Volume 7 (2024) Issue 4 No.6 Pages 124-128
Abstract
Introduction: The first-line treatment of moderate-severe chronic graft versus host disease (cGVHD) involves systemic corticosteroids ± calcineurin inhibitors. Around half of the patients will need second-line agents for corticosteroid-refractory/dependent (SR/SD) cGVHD. Herein, we report our experience using sirolimus as an add-on agent to corticosteroids in moderate-severe cGVHD.
Methods: This was a single-center study of allogeneic cell transplant recipients aged ≥ 12 during 2016-2022. The diagnosis and severity of cGVHD were as per the NIH-2014 criteria. At the physician's discretion, sirolimus was added to corticosteroids for moderate to severe cGVHD. The GVHD response was classified based on the EBMT-NIH-CIBMTR criteria.
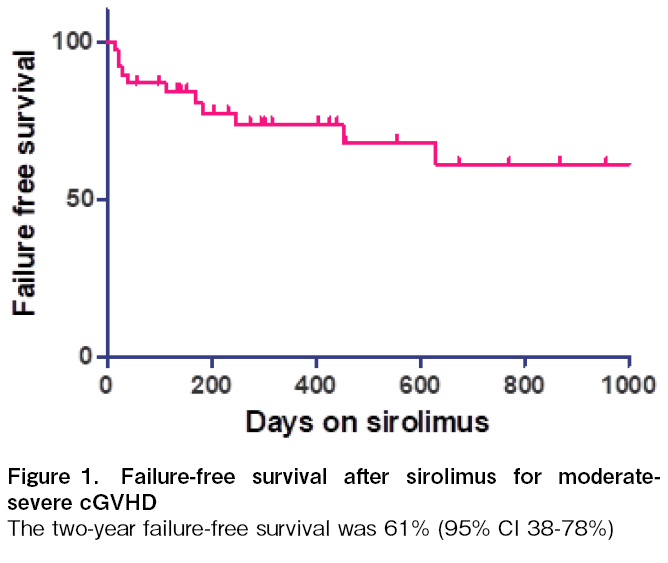
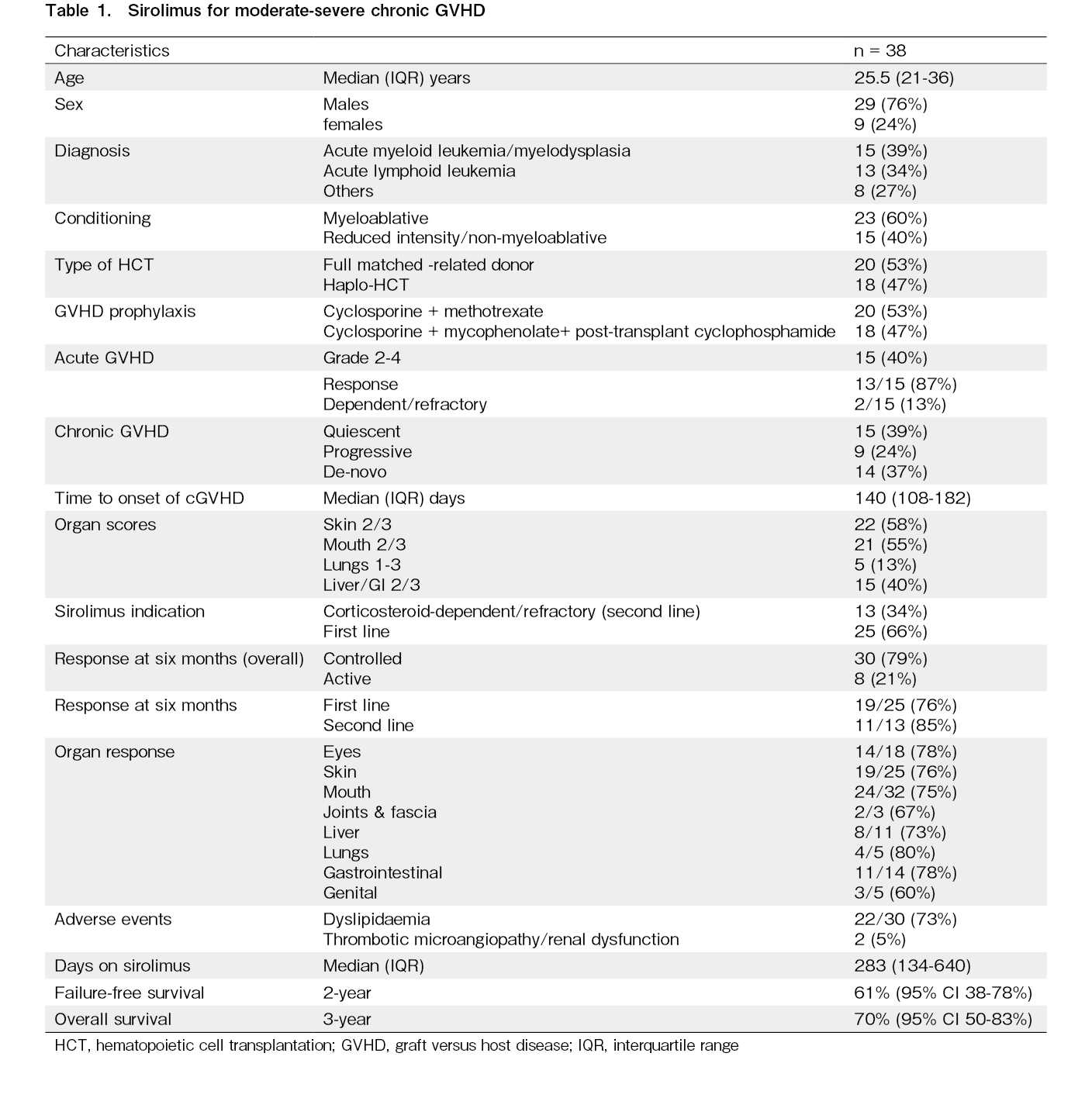
Results: cGVHD occurred in 66 (49%) out of 134 recipients. It was mild in 13 (10%) and moderate-severe in 53 (39%) recipients. Sirolimus was used in 38 out of 53 (71.6%) patients with moderate-severe cGVHD, with equal proportions of matched-related (53%) and haploidentical HCT (47%) recipients. The median time to onset of cGVHD was 140 days (IQR 108-182). The onset was de novo in 14 (37%), quiescent in 15 (39%), and progressive in 9 (24%) patients. The median duration on sirolimus was 283 days (134-640). cGVHD was controlled in 30 (79%) and active in 8 (21%) recipients at 6 months. Dyslipidemia was the most common (73%) adverse event. Failure-free survival at two years was 61% (95% CI 38-78%).
Discussion: This study demonstrates the safety and efficacy of sirolimus as an add-on agent to systemic corticosteroids in managing moderate-severe cGVHD. This strategy can reduce the burden of SR/SD cGVHD.
