Volume 8 (2025) Issue 1 No.1 Pages 138-146
Abstract
Hodgkin Lymphoma is a highly curable malignancy, and relapsed/refractory Hodgkin lymphoma (RRHL) is treated with salvage chemotherapy, followed by autologous stem cell transplant (ASCT). This single-center, retrospective study included patients with RRHL who underwent ASCT. The study included 62 patients with a median age at diagnosis of 22.5 years and a median age at transplant of 24.5 years. Advanced disease at presentation was observed in 64% of the patients. At relapse, 33.3%, 36.6%, and 30% had stage 2, 3, and 4 disease, respectively. Multiple salvage regimens were administered as per the practices at our institution. Among the patients, 63% had complete remission prior to transplant, and 74% underwent ASCT after first-line salvage. Different conditioning regimens were chosen according to institutional practice. Febrile neutropenia was observed in 93.5% of the patients. The treatment-related mortality rate was 4.8% (n=3). The median follow-up duration was 30 months. Median relapse-free survival (RFS) and overall survival (OS) were not reached, and the 3-year RFS and OS rates were 67.1% and 78%, respectively. Factors that adversely influenced OS and RFS were the type of last salvage used and the number of salvage lines prior to transplantation. An advanced stage at the diagnosis was associated with inferior OS but not with RFS. Patients with the refractory disease had inferior RFS and OS rates, of 25.2% and 56.6%, compared with patients with chemoresponsive disease, who had RFS and OS rates of 77% and 84.7%, respectively. ASCT in RRHL showed promising survival, with over two-thirds of the patients surviving without relapse after 3 years.
Introduction
Hodgkin lymphoma (HL) is a highly curable malignancy with 5-year survival rates of 96.2% and 90% in the 0-19-year and 20-64-year age groups, respectively, in Western countries1. In the Indian setting, earlier studies had shown 5-year progression-free survival (PFS)/freedom from treatment failure rates in the range of 66.3-78.3% and 5-year overall survival (OS) rate in the range of 79.7-86.6%2, 3. Data from a multicenter registry in India, of 939 patients between 2011 and 2017, reported a 5-year event-free survival (EFS) rate of 83.5% for early HL and 73.5% for advanced HL and a 5-year OS rate of 83.6%4. The long-term outcomes of 172 patients with pediatric HL treated at our center demonstrated a 5-year PFS rate of 83.1% and 5-year OS rate of 92.9%5.
Approximately 15-25% of the patients remain refractory or undergo early relapse after first-line therapy6. The standard of care for relapsed/refractory Hodgkin lymphoma (RRHL) is salvage chemotherapy, followed by autologous stem cell transplant (ASCT). Two randomized trials have shown the benefit of ASCT in HL with a 3-year EFS rate of approximately 50%7. Common salvage regimens include DHAP (dexamethasone, high-dose cytarabine, cisplatin); ICE (ifosfamide, carboplatin, etoposide); GVDexa (gemcitabine, vinorelbine, dexamethasone); VIBE (vinorelbine, ifosamide, bendamustine, etoposide) and GVDoxil (gemcitabine, vinorelbine, liposomal doxorubicin)8–10. Previous studies have shown that the risk factors affecting outcomes in RRHL are refractoriness to the last chemotherapy regimen, short time to relapse, and advanced stage at relapse. Tailoring salvage therapy depends on upfront therapy, non-hematological toxicity risk, and the possibility of harvesting stem cells. No prospective studies have compared the effectiveness of salvage regimens in RRHL11. The long-term outcome of RRHL post-ASCT is 54% at 15 years12. Complete response (CR) after salvage therapy before ASCT is associated with superior PFS13. In a study, the use of novel agents, such as brentuximab, resulted in long-term durable remission in only 9% of the patients14. Even in the era of novel agents, avoiding ASCT has not yet become the standard of care. Novel agents are currently being used in various settings, such as post-ASCT relapse, for ASCT-ineligible patients, and as first-line salvage regimens along with chemotherapy in RRHL. There are only a few published studies on ASCT outcomes in patients with RRHL from developing countries, such as India15, 16. In developing countries, access to novel agents is low and there is a high incidence of Multi-Drug resistant infections that could compromise the outcomes of patients with RRHL. Hence, we emphasize that more data from developing countries, such as India, are needed to ascertain the outcomes in a real-world setting. Hence, we present the outcomes of patients with RRHL who underwent ASCT at our center.
Materials and Methods
This was a retrospective single-institution study conducted at a tertiary care hospital in South India on patients with RRHL of all age groups who underwent ASCT between January 1997 and December 2021. An approval for conducting this study was obtained from the Institutional Ethics Committee, Cancer Institute (WIA) Chennai, India. The Institutional Ethics Committee waived the requirement for Informed Consent for this study. Patients who did not undergo adequate mobilization or allogeneic stem cell transplantation were excluded. A list of the patients was obtained from the stem cell transplant register maintained at our institution. Baseline characteristics, such as age at diagnosis and transplant, stage of disease upfront and at relapse, salvage regimen used, disease response prior to transplantation, number of lines of salvage before transplantation, conditioning regimen used, details on stem cell dose, plerixafor use, neutrophil and platelet recovery times, treatment-related morbidity, and follow-up, were collected from patient records. Survival data were censored in June 2022. Survival analysis was performed using the Kaplan-Meier (KM) method, and comparisons between variables were performed using the log-rank test. An association was considered statistically significant if the p-value was less than 0.05. Statistical analyses were performed using IBM SPSS Version 21 software.
