Volume 8 (2025) Issue 1 No.3 Pages 160-166
Abstract
Introduction: Poor graft function (PrGF) is a serious complication of allogeneic hematopoietic cell transplantation (allo-HCT), and current therapies are only partially effective. Eight published reports on the effect of eltrombopag on PrGF showed encouraging results. This study aimed to assess the safety and efficacy of eltrombopag for the treatment of PrGF after allo-HCT.
Methods: A retrospective study was conducted on 23 patients with PrGF following allo-HCT at the King Hussein Cancer Center (KHCC), Amman, Jordan between January 2013 and December 2023. The patient-, disease-, and transplant-related characteristics were obtained from the KHCC-BMT database.
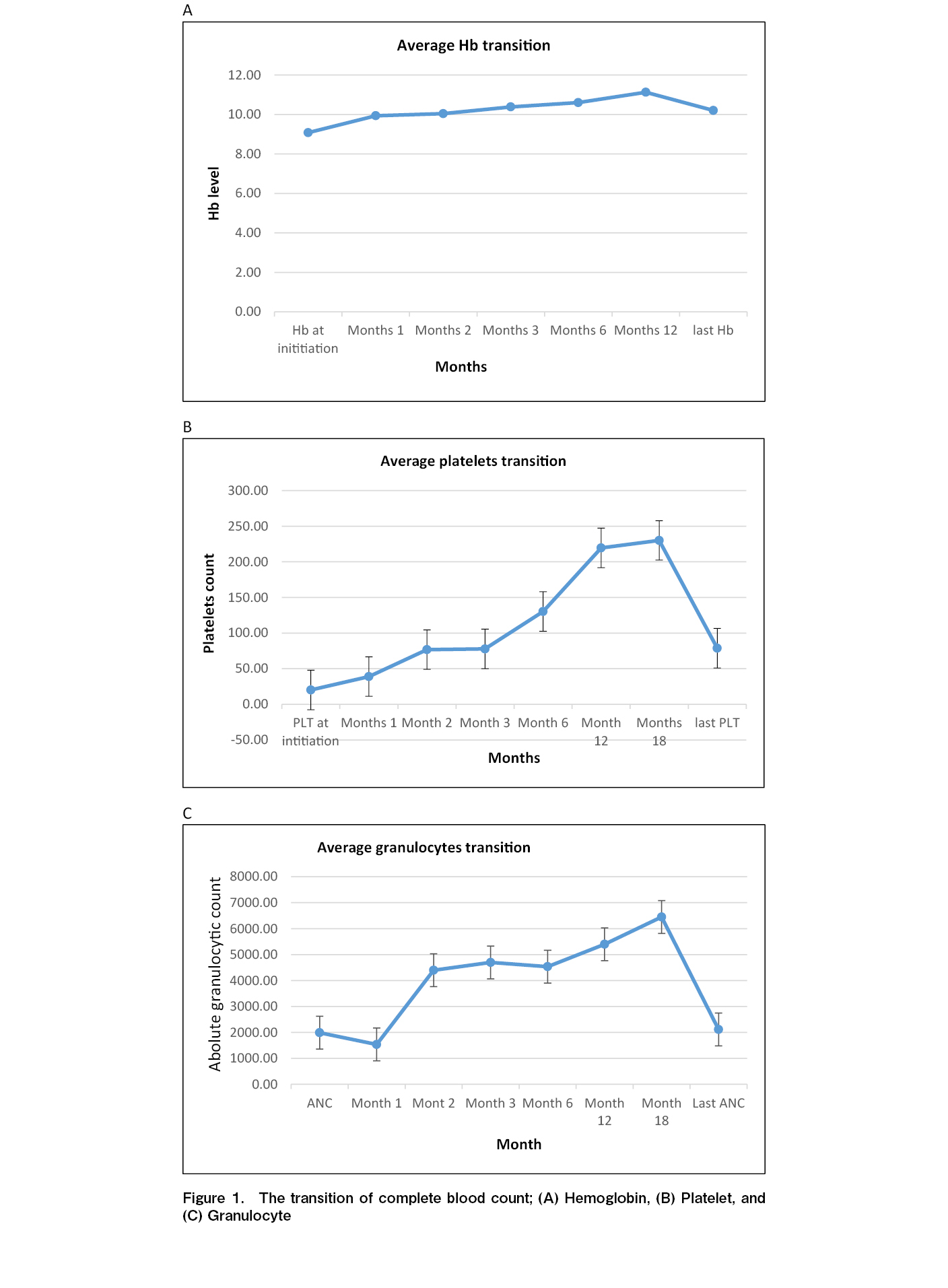
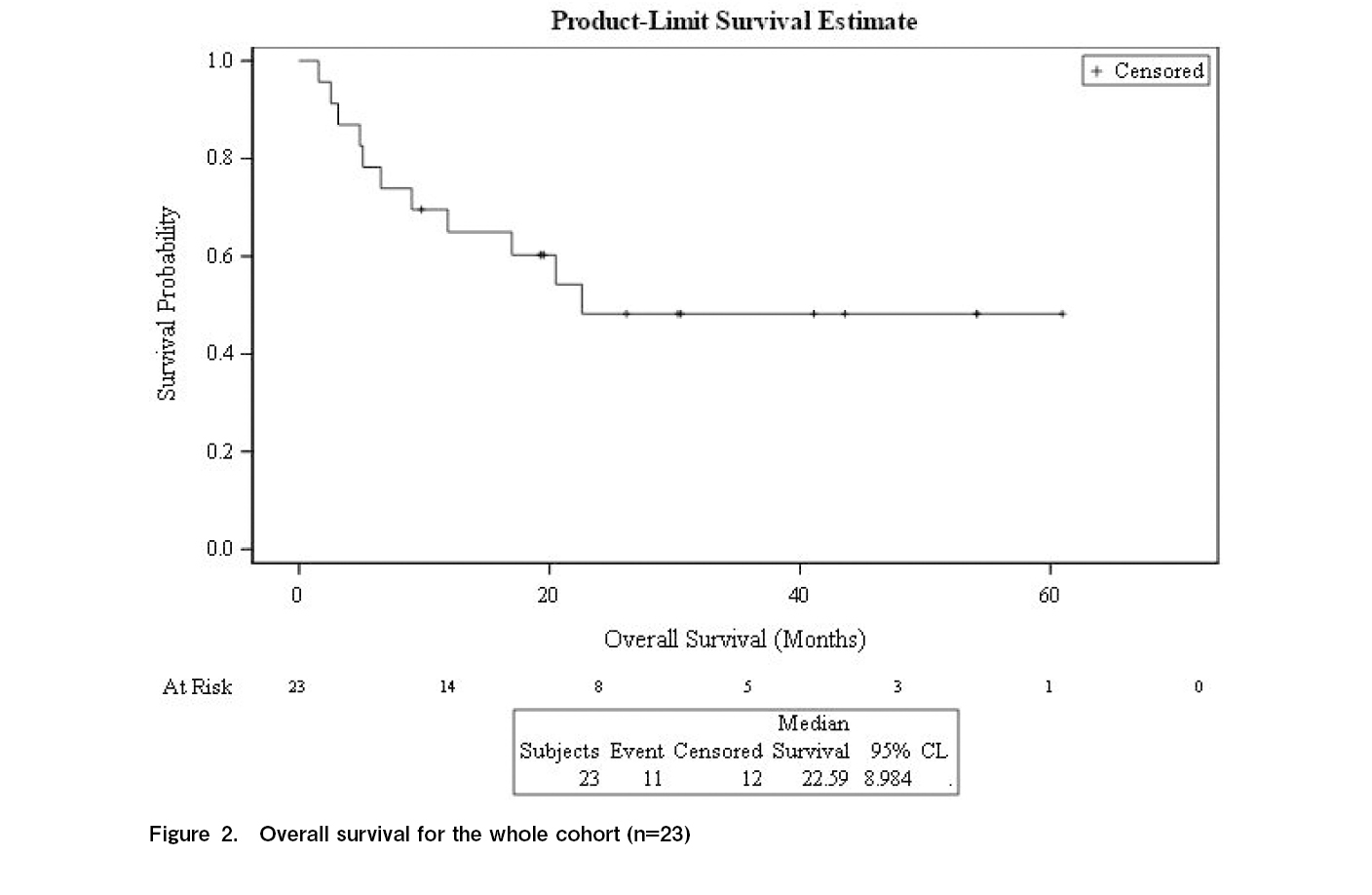
Results: The median age was 47 years (range, 26-68 years), and 13 patients were female. Fourteen patients received reduced-intensity conditioning, and 14 donors were human-leukocyte antigen (HLA) -identical siblings. Median hemoglobin (Hb) concentration pre-eltrombopag administration was 8.5 g/dL (range: 6-14.2 g/dL), granulocytes 7.1 × 109/L (range: 1.6-85 × 109/L), and platelets 18 × 109/L (range: 6-50 × 109/L). Thirteen patients had platelets < 20 × 109/L, and 15 had reduced megakaryocytes. The