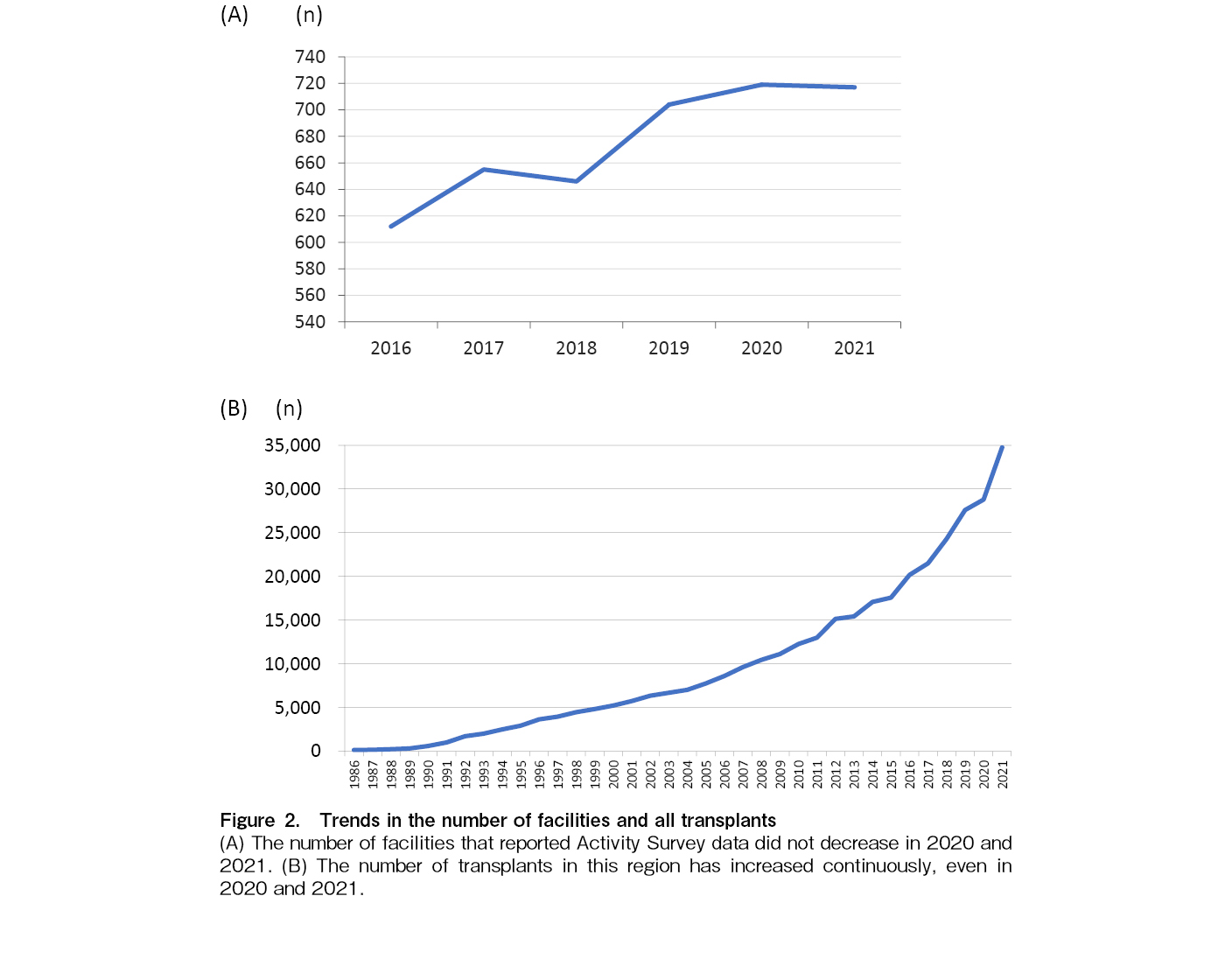
The number of facilities reporting data was 719 and 722 in 2020 and 2021, respectively (Table 1). Despite the COVID-19 pandemic, the number of transplant facilities in this region has increased continuously since 2016 (Figure 2A). As shown in Figure 2B, the total number of annual transplants did not decrease in 2020 and increased steeply in 2021. The total number of transplants in 2020 was 28,793, an increase of 1,204 compared to 2019, and that in 2021 was 34,754, an increase of 5,961 compared to 2020 (Figure 2B).