Relapse-free survival (RFS) was defined as the duration from the day of stem cell infusion to the date of relapse or death from any cause. OS was defined as the duration from the day of stem cell infusion to the date of death from any cause. The median follow-up duration was calculated using the reverse KM Method. Primary progressive Hodgkin's lymphoma (PPHL) was defined as disease refractory to first-line treatment or disease progression within 3 months of completion of first-line treatment. Refractory disease (RD) was defined as a primary progressive disease refractory to first- or second-line salvage. ASCT was performed only after achieving a CR or partial response (PR) after subsequent salvage therapy in patients with RD. Early relapse (ER) was defined as relapse/progression within 12 months of the completion of first-line treatment and late relapse (LR) was defined as relapse after 12 months of completion of first-line treatment. Treatment-related mortality (TRM) was defined as death due to a non-relapse cause, within 6 months of ASCT.
Results
Baseline characteristics
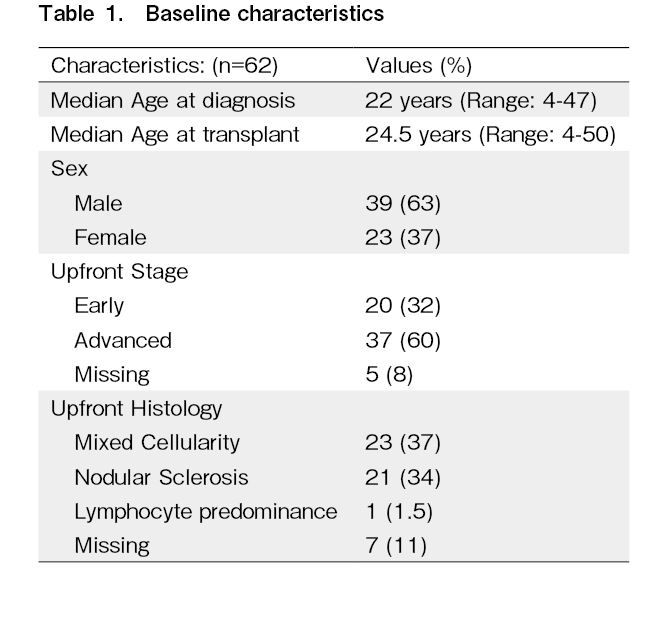
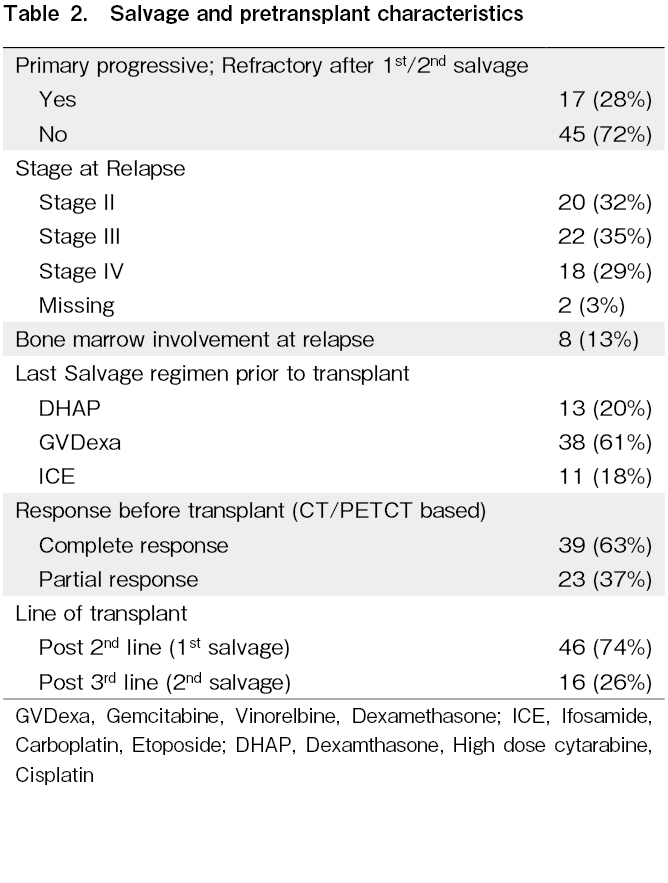
Out of 62 patients included in this study, 39 (63%) were male. The median age at diagnosis was 22 (range: 4-47) years. Thirty-eight percent of the patients had B symptoms at the time of diagnosis. The median age at transplant was 24.5 (range: 4-50) years. The upfront regimen used was ABVD/AVD (Adriamycin, Bleomycin, Vinblastine, Dacarbazine) in 51/62 (82%) patients (Table 1). Seventeen patients (28%) had refractory disease. The stage at relapse was II, III, and IV in 32%, 35%, and 29% of the patients, respectively (Table 2).
Salvage regimen and pretransplant characteristics
Sixty-three percent of the patients had CR and 37% had PR prior to transplantation. Seventy-four percent of the patients underwent ASCT after second-line (first salvage) therapy and 26% underwent ASCT after third-line (second salvage) therapy (Table 2). The median waiting time for transplantation was approximately 2 months (62 days; range: 27-406 days).