median CD34-positive cell dose was 6.10 × 106/kg (range: 2.75-9.78 × 106/kg). Eltrombopag was initiated at a median of 105 day (d) post-transplant (range: 29-800 d). The median weekly dose was 488 mg (range: 350-700 mg), and the median treatment duration was 75 d (range: 5-446 d). In total, 65% of patients (n=15) responded at a median of 30 d (range, 7-122 d). Twelve responders were alive at a median follow-up of 30 months (range: 7.0-122 months) with normal blood counts. The 2-year overall rate was 48% (95% confidence intervals, 27-70%), and seven non-responders died because of relapse. No major adverse events were reported.
Conclusion: Eltrombopag improved peripheral blood counts in patients with PrGF following allo-HCT. Thus, response to eltrombopag was predictable and has a significant effect on survival.
Introduction
Poor graft function (PrGF) is a serious complication of allogeneic hematopoietic cell transplantation (allo-HCT), and current PrGF therapies are only partially effective. PrGF diagnosis is based on the presence of two or more of the following criteria: hemoglobin (Hb) concentration < 100 g/L, neutrophil count < 1.0 × 109/L, or platelet count < 30 × 109/L, occurring ≥ 30 d post-transplant1. Additional criteria include the need for red blood cell (RBC) and platelet transfusions, decreased bone marrow cellularity, complete donor chimerism, and absence of graft-versus-host disease (GvHD) or relapse.
PrGF is distinct from graft failure, which is due to retained recipient immune cells, and manifests as a loss of donor chimerism or late platelet recovery failure. PrGF incidence varies widely, with reports ranging from 5% to 27%, and its clinical course can range from spontaneous recovery to death due to cytopenia-related complications1–3.
Treatment options for PrGF include RBC and platelet transfusions, intravenous immunoglobulin, hematopoietic growth factors (e.g., granulocyte-colony stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF)), donor blood or bone marrow infusions, mesenchymal cell infusions, or a second transplant from the same or a different donor4–8. However, these treatments are often partially effective. Eltrombopag, an oral thrombopoietin receptor agonist, has shown efficacy in the treatment of severe aplastic anemia, idiopathic thrombocytopenic purpura, thrombocytopenia associated with hepatitis C, myelodysplastic syndrome, and acute myeloid leukemia9–15. Eltrombopag has also been reported to improve PrGF-induced cytopenia16–24. This study aimed to enhance our understanding of eltrombopag use in the treatment of PrGF post-allo-HCT.