Trends of HCT by stem cell sources
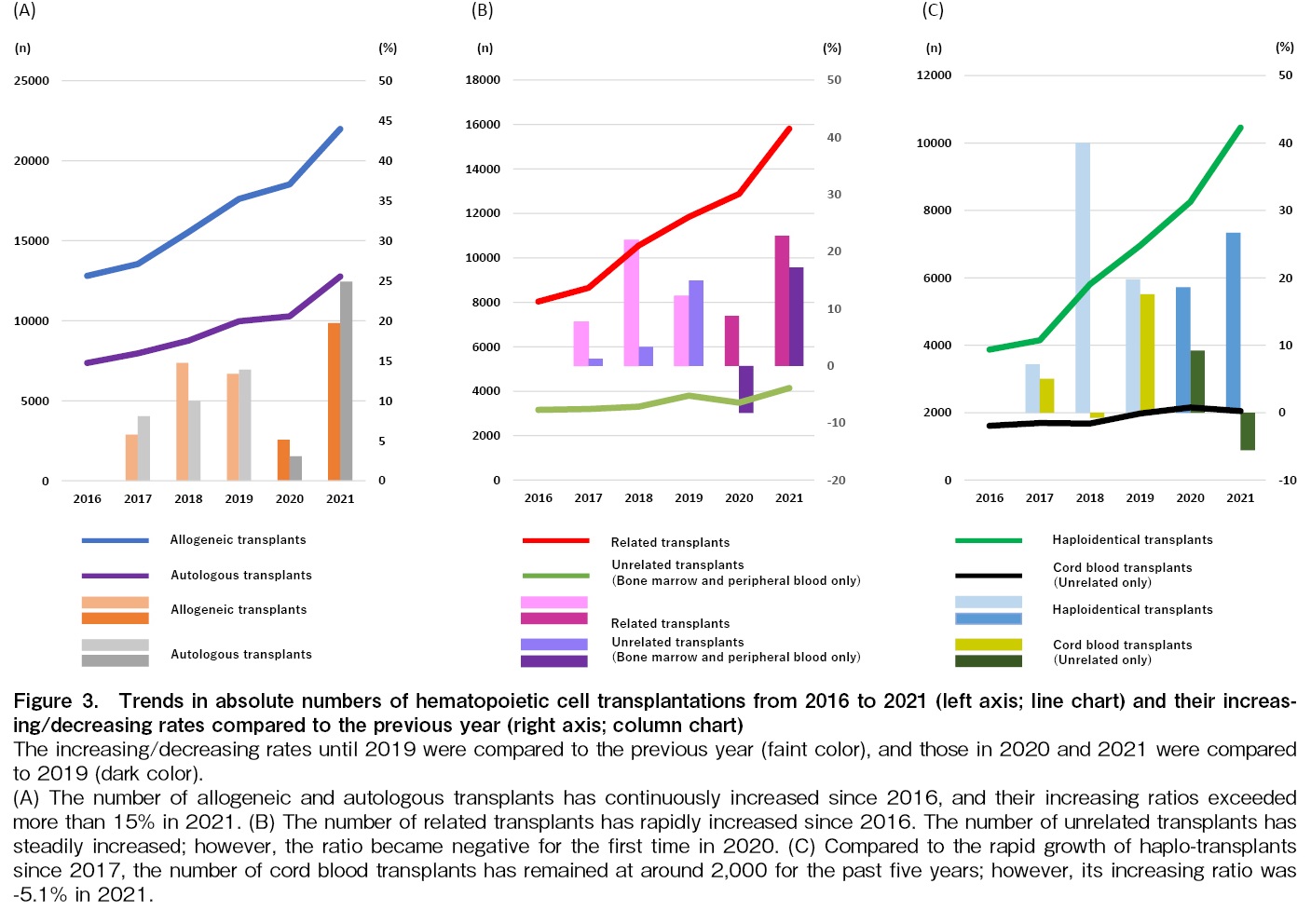
The number of allogeneic and autologous transplants has continuously increased in 2020 and 2021. Although the increase rate of allogeneic and autologous transplants was lower in 2020 than in the previous three years, it increased steeply in 2021; it was 19.7% and 24.9% for allogeneic and autologous transplants, respectively (Figure 3A). The change in unrelated transplants in 2020 was -8.2%; however, it recovered steeply in 2021, and the rate raised to 17.2%. Compared with unrelated transplants, the number of related transplants increased in 2020 and 2021, and the number of related transplants exceeded 15,000 for the first time in 2021 (Figure 3B). The number of haploidentical transplants has also steadily increased in 2020 and 2021. The growth rate of CBT was 9.2% in 2020; however, it decreased by 5.6% in 2021 (Figure 3C).