Transplant characteristics
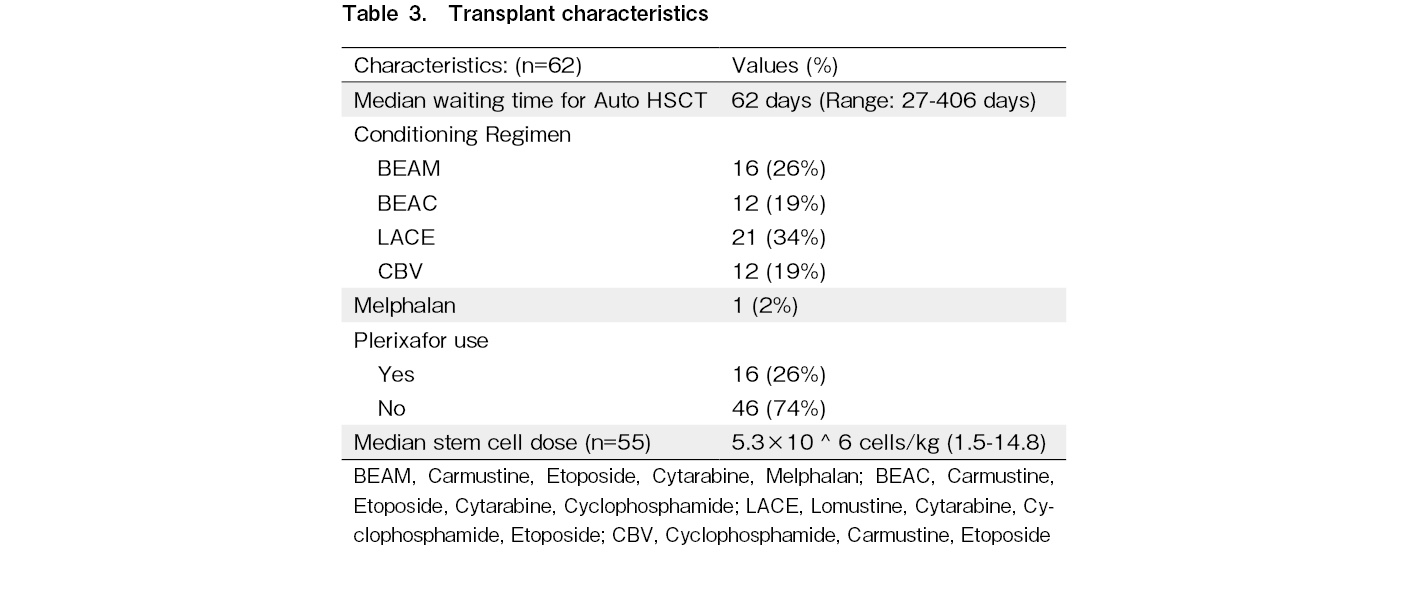
The conditioning regimens are shown in Table 3. Plerixafor was used for stem cell mobilization in 26% of the patients. Three patients underwent chemo-mobilization using high-dose etoposide along with filgrastim and plerixafor. Sixty-one patients underwent peripheral blood stem cell collection and one patient underwent bone marrow stem cell collection. All the collected stem cells were cryopreserved using dimethyl sulfoxide in a -80° deep freezer and used after high-dose chemotherapy. The median stem cell dose used was 5.3 × 106 (range: 1.5× 106 -14.8× 106) cells/kg and 62% of the patients received a dose of more than 4 × 106 cells/kg. The median neutrophil recovery time was 11 (range: 8-18) days, and the median platelet recovery time was 14 (8-38) days. Febrile neutropenia was observed in 93.5% of the patients. The TRM rate was 4.8% (three patients). The causes of TRM were febrile neutropenia, sepsis, and refractory septic shock in two patients and delayed-onset acute pneumonitis in one patient.