Materials and Methods
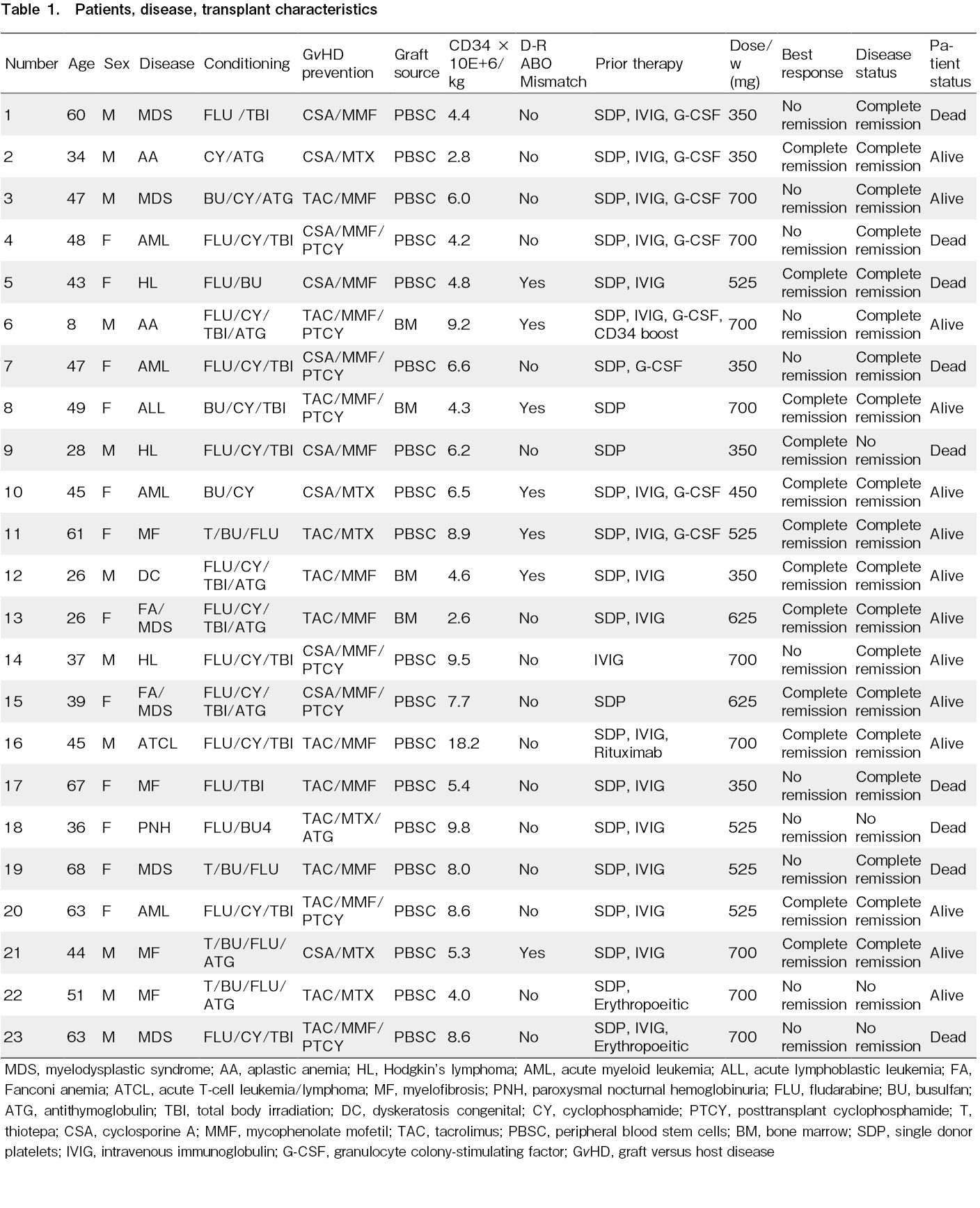
This retrospective, single-center study evaluated the safety and efficacy of eltrombopag in patients with PrGF post-allo-HCT. After obtaining approval from the Institutional Review Board of the King Hussein Cancer Center (18 KHCC 146) and exemption of consent, data were collected from electronic medical records. Patient-, disease-, and transplant-related variables are presented in Table 1. Complete response was defined as the normalization of blood counts, and partial response as transfusion independence25. Statistical analyses were performed using IBM SPSS Statistics for Windows version 26, SPSS Inc. Here, we report 82 patients with PrGF, 23 of whom received eltrombopag from a single center in Jordan25.