Trends of all transplants in each country/region
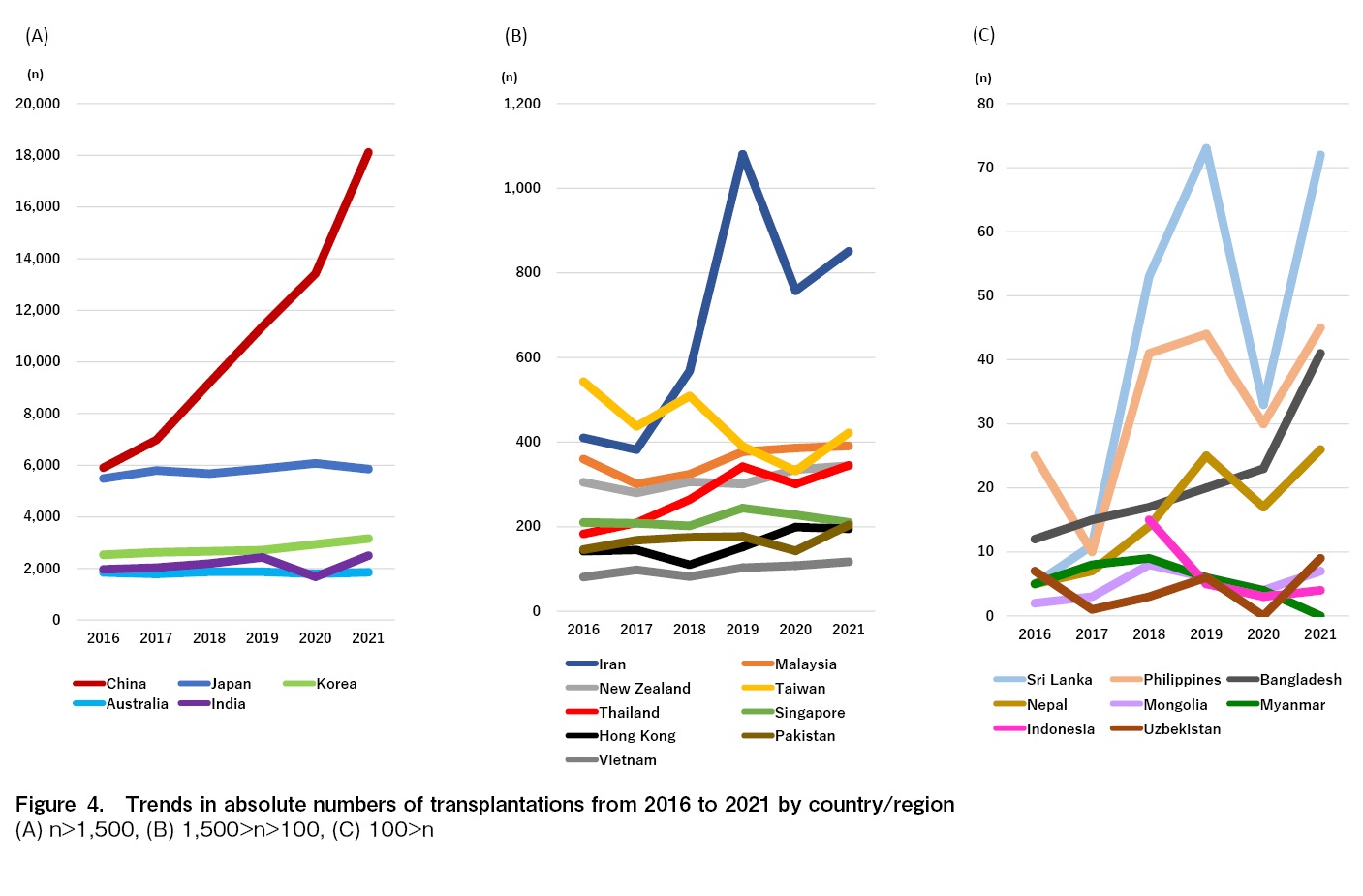
Figure 4 shows the trends in the number of all transplants by country and region since 2017. Excluding Indonesia, Mongolia, Myanmar, and Uzbekistan, which had ten or fewer transplants per year in 2020, the number of all transplants per year in 2020 did not decrease in Bangladesh, China, Hong Kong, Japan, Korea, Malaysia, New Zealand, and Vietnam. As opposed to this, the number of transplants temporally decreased in Australia, India, Iran, Nepal, Pakistan, the Philippines, Singapore, Sri Lanka, Taiwan, and Thailand in 2020 compared to the previous year, and then recovered in 2021, except for Singapore. Notable changes were found in India and China. India saw a decrease