Survival
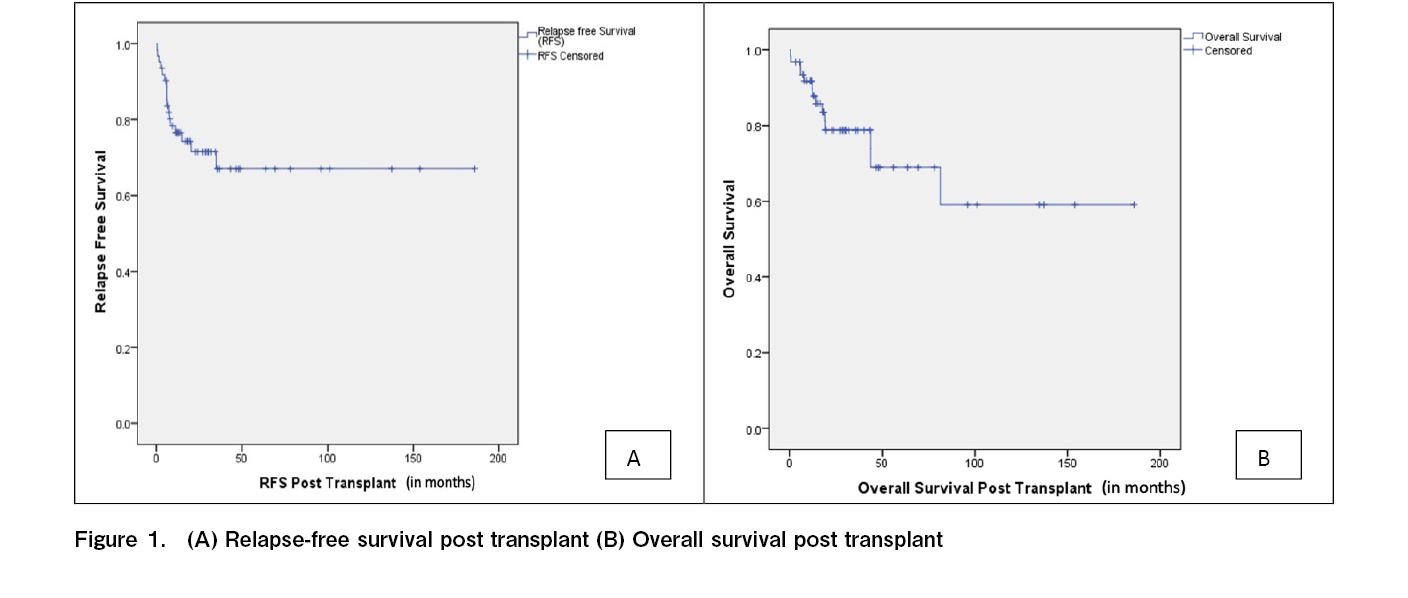
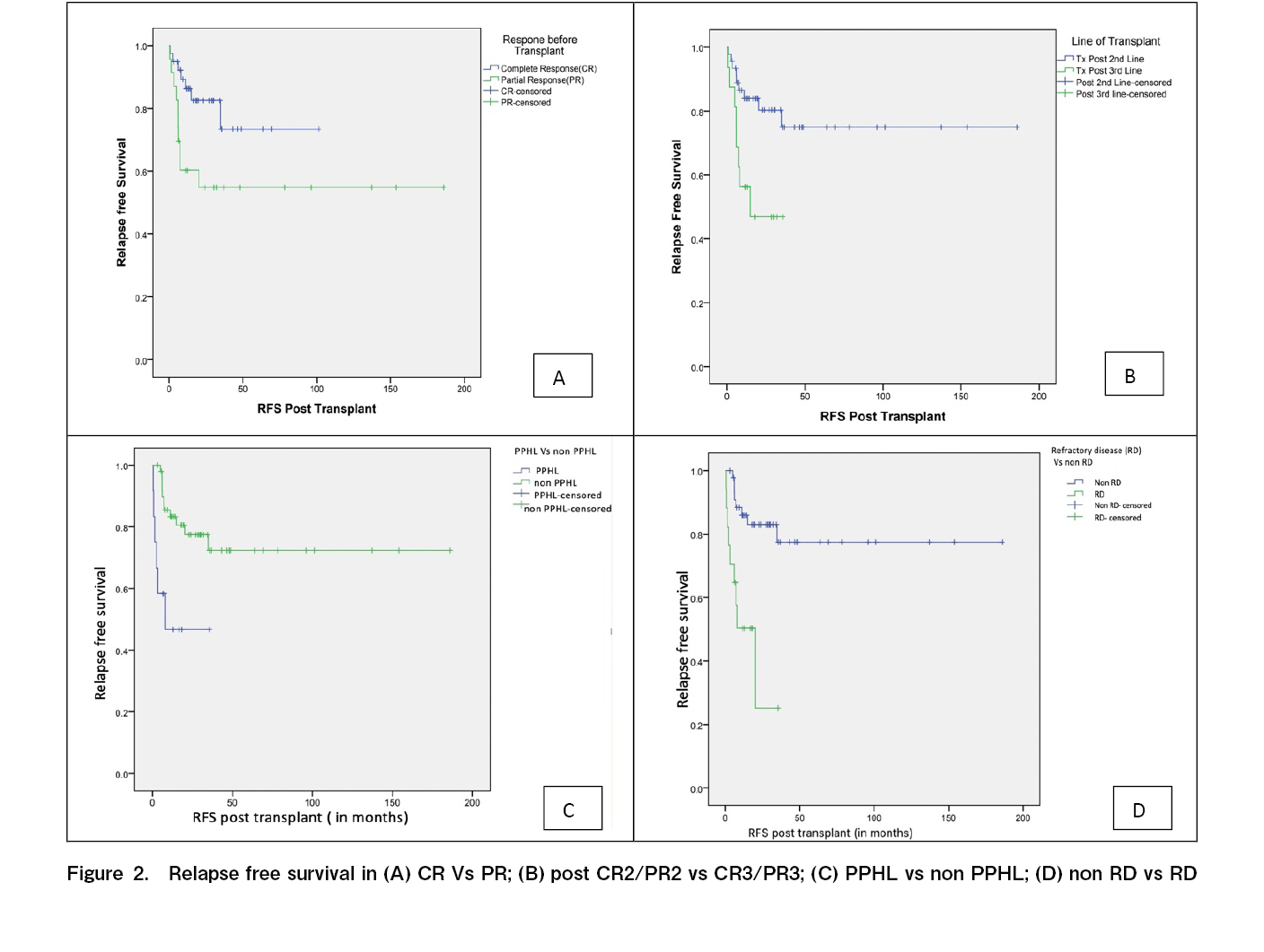
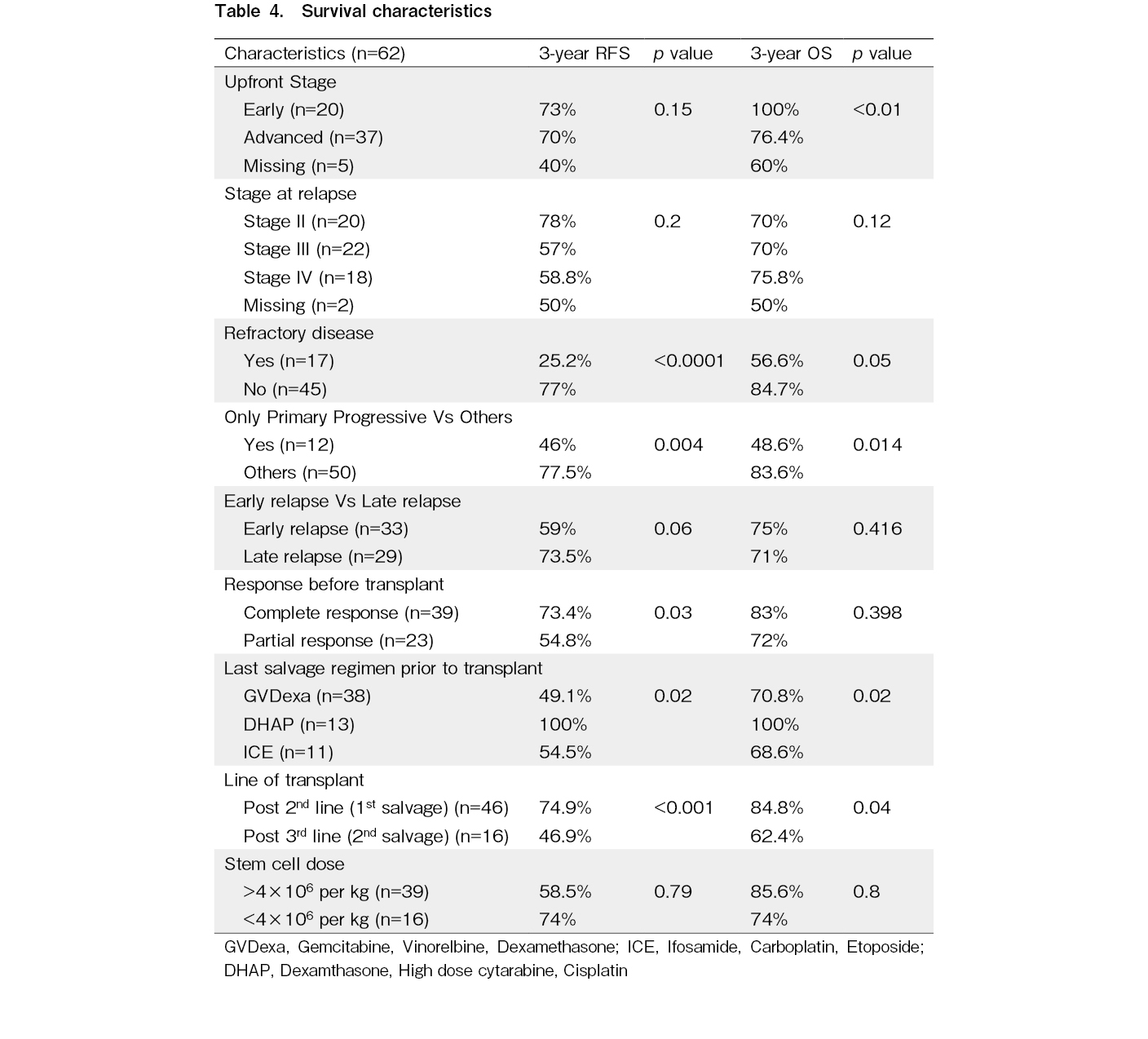
The median post-transplantation follow-up duration was 30 (range: 3-185) months. Forty-five patients were alive without disease at the time of analysis. The median RFS and OS were not reached at the time of analysis. Three-year RFS (RFS3) rate was 67.1% and 3-year OS (OS3) rate was 78% (Table 4 and Figure 1).
Univariate analysis using the log-rank test on RFS (Table 4) showed that patients with PPHL had an RFS3 rate of 46%, compared with 77.5% in other patients who had responded to primary therapy (p=0.004) (Figure 2). Patients with RD had an RFS3 rate of 25.2%, compared with 77% in other patients (p<0.001). The RFS3 rate in patients with ER and LR were 59% and 73.5%, respectively (p=0.06). Patients with CR prior to transplantation had an RFS3 rate of 73.4% and those with PR prior to transplantation had an RFS3 rate of 54.8% (p=0.03). The RFS3 rates based on the last salvage regimen before transplantation with GVDexa, DHAP, and ICE were 49.1%, 100%, and 54.5%, respectively (p=0.02). The RFS3 rates of the patients who underwent transplantation after second-line (first salvage) and third-line (second salvage) therapy were 74.9% and 46.9%, respectively (p<0.001).