Introduction
Graft-versus-host disease (GVHD) is a major contributor to mortality and morbidity following allogeneic stem cell transplantation (HCT). Chronic GVHD (cGVHD) affects 35-70% of recipients and remains the Achilles heel of HCT1. The standard first-line treatment strategy for cGVHD has been systemic corticosteroids, with or without calcineurin inhibitors (CNI)2. Prednisone is started at a dose of 0.5-1 mg/kg and tapered primarily as per the prototype schedules to achieve a dose of less than 0.5 mg/kg alternate day by 12 weeks2. While trials showed no benefit of adding CNI to prednisone, this does appear to reduce the incidence of corticosteroid side effects3. Therefore, most guidelines recommend adding a CNI for moderate-severe cGVHD as a corticosteroid-sparing agent to minimize the toxicity associated with prolonged corticosteroid treatment4. Another trial suggested that sirolimus in combination with prednisone is an equivalent therapeutic alternative to the prednisone/CNI/sirolimus combination based on similar failure-free survival rates5. Despite this, adding sirolimus to prednisone has not been standard practice. This drug has been overshadowed by the newer drugs used in the management of corticosteroid-refractory (SR) GVHD namely ruxolitinib, ibrutinib, and belumosudil, the only agents approved for this indication. The incidence of SR cGVHD is ~50% and these newer drugs are targeted to meet this huge unmet need. The response rates with these agents vary from 30% to 60%6. Herein, we report our experience using sirolimus as an add-on agent to corticosteroids in moderate-severe cGVHD.