Continuous variables are presented as medians and ranges, and categorical variables are presented as frequencies (percentages). To compare groups, we used the chi-square or Fisher's exact test for categorical variables, and the Mann-Whitney U test for continuous variables. P values < 0.05 are considered statistically significant.
Results
Between January 2013 and December 2023, we documented that 82 of 1,000 consecutive allotransplant recipients (8.2%) who developed PrGF. Thirty-five patients received a second transplant, all for aplastic anemia or B-thalassemia, and 23 for eltrombopag therapy, all of which were female. The median patient age was 47 years (range: 26-68 years). Fourteen patients received reduced-intensity preparative conditioning and nine received conventional myeloablative conditioning. Fourteen donors were HLA-identical siblings and nine were HLA-mismatched related relatives. The median Hb concentration pre-eltrombopag administration was 8.5 g/dL (range: 6-14.2 g/dL), granulocyte count was 7.1 × 109/L (range: 1.6-85 × 109/L), and platelet count was 18 × 109/L (range: 6-50 × 109/L). Thirteen patients had platelet counts < 20 × 109/L. Pre-eltrombopag administration, bone marrow aspirations and biopsies were normocellular with trilineage hematopoiesis in six patients and hypocellular with trilineage hypoplasia in 17 patients, and 15 patients had decreased or absent megakaryocytes. Chimerism was complete (
Eltrombopag was initiated at a dose of 50 mg/day and increased in 25 mg increments every 2 weeks if no response was achieved. Fifteen allotransplant recipients (65%) responded to the treatment. The median interval to response was 30 d (range: 7-122 d). Eltrombopag was gradually tapered off after achieving a stable platelet count of 25 mg every 1-2 weeks. One patient lost response after eltrombopag discontinuation because of primary disease relapse and dense marrow fibrosis. Adequate bone marrow megakaryocytes were predictive of response (p=0.019). There was no statistically significant difference in the objective response between patients with primary and secondary graft failure (p=0.074).
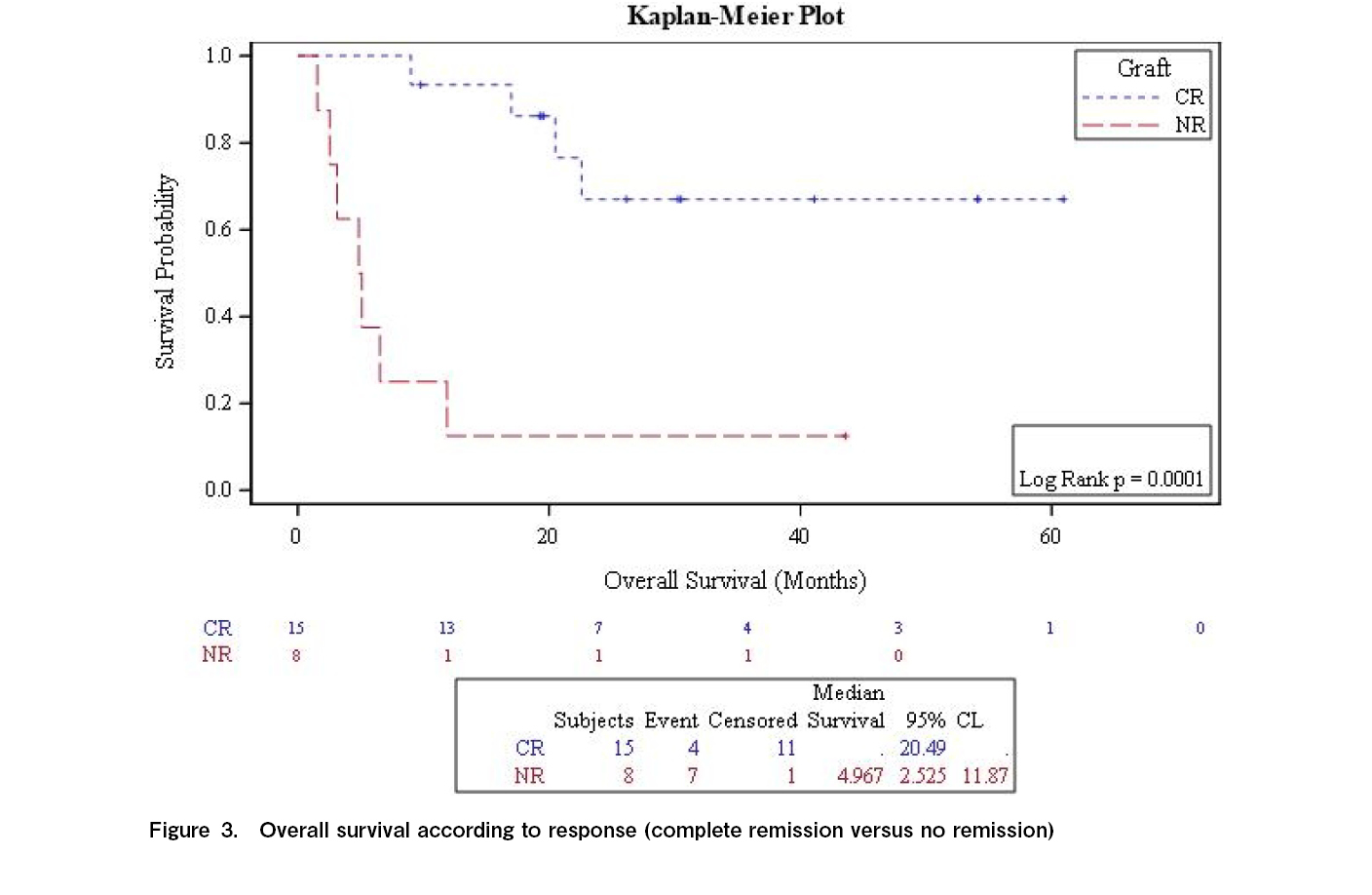