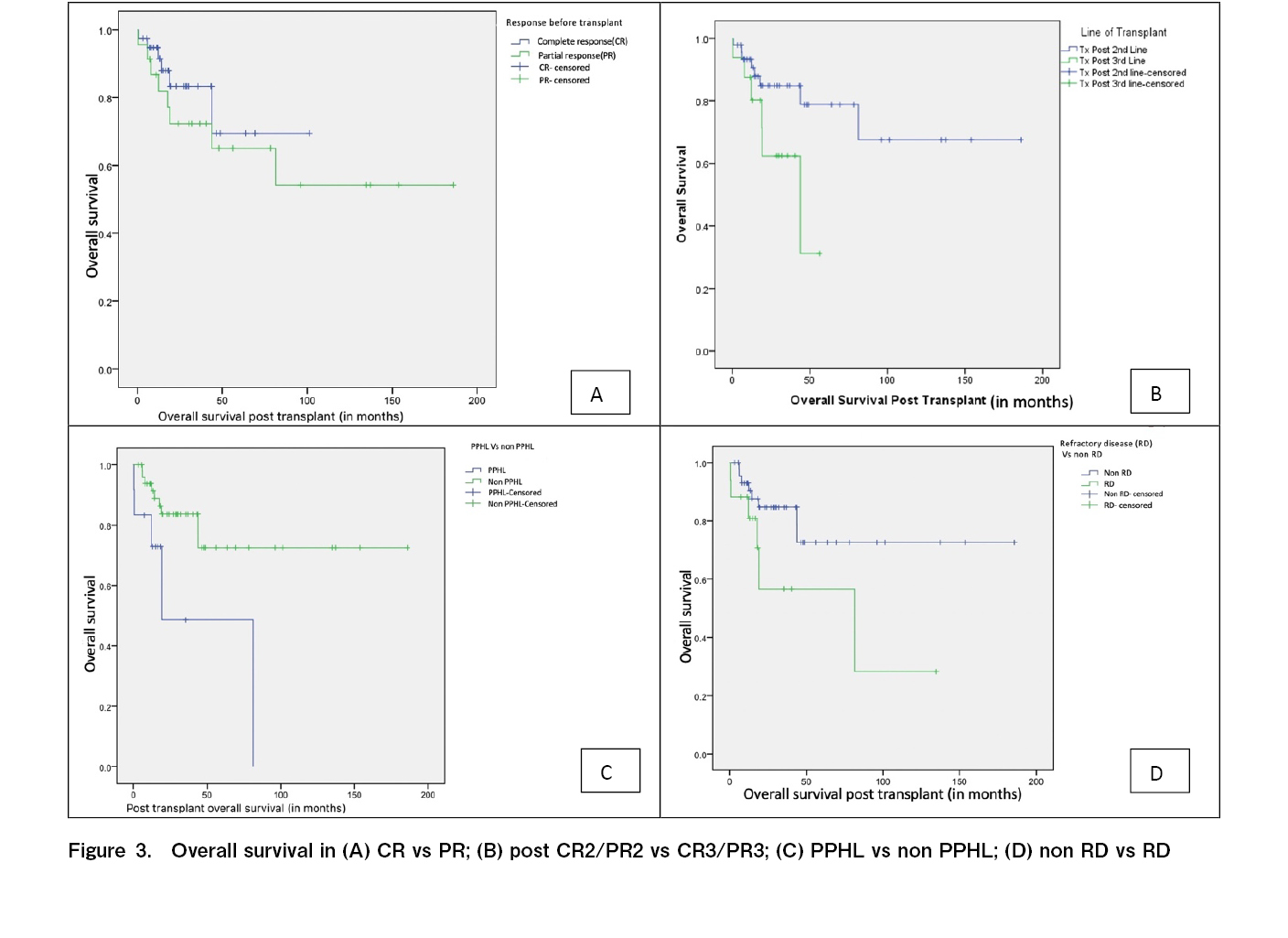
In terms of OS, patients with early stage and advanced stage disease at the time of diagnosis had OS3 rates of 100% and 76.4%, respectively (p<0.01). Patients with PPHL had an OS3 rate of 48.6%, whereas those without PPHL had an OS3 rate of 83.6% (p=0.014) (Figure 3). Patients with RD had an OS3 rate of 56.6%, whereas those who were not chemo-refractory had an OS3 rate of 84.7% (p=0.05). Patients in CR before transplantation had an OS3 rate of 83% and those in PR before transplantation had an OS3 rate of 72% (p=0.39). The OS3 rates of the patients who underwent transplantation after second-line (first salvage) and third-line (second salvage) therapy were 84.8% and 62.4%, respectively (p=0.04).
Post-relapse treatment
At the time of the analysis, there were 17 RFS events; three were due to TRM. Fourteen patients experienced disease relapse post-ASCT. Seven of the fourteen patients received only palliative oral chemotherapy. All seven either died or were lost to follow-up.
The remaining seven patients received subsequent salvage chemotherapy (brentuximab with bendamustine, GVDexa, and multiple lines of chemotherapy in four, two, and one patient respectively). Of the seven patients who underwent salvage therapies, three underwent allogeneic stem cell transplantation (allo-SCT), one was awaiting allo-SCT, one was lost to follow-up, one had progressive disease, and one was unwilling to undergo allo-SCT (was subsequently treated with multiple lines of chemotherapy).
Of the three patients who underwent allo-SCT, two were progression-free and alive. One patient had progressive disease after allo-SCT and received nivolumab as subsequent treatment. The patient achieved CR after 3 months of nivolumab treatment and hence was planned for completion of 2 years of nivolumab treatment.