Materials and Methods
This was a single-center partial retrospective and prospective study as part of the long-term follow-up of consecutive patients receiving allogeneic HCT from 2016 to 2022. The Institute's Ethics Committee approved the study (INT/IEC/2021/SP2-982). Informed consent was obtained from patients prospectively enrolled in the study and was waived for the retrospective cohort. The study enrolled all patients aged ≥ 12 years. All patients received peripheral blood grafts. The GVHD prophylaxis included CNI/Methotrexate for full-matched related donors and post-transplantation cyclophosphamide/CNI/Mycophenolate in haploidentical HCT. The immunosuppressive therapy (IST) was tapered between 60 and 120 days depending upon the disease risk, conditioning intensity, chimerism, and acute GVHD at the physician's discretion. The diagnosis and severity of GVHD were as per the Mount Sinai Acute GVHD International Consortium (MAGIC) and National Institute of Health (NIH) 2014 consensus criteria7, 8. The eGVHD app was used at the bedside for accurate scoring of GVHD severity during each patient visit9. At the physician's discretion, sirolimus was added to corticosteroids for moderate to severe cGVHD. Prednisone was started at 0.5-1 mg/kg/day and tapered by 20-30% every two weeks to reach half of the starting dose alternate day by 12 weeks and 0.1 mg/kg alternate day by 24 weeks4. Sirolimus was dosed at 2 mg PO once daily without a loading dose, and levels were monitored twice a month to target trough levels of 7-12 ng/mL10. The lipid profile was monitored per the standard long-term follow-up guidelines for HCT survivors11. The GVHD response was classified according to the EBMT-NIH-CIBMTR criteria12. Corticosteroid refractoriness was defined as progression of cGVHD on prednisone dose ≥ 1 mg/kg/day for 1-2 weeks or stable GVHD on prednisone dose ≥ 0.5 mg/kg/day for 1-2 months. Corticosteroid dependence was defined as the inability to taper prednisone < 0.25 mg/kg/day in two attempts over 8 weeks. The overall response was assessed at 6 months. The GVHD status was defined as active if inflammatory or worsening cGVHD manifestations were present, controlled if these manifestations were absent while on IST or IST stopped for < 24 weeks, and resolved if these manifestations were absent off IST (stopped for > 24 weeks). Failure-free survival (FFS) was defined by the absence of second-line treatment, non-relapse mortality, and relapse during treatment on sirolimus13. Overall survival (OS) was defined as the time from transplant to death due to any cause. GraphPad Prism Software was used for statistical analysis.