At 2 years, graft function was better preserved in the responders than in the non-responders (67% vs 12.5%; p=0.0001). The median follow-up period for responders was 30 months (range, 7-122 months). Twelve of the 15 responders survived with normal blood cell concentrations. The 2-year survival rate was 48% (range: 27%-70%) (Figure 2), which was significantly improved in responders compared to that in non-responders (67.0% vs 12.5%; p=0.0001; Figure 3). Three responding patients and seven non-responders died (none owing to relapse). The causes of death, GvHD (n=3), infection (n=3), and pulmonary hemorrhage (n=2). One patient responded, but died due to disease progression. No major adverse events were reported.
Conclusion
Twenty-three patients with PrGF following allo-HCT in whom prior therapies failed responded to eltrombopag treatment, as it improved their peripheral blood cell concentration. Thus, patient response to eltrombopag treatment is predictable, and eltrombopag has significant impact on patient survival outcomes.
Discussion
Our study reports the use of eltrombopag to resolve PrGF in patients post-allo-HCT. Fifteen patients (65%) achieved an objective response and became transfusion independent after a median treatment duration of 75 d. Twelve patients were alive with normal blood cell concentrations, and the 2-year survival rate was 48% (range, 27%-70%). Therefore, eltrombopag administration is safe, with no reported adverse events at the highest daily dose of 100 mg.
The efficacy of thrombopoietic agonists in severe aplastic anemia, in which the bone marrow is hypocellular, led to their use in the management of post-allo-HCT cytopenias26. Giammarco et al. reported the largest cohort of patients with PrGF-induced post-transplant cytopenia (n=48) who were treated with eltrombopag (50-150 mg) for a median of 120 d. They reported an overall response rate of 75% (n=36), with complete normalization of blood counts in 24 patients (50%). This multicenter study included prolonged idiopathic thrombocytopenia as a criterion for PrGF27. In our cohort, eltrombopag was used to treat PrGF-induced post-transplant cytopenia (uni-, bi-, or tri-lineage cytopenia). We observed an increase in platelet, hemoglobin, and granulocyte counts. Most of our cohort was on supportive G-CSF, and a few were on erythropoietin agonists.