of 757 transplant cases in 2020 compared to the previous year, followed by an increase of 822 cases in 2021. In China, which increased by 2,048 cases in 2020 compared to 2019 and saw a greater increase of 4,695 cases in 2021. The detailed number of facilities and transplants in 2020 and 2021 and the differences between the previous year in each country and region are shown in Table 1.
Relationship between economic indicators of figures or index and the increase/decrease of the transplants number in each country/region
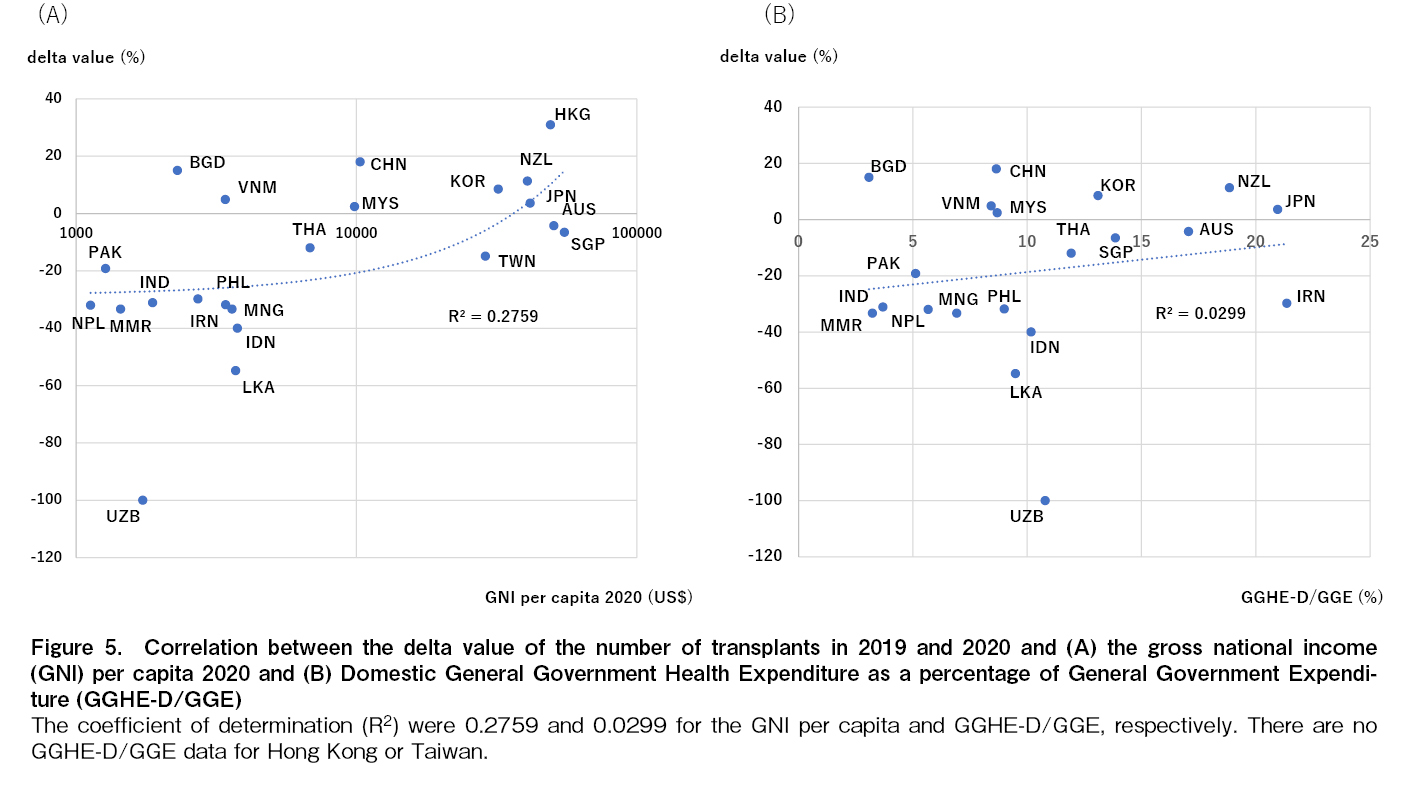
No correlation was found between the GNI per capita of each country/region in 2020 or the GGHE-D/GGE and the delta value of the number of transplants in 2020 compared to 2019 (Figure 5A and B).