Results
A total of 134 allo-HCT recipients were enrolled during the study period. cGVHD occurred in 66 (49.2%) recipients during the study. cGVHD was mild in 13 (9.7%) and moderate-severe in 53 (40.2%) recipients. Sirolimus was used in 38 out of 53 (71.6%) patients with moderate-severe cGVHD. It was used as primary treatment with corticosteroids in 25 (66%) and as a second-line agent in corticosteroid-dependent/refractory cases in 13 (34%) recipients. Cyclosporine or mycophenolate was used as the first-line therapy in these 13 patients. The median age of this cohort was 25.5 years (IQR 21-36) (Table 1). Most patients had malignant conditions and received myeloablative conditioning (60%). There were equal proportions of matched-related (53%) and haploidentical HCT (47%). Forty percent of this cohort of patients had grade 2-4 acute GVHD, of which only 13% were corticosteroid-dependent/refractory. The median time to onset of cGVHD was 140 days (IQR 108-182). The onset was de novo in 14 (37%), quiescent in 15 (39%), and progressive in 9 (24%) patients. GVHD organ severity scores of ≥2 were found for the skin (58%), mouth (55%), and liver/gastrointestinal tract (40%) of the recipients. Lung GVHD occurred in 13% of the recipients. The median duration on sirolimus was 283 days (134-640). cGVHD was controlled in 30 (79%) and active in 8 (21%) recipients at 6 months. The response rates were very similar when used as first and second line treatments (76% and 85%, respectively). Organ response rates were the lowest for genital (60%) and joint & fascia GVHD (67%). The response rates in other organs were close to 75%. At the last follow-up, GVHD was resolved in 5 patients. Patients with active GVHD (n = 8) showed the following outcomes: There were two deaths and relapses within 6 months. The two patients who died on treatment showed organ scores of mouth-3, eye-1, and skin-2, mouth-2, eye-2, respectively. Two recipients (5.2%) required another agent for severe adverse events (thrombotic microangiopathy leading to acute kidney injury) and corticosteroid-refractory/dependent GVHD. The two patients with SR-cGVHD had severe cGVHD with organ scores of mouth-3, liver-3, skin-2 and mouth-2, skin-3, and genital-1, respectively. Dyslipidemia was the most common (73%) adverse event related to sirolimus but was manageable using lipid-lowering therapy. Failure-free survival at two years was 61% (95% CI 38-78%). The 3-year overall survival was 70% (95% CI 50-83%) (Figure 1).
Discussion
The first-line treatment of moderate-severe cGVHD involves systemic corticosteroids with or without calcineurin inhibitors. Around half of the patients will need second-line agents for corticosteroid-refractory/dependent (SR/SD) cGVHD. Currently, three drugs (ruxolitinib, ibrutinib, and belumosudil) are FDA-approved for the management of SR cGVHD. These newer drugs have overshadowed sirolimus, which has recently gone off-patent. Sirolimus is a mammalian target of rapamycin (mTOR) inhibitor14. It has been studied as GVHD prophylaxis and in the management of SR aGVHD and cGVHD15. While studies have shown high (63-81%) overall response rates in SR cGVHD, there were few complete responses (17-38%)10, 16. These studies were done over a decade back, and sirolimus was used much later (≥ three lines in 63-75%). This study demonstrated that using sirolimus at earlier lines as an add-on therapy to corticosteroids in cGVHD was associated with a reasonable response rate (79%). Though the patient numbers for second-line therapy were smaller, the response rates were similar to those of patients receiving first-line therapy. Sirolimus was effective across all organs, but the patient numbers for lung, genital, joints & fascia GVHD were lower. While there were two deaths and relapses and two patients had thrombotic microangiopathy necessitating treatment change, there were only two patients with true SR-cGVHD. The rest of the patients could taper corticosteroid doses as per the scheduled plan. While the renal toxicity rates were lower in our study without the use of the loading dose, the incidence of dyslipidemia was similar15. The patients were on sirolimus for a median duration of 40 weeks (max 5.6 years). There is also data showing that prolonged sirolimus administration (≥ 1-year post-transplant) was associated with a lower risk of moderate-severe cGVHD17. The failure-free survival rates were similar to those reported with ruxolitinib and belumosudil18,19. The track record of safety and tolerance of sirolimus is much longer than those of the newer drugs. Limitations of this study include the single-center study and heterogeneous use of sirolimus at the physician's discretion as frontline therapy or after corticosteroid refractoriness. Nevertheless, this study demonstrates the safety and efficacy of early sirolimus application in managing moderate-severe cGVHD. This strategy has the potential to reduce the burden of SR/SD cGVHD and the need for expensive second-line treatment options. The cost of generic sirolimus is a fraction of that of newer therapies for SR-GVHD. In the era of newer drugs for cGVHD, sirolimus should not be forgotten as a cost-effective therapeutic alternative, and efforts to study it in RCTs should be continued.