Discussion
Our study had a median follow-up of approximately 30 months showing RFS3 and OS3 rates of 67% and 78%, respectively. We found that patients with PPHL who underwent transplantation after the third-line therapy had poor outcomes in terms of RFS and OS.
The patients in our study had a median age at diagnosis of 26 years; they were a decade younger than those from OncoCollect, a multicenter registry in India, where the median age at presentation was 38 years4. In our study, 60% of the patients had advanced stage disease at presentation. Those with advanced stage disease and those with early stage disease at presentation exhibited a significant difference in the OS3 rate (76.4% vs. 100%; p<0.01); however, there was no significant difference in the RFS3 between these groups of patients. Improved outcomes in early stage HL are possibly due to a lower tumor burden and better response to therapy17. There were no significant differences between the OS3 and RFS3 regardless of the stage of relapse. Our findings did not align with those of previous studies that showed the stage at relapse as an adverse prognostic factor11.
Patients with RD (n=17) had inferior outcomes, compared with other patients, with an RFS3 rate of 25.2% vs. 77% (p<0.001) and OS3 rate of 56.6% vs. 84.7% (p=0.05) which is similar to reports from other studies. A retrospective study of 100 patients who underwent ASCT showed a 10-year OS rate of 39% in primary refractory disease12. Retrospective evidence from Germany from a cohort of 656 patients has shown that patients with stage IV disease, poor performance status, a bulky disease, time to relapse ≤ 3 months, and poor responders have a higher risk of relapse18.
Compared with other patients, patients with PPHL had poorer outcomes in terms of RFS3 and OS3. Refractory disease and PPHL were both adverse factors associated with RFS3 and OS3. A retrospective study of 357 patients previously reported that a short CR duration was an adverse prognostic factor for time to treatment failure19. In our study, there were no significant differences in the OS or RFS between patients with early and late relapse.
Numerous studies have shown that a CR before ASCT is a strong prognostic factor for both PFS and OS9, 20–24. In our study, 37% of the patients had PR prior to ASCT either as per CT or PET/CT imaging findings, and 63% had CR prior to ASCT. When comparing those had CR with those who had PR prior to ASCT, the RFS3 rate was significantly better (73.4% vs. 54.8%, p=0.03) and the OS was numerically better for the patients with CR compared to those with PR, but not significant (83% vs. 72%, p=0.398). We believe that PET/CT imaging is crucial for response assessments prior to transplantation.
The salvage regimen used prior to transplantation was based on the preference of the consultants at our transplant center, and no one regimen was considered superior to the other. Platinum-containing salvage chemotherapy regimens for RRHL have shown a response rate of 70-90% with 10-30% of the patients remaining refractory to therapy25. In our center, we commonly used GVDexa (61%) as the last salvage regimen prior to transplantation. A previously published phase II study showed that GVDexa is a cost-effective and less toxic salvage regimen with a response rate of 63%, and the majority of the treated patients (86%) had successful stem cell collection8. Other regimens included DHAP (20%) and ICE (18%), with comparable response rates. In our study, the DHAP regimen, when used as the last salvage therapy had 100% RFS3 and OS3 rates which were better and statistically significant compared to the use of the other two regimens. This finding needs to be confirmed using multivariate analysis. However, a multivariate analysis of a small number of patients would be futile.
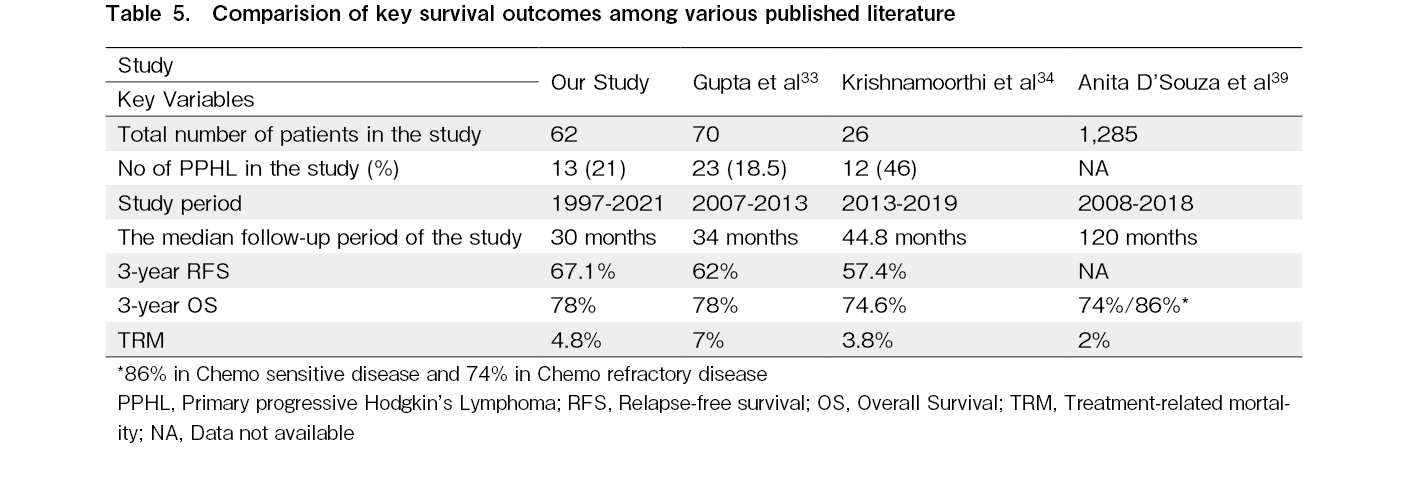
In our study, 74% of the patients underwent ASCT after first-line salvage therapy. Compared with those who underwent ASCT after second salvage therapy, these patients had an RFS3 rate of 74.9% (vs. 46.9%, p<0.001) and OS3 rate of 84.8% (vs. 62.4%, p=0.04), respectively. In contrast to our findings, a retrospective study of 55 patients from Saudi Arabia showed no difference in outcomes in terms of RFS and OS with respect to the line of salvage regimen. However, 55% of these patients received brentuximab as part of the salvage regimen and also as post-ASCT consolidation, which is the reason for comparable outcomes26. The use of two or more lines of salvage therapy before ASCT has also been identified as an adverse prognostic factor for OS27. A retrospective study of 19 patients who received second-line salvage chemotherapy to achieve disease response prior to ASCT had very poor outcomes, with 5-year PFS and OS rates of 11% and 20%, respectively28. Overall, our outcomes were similar to those of other centers across India and the Center for International Blood & Marrow Transplant Research (CIBMTR) data (Table 5).