Discussion
The APBMT Data Center commenced the HCT Activity Survey in 2007 and the initial report was published in 20107. Since then, we conducted the survey every year and reported on trends in HCT in the AP region8–11. During this period, the total annual number of all kind of HCTs exceeded 10,000 in 20087 and 20,000 in 20169, indicating that it took eight years for the number of transplants to increase from 10,000 to 20,000. In this report, the total number of transplants in 2021 exceeded 30,000 for the first time; however, it took only five years to increase the number from 20,000 to 30,000. This finding indicates that HCT has recently become increasingly active in the AP region.
The COVID-19 pandemic caused by SARS-CoV-2 has spread throughout the world since 2020, both in developed and emerging countries/regions, and has had a significant impact on the activity of HCT. EBMT Activity Survey reported a 6.5% decrease in total transplants, 5.1% in allogeneic transplants, and 7.5% in autologous transplants in 202012. In the United States, the number of allogeneic and autologous transplants has also decreased in 2020 compared to 201913. As opposed to those reports, we found that the number of HCTs in the AP region did not decrease in the number of autologous and allogeneic transplants, and both of which recorded the highest activity ever in 2020 and 2021. Despite the COVID-19 epidemic, the number of autologous and allogeneic transplants in this region did not decrease because the number of both transplants in China showed a significant increase, covering a decrease in the number of transplants in other countries/regions. Xu et al. explained the reason for their uptrend that the number of medium and large HCT groups increased, and transplant care was carried out smoothly as usual because of the basic principles of infection prevention, namely social distancing, masking, and thorough education of patients and donors14. As we previously reported9, the number of haploidentical transplants, which had been on the rise before 2020, has continued to increase in 2020 and 2021. One possible reason is that donors for haploidentical transplants are relatively easier to select than unrelated donors, even under social restrictions. On the other hand, the growth rate of unrelated transplants in 2020 was negative compared to the previous year for the first time. It is because of delays in transplant coordination to prevent infection and cancellations and postponements of transplants due to donor infection. Like haploidentical transplants, since CB was easy to use as a transplant source even in social restrictions and complemented other donor sources, the number of CBTs in 2020 increased from the previous year. However, it decreased in 2021, which may reflect a decrease in CBT due to the increase in haploidentical transplants in Japan15. In other words, as we reported in our most recent publication11, the choice of stem cell sources in this region varies significantly across countries and regions, and these differences may have influenced the increase or decrease in HCT numbers during the COVID-19 pandemic in each country and region.
Atsuta et al. have shown the correlation between economic growth indicators and the number of transplants by transplant type in each region of Europe, America, Asia-Pacific, the Middle East, and Africa and they have reported that the number of autologous transplants tends to be more strongly correlated with GNI per capita in the region compared to allogeneic transplants16; however, there was no direct implication between the increase and decrease in the number of transplants and the economic situation of the country and region in this analyses. The economic situation obviously impacts transplant medicine, but the Asia-Pacific region is vast and has a wide variety of populations, age structures, politics, and financial systems, the increase or decrease in the number of transplants in each country and region is thought to be the result of not only the economic situation but also a complex interaction of multiple factors, such as when the government started substantial restrictions on going out, how strictly they were implemented, thorough implementation of basic infection prevention measures, and the availability of human and material medical resources.