Kırcalı et al. documented 39 patients with post-transplant cytopenia (8 with isolated thrombocytopenia, 19 with bi-lineage cytopenia, and 12 with pancytopenia), including nine patients after autologous bone marrow transplants. In their cohort, 84.6% (n=33) of patients responded to eltrombopag after a median of 82 d. Furthermore, they observed a trend toward improved 1-year overall survival rates for responders (75% vs 66.7%; p=0.3), with no major safety concerns. This is consistent with our results, which showed that survival at 2 years was significantly better in responders than in non-responders (67.0% vs 12.5%; p=0.0001). Additionally, 61.5% of patients in their study discontinued eltrombopag successfully after a median of 82 d, which is comparable to that in our cohort (65%) at a median of 75 d.
This study has many limitations, including its retrospective single-center design and small sample size. Moreover, the variable definitions of PrGF across different studies made comparing results challenging. Larger real-world data, registry reports, and randomized studies are warranted to draw stronger conclusions.
Acknowledgments
We acknowledge healthcare providers, patients and their families
Author Contributions
KH contributed to the conception, design of the study, and interpretation of results, wrote the first draft and final manuscript. MA, IM, RA, WD, and RA did the data acquisition. MM, HA, and MS, designed the study. AT analyzed results. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
We acknowledge healthcare providers, patients and their families
Data Sharing Statement
All data generated or analyzed during this study are included in this article.
References
1.Lee KH, Lee JH, Choi SJ, Lee JH, Kim S, Seol M, et al. Failure of trilineage blood cell reconstitution after initial neutrophil engraftment in patients undergoing allogeneic hematopoietic cell transplantation―frequency and outcomes. Bone Marrow Transplant. 2004; 33: 729-34.
2.Zhao Y, Gao F, Shi J, Luo Y, Tan Y, Lai X, et al. Incidence, risk factors, and outcomes of primary poor graft function after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2019; 25: 1898-907.
3.Halahleh K, Arai Y, Gavriilaki E. Post-Transplant Complications. Blood Cell Ther. 2023; 6: 23-9.
4.Stasia A, Ghiso A, Galaverna F, Raiola AM, Gualandi F, Luchettie S, et al. CD34 selected cells for the treatment of poor graft function after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2014; 20: 1440-3.
5.Ghobadi A, Fiala MA, Ramsingh G, Gao F, Abboud CN, Stockerl-Goldstein K, et al. Fresh or cryopreserved CD34 (+)-selected mobilized peripheral blood stem and progenitor cells for the treatment of poor graft function after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2017; 23: 1072-7.
6.Bittencourt H, Rocha V, Filion A, Ionescu I, Herr AL, Garnier F, et al. Granulocyte colony-stimulating factor for poor graft function after allogeneic stem cell transplantation: 3 days of G-CSF identifies long-term responders. Bone Marrow Transplant. 2005; 36: 431-5.
7.Guardiola P, Kuentz M, Garban F, Blaise D, Reiffers J, Sttal M, et al. Second early allogeneic stem cell transplantations for graft failure in acute leukaemia, chronic myeloid leukaemia and aplastic anaemia. French Society of Bone Marrow Transplantation. Br J Haematol. 2000; 111: 292-302.
8.Liu X, Wu M, Peng Y, Chen X, Sun J, Huang F, et al. Improvement in poor graft function after allogeneic hematopoietic stem cell transplantation upon administration of mesenchymal stem cells from third-party donors: a pilot prospective study. Cell Transplant. 2014; 23: 1087-98.
9.Desmond R, Townsley DM, Dumitriu B, Olnes MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014; 123: 1818-25.
10.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017; 376: 1540-50.
11.McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated hepatitis C. N Engl J Med. 2007; 357: 2227-36.