Our study had a TRM rate of 4.8%, which is comparable to the outcomes in the Western population, ranging from 1.4-3.7%, and those from studies in Europe reporting a TRM rate of 5-13%29–32. Centers across India have reported TRM rates ranging from 0-11.2%33–36.
Only 50% (7/14) of the patients who relapsed after ASCT could receive an effective salvage treatment. Of these seven patients, only four survived until receiving allogeneic stem cell transplantation. The remaining patients who relapsed (7/14) were either unfit or unwilling to undergo further intensive therapy. This depicts the real-world situation in developing countries, where access to novel agents is problematic. The outcomes of post-ASCT relapse patients could be improved only with the use of novel agents, such as brentuximab or immune checkpoint inhibitors, either as consolidation or as a subsequent line of therapy37, 38.
In this study, the data of patients with RRHL who did not undergo ASCT due to financial constraints, poor stem cell mobilization, or unwillingness to undergo transplantation were not analyzed. The strength of our study is that it is an outcome report of an autologous transplant for RRHL at a tertiary cancer care center in a developing country. The outcomes of patients with PPHL and RD at our center were poor because of the lack of access to newer agents. Our study has some limitations, including its retrospective design, small sample size, and short follow-up period.
ASCT in RRHL showed good survival outcomes, with more than two-thirds of patients surviving without relapse after 3 years, even in developing countries. However, patients with PPHL and RD have inferior outcomes, compared with chemo-responsive patients. These patients require a better approach for salvage or consolidation therapy using novel agents to achieve good outcomes.
Acknowledgments
We would like to acknowledge and thank Ms. Vanitha our stem cell transplant coordinator, the tumor registry department, and all the residents of the Department of Medical Oncology.
Author Contributions
MM and JPK collected data, prepared, revised manuscript, and approved finalized version. JPK also analyed data. NM, PK, VR, and KK corrected manuscript and approved finalized version.
Conflicts of Interest
JPK received Travel Grant and Speaker Honoraria from Gilead.
Other authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.SEER. SEER Cancer Statistics Review, 1975-2018. Availabe from: http://seer.cancer.gov/scr/1975_2018/index.html [Accessed: 12 October 2023]
2.Ganesan P, Kumar L, Raina V, Sharma A, Bakhshi S, Sreenivas V, et al. Hodgkin's lymphoma–long-term outcome: an experience from a tertiary care cancer center in North India. Ann Hematol. 2011; 90: 1153-60.
3.Maddi RN, Linga VG, Iyer KK, Chowdary JS, Gundeti S, Digumarti R, et al. Clinical profile and outcome of adult Hodgkin lymphoma: Experience from a tertiary care institution. Indian J Med Paediatr Oncol. 2015; 36: 255-60.
4.Bhurani D, Nair R, Rajappa S, Rao SA, Sridharan N, Boya RR, et al. Real-World Outcomes of Hodgkin Lymphoma: A Multi-Centric Registry From India. Front Oncol. 2022; 11: 799948.
5.Radhakrishnan V, Dhanushkodi M, Ganesan TS, Ganesan P, Sundersingh S, Selvaluxmy G, et al. Pediatric Hodgkin Lymphoma Treated at Cancer Institute, Chennai, India: Long-Term Outcome. J Glob Oncol. 2016; 3: 545-54.
6.Connors JM, Ansell SM, Fanale M, Park SI, Younes A. Five-year follow-up of brentuximab vedotin combined with ABVD or AVD for advanced-stage classical Hodgkin lymphoma. Blood. 2017; 130: 1375-7.
7.Josting A, Rudolph C, Reiser M, Mapara M, Sieber M, Kirchner HH, et al. Time-intensified dexamethasone/cisplatin/cytarabine: an effective salvage therapy with low toxicity in patients with relapsed and refractory Hodgkin's disease. Ann Oncol Off J Eur Soc Med Oncol. 2002; 13: 1628-35.
8.Ganesan P, Mehra N, Joel A, Radhakrishnan V, Dhanushkodi