The guidelines have played a major role in continuing transplant medicine during the COVID-19 pandemic. In India, the Indian Society for Blood and Marrow Transplantation (ISBMT) took the lead in publishing guidelines for COVID-1917, and as a result, the number of transplants recovered to pre-COVID-19 levels in 2021. By December 2020, guidelines were issued by EBMT18, WMDA19, ASTCT20, and other organizations in the field of HCT. Because transplantation, especially allogeneic transplantation, requires the involvement of various allied health professionals and donors, these guidelines helped create a system in which patients with blood diseases could receive transplantation. It is thought that each country and region used them as a reference to modify their own transplantation care.
There are some limitations to our report. Our survey covered data from the first two years of the COVID-19 pandemic, so long-term data collection and analysis will be necessary to see how the COVID-19 pandemic has affected transplant care in this region. Secondly, the AP region is vast, and it is likely that there were significant differences in the approach to transplant care in each country/region during the pandemic, so this report cannot be used to discuss its success or failure. Thirdly, the Activity Survey only collects data on the number of transplants, so the results cannot reflect the disease background of individual patients.
The COVID-19 pandemic showed us how we should control the supply chain and logistics involved in transplantation under unexpected circumstances and overcome some challenges so that patients can receive the transplant care they need. Taking the above limitations into consideration, APBMT will continue analyzing the Activity Survey and start patient-specific data analyses in the future.
Acknowledgments
We sincerely thank all the members of the APBMT, especially the Scientific Committee members and their data managers, who gathered data from each registry, country, facility, and hospital annually. We are also grateful for the cooperation of all participating teams, countries and regions, organizations, and their staff, especially the Australasian Bone Marrow Transplant Recipient Registry (ABMTRR), Chinese Blood and Marrow Transplantation Registry (CBMTR), Indian Society for Blood and Marrow Transplantation Registry (ISBMT), Korean Society of Blood and Marrow Transplantation (KSBMT), Japan Society for Transplantation and Cellular Therapy (JSTCT)/Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT), Malaysian Society of Transplantation (MST), and Taiwan Society of Blood and Marrow Transplantation (TBMT), and Ministry of Health and Medical Education of the Islamic Republic of Iran. This study was conducted by the APBMT Registry Committee and Data Center. We also thank Yukari Nakao, Yuko Taki, and Shinobu Herron of the APBMT Data Center for their assistance. All facilities that contributed to the Activity Survey by reporting for this study are listed in
Author Contributions
MI and YA designed the study, and MI wrote the manuscript. LW, AD, MA, ML, JS, VL, KML, DA, AAH, YA, JHM, YSC, KWH, KB, AAG, BSP, TF, MRB, UB, SH, MD, and PCD submitted the data. MI and APBMT Data Center analyzed the data. All co-authors reviewed the manuscript, and YA, AH, AD, DS, YSC, CCL, UA, MD, PCD, and SO revised it.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
MI, AS, AAH, NK, MB, and SO are editors of Blood Cell Therapy. They were not involved in the editorial evaluation and the decision to accept this article for publication.
References
1.Banerjee A, Pasea L, Harris S, Gonzalez-Izquierdo A, Torralbo A, Shallcross L, et al. Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020; 395: 1715-25.
2.Algwaiz G, Aljurf M, Koh M, Horowitz MM, Ljungman P, Weisdorf D, et al. Real-World Issues and Potential Solutions in Hematopoietic Cell Transplantation during the COVID-19 Pandemic: Perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant. 2020; 26: 2181-9.
3.Shimomura Y, Kitamura T, Nishikubo M, Sobue T, Uchida N, Doki N, et al. Effect of the COVID-19 pandemic on allogeneic stem cell transplantation in Japan. Int J Hematol. 2023; 117: 590-7.