Author Contributions
DPL and AK conceived the study. RS, AK, and DPL analyzed the data and drafted the manuscript. All authors were involved in patient recruitment, clinical care of the patients, and manuscript writing. DPL and RS confirm full access to the study data and final responsibility for the manuscript.
Financial Support
This work was done as a part of the American Society of Hematology Global Research Award to DPL.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website. DPL is one of the Editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
References
1.Pidala JA, Gooley TA, Luznik L, Blazar BR. Chronic graft-versus-host disease: Unresolved complication or ancient history?. Blood. 2024; 144: 1363-73.
2.Flowers ME, Martin PJ. How we treat chronic graft-versus-host disease. Blood. 2015; 125: 606-15.
3.Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002; 100: 48-51.
4.Carpenter P, Boeckh M, Deeg J, Cheng GS, Stern J, Holmberg L. LONG-TERM FOLLOW-UP AFTER HEMATOPOIETIC STEM CELL TRANSPLANT GENERAL GUIDELINES FOR REFERRING PHYSICIANS. FRED HUTCHINSON CANCER CENTER. 2023. https://www.fredhutch.org/content/dam/www/research/patient-treatment-and-support/ltfu/LTFU_HSCT_guidelines_physicians.pdf [Accessed: 21 July 2024]
5.Carpenter PA, Logan BR, Lee SJ, Weisdorf DJ, Johnston L, Costa LJ, et al. A phase II/III randomized, multicenter trial of prednisone/sirolimus versus prednisone/sirolimus/calcineurin inhibitor for the treatment of chronic graft-versus-host disease: BMT CTN 0801. Haematologica. 2018; 103: 1915-24.
6.Martini DJ, Chen B, DeFilipp Z. Recent FDA Approvals in the Treatment of Graft-Versus-Host Disease. Oncologist. 2022; 27: 685-93.
7.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016; 22: 4-10.
8.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21: 389-401.e1.
9.Chhabra P, Kasudhan KS, Khaire N, Kaundal S, Chopra M, Singh C, et al. Utility of eGVHD App for bedside GVHD assessment in a high-volume BMT center. Blood Cell Ther. 2023; 6: 18-22.
10.Jurado M, Vallejo C, Pérez-Simón JA, Brunet S, Ferra C, Balsalobre P, et al. Sirolimus as part of immunosuppressive therapy for refractory chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007; 13: 701-6.
11.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18: 348-71.
12.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018; 53: 1401-15.
13.Inamoto Y, Flowers ME, Sandmaier BM, Aki SZ, Carpenter PA, Lee SJ, et al. Failure-free survival after initial systemic treatment of chronic graft-versus-host disease. Blood. 2014; 124: 1363-71.
14.Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003; 35 (Suppl 3): 7s-14s.
15.Abouelnasr A, Roy J, Cohen S, Kiss T, Lachance S. Defining the role of sirolimus in the management of graft-versus-host disease: from prophylaxis to treatment. Biol Blood Marrow Transplant. 2013; 19: 12-21.
16.Couriel DR, Saliba R, Escalón MP, Hsu Y, Ghosh S, Ippoliti C, et al. Sirolimus in combination with tacrolimus and corticosteroids for the treatment of resistant chronic graft-versus-host disease. Br J Haematol. 2005; 130: 409-17.
17.Pidala J, Kim J, Alsina M, Ayala E, Betts BC, Fernandez HF, et al. Prolonged sirolimus administration after allogeneic hematopoietic cell transplantation is associated with decreased risk for moderate-severe chronic graft-versus-host disease. Haematologica. 2015; 100: 970-7.
18.Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2021; 385: 228-38.
19.Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021; 138: 2278-89.
Search
News