12.Fattizzo B, Levati G, Cassin R, Barcellini W. Eltrombopag in immune thrombocytopenia, aplastic anemia, and myelodysplastic syndrome: From megakaryopoiesis to immunomodulation. Drugs. 2019; 79: 1305-19.
13.Strickland SA, Wang XV, Cerny J, Rowe JM, Rybka W, Tallman MS, et al. A Novel PrECOG (PrE0901) Dose-Escalation Trial Using Eltrombopag: Enhanced Platelet Recovery During Consolidation Therapy in Acute Myeloid Leukemia. Leuk Lymphoma. 2020; 61: 2191-9.
14.Yuan C, Boyd AM, Nelson J, Patel RD, Varela JC, Goldstein SC, et al. Eltrombopag for Treating Thrombocytopenia after Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2019; 25: 1320-4.
15.Giammarco S, Sica S, Chiusolo P, Laurenti L, Sorá F, Martino M, et al. Eltrombopag for the treatment of poor graft function following allogeneic stem cell transplant: a retrospective multicenter study. Int J Hematol. 2021; 114: 228-34.
16.Mahat U, Rotz SJ, Hanna R. Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2020; 26: e65-73.
17.Marotta S, Marano L, Ricci P, Cacace F, Frieri C, Simeone L, et al. Eltrombopag for post-transplant cytopenias due to poor graft function. Bone Marrow Transplant. 2019; 54: 1346-53.
18.Popat UR, Ray G, Bassett RL Jr., Poon M-YC, Valdez BC. Eltrombopag for post-transplant thrombocytopenia: results of phase II randomized double blind placebo controlled trial. Blood. 2015; 126: 738. https://ashpublications.org/blood/article/126/23/738/136431/Eltrombopag-for-Post-Transplant-Thrombocytopenia [Accessed: 7 August 2024].
19.Tang C, Chen F, Kong D, Ma Q, Dai H, Yin J, et al. Successful treatment of secondary poor graft function post allogeneic hematopoietic stem cell transplantation with eltrombopag. J Hematol Oncol. 2018; 11: 103.
20.Bento L, Bastida JM, García-Cadenas I, García-Torres E, Rivera D, Bosch-Vilaseca A, et al. Thrombopoietin receptor agonists for severe thrombocytopenia after allogeneic stem cell transplantation: Experience of the Spanish Group of Hematopoietic Stem Cell Transplant. Biol Blood Marrow Transplant. 2019; 25: 1825-31.
21.Karataş A, Göker H, Demiroğlu H, Malkan ÜY, Velet M, Çınar OE, et al. Efficacy of eltrombopag in thrombocytopenia after hematopoietic stem celltransplantation. Turk J Med Sci. 2022; 52: 413-9.
22.Nampoothiri RV, Kumar R. Eltrombopag: role in cytopenias following hematopoietic stem cell transplantation. Indian J Hematol Blood Transfus. 2020; 36: 238-45.
23.Kırcalı E, Seval GC, Öztürk C, Yılmaz H, Koyun D, Bozdağ SC, et al. Eltrombopag for the Treatment of Poor Graft Function Following Haematopoietic Cell Transplantation: Real-Life Data. Balkan Med J. 2023; 40: 51-6.
24.Davulcu EA, Soyer NA, Vural F. Eltrombopag for the Treatment of Allogeneic Hematopoietic Stem Cell Transplantation-Related Poor Graft Function. Cureus. 2023; 15: e44555.
25.Giammarco S, Sica S, Chiusolo P, Laurenti L, Sorá F, Martino M, et al. Eltrombopag for the treatment of poor graft function following allogeneic stem cell transplant: a retrospective multicenter study. Int J Hematol. 2021; 114: 228-34.
26.Halahleh K, Gale RP, Da'na W, Ma'koseh M, Saadeh S, Alan W, et al. Therapy of posttransplant poor graft function with eltrombopag. Bone Marrow Transplantation. 2021; 56: 4-6.
27.Desmond R, Townsley DM, Dumitriu B, Olne MJ, Scheinberg P, Bevans M, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014; 123: 1818-25.
Search
News