4.Bank TW. Population, total. In, 2021. https://data.worldbank.org/indicator/NY.GDP.MKTP.CD [Accessed: 16 July 2024]
5.Group WB. GNI percapita (current US$). In, 2024. https://data.worldbank.org/indicator/NY.GNP.ATLS.CD?skipRedirection=true&view=map [Accessed: 16 July 2024]
6.WHO. Domestic general government health expenditure (GGHE-D) as a percentage of general government expenditure (GGE) (%). In, 2024. https://www.who.int/data/gho/data/indicators/indicator-details/GHO/domestic-general-government-health-expenditure-(gghe-d)-as-percentage-of-general-government-expenditure-(gge) [Accessed: 16 July 2024]
7.Yoshimi A, Suzuki R, Atsuta Y, Iida M, Lu DP, Tong W, et al. Hematopoietic SCT activity in Asia: a report from the Asia-Pacific Blood and Marrow Transplantation Group. Bone Marrow Transplant. 2010; 45: 1682-91.
8.Iida M, Kodera Y, Dodds A, Ho AYL, Nivison-Smith I, Akter MR, et al. Advances in hematopoietic stem cell transplantation in the Asia-Pacific region: the second report from APBMT 2005-2015. Bone Marrow Transplant. 2019; 54: 1973-86.
9.Iida M, Dodds A, Akter M, Srivastava A, Moon JH, Dung PC, et al. The 2016 APBMT Activity Survey Report: Trends in haploidentical and cord blood transplantation in the Asia-Pacific region. Blood Cell Ther. 2021; 4: 20-8.
10.Iida M, Liu K, Huang XJ, Depei W, Kuwatsuka Y, Moon JH, et al. Trends in disease indications for hematopoietic stem cell transplantation in the Asia-Pacific region: A report of the Activity Survey 2017 from APBMT. Blood Cell Ther. 2022; 5: 87-98.
11.Iida M, Liu K, Huang XJ, Huang H, Kuwatsuka Y, Moon JH, et al. Report on hematopoietic cell transplantations performed in 2018/2019 focusing on the trends of selection of stem cell sources in the Asia-Pacific region: APBMT Activity Survey. Blood Cell Ther. 2023; 6: 114-23.
12.Passweg JR, Baldomero H, Chabannon C, Corbacioglu S, de la Cámara R, Dolstra H, et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022; 57: 742-52.
13.CIBMTR. Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT) in the U.S. 2022 Summary Slides. In, 2022. https://cibmtr.org/CIBMTR/Resources/Summary-Slides-Reports [Accessed: 16 July 2024]
14.Xu LP, Lu DP, Wu DP, Jiang EL, Liu DH, Huang H, et al. Hematopoietic Stem Cell Transplantation Activity in China 2020-2021 During the SARS-CoV-2 Pandemic: A Report From the Chinese Blood and Marrow Transplantation Registry Group. Transplant Cell Ther. 2023; 29: 136.e1-.e7.
15.JSTCT/JDCHCT. Hematopoietic Cell Transplantation in Japan Annual Report of Nationwide Survey 2022 Summary Slide. In, 2022. https://www.jdchct.or.jp/en/data/slide/2022/ [Accessed: 16 July 2024]
16.Atsuta Y, Baldomero H, Neumann D, Sureda A, DeVos JD, Iida M, et al. Continuous and differential improvement in worldwide access to hematopoietic cell transplantation: activity has doubled in a decade with a notable increase in unrelated and non-identical related donors. Haematologica. 2024; 109: 3282-94.
17.Damodar S, Radhakrishnan VS, John MJ, Malhotra P, Jain R, Melinkeri S, et al. HSCT Guidelines for Transplant Practices During the COVID-19 Pandemic in India ISBMT DOCUMENT Version1.0. Blood Cell Ther. 2020; 3: 59-70.
18.Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020; 55: 2071-6.
19.WMDA. WMDA Rapid Alert May 2020 In, 2020. https://wmda.info/wp-content/uploads/2020/07/20200611-SEAR-Rapid-Alert.pdf [Accessed: 16 July 2024]
20.Waghmare A, Abidi MZ, Boeckh M, Chemaly RF, Dadwal S, El Boghdadly Z, et al. Guidelines for COVID-19 Management in Hematopoietic Cell Transplantation and Cellular Therapy Recipients. Biol Blood Marrow Transplant. 2022; 26: 1983-94.
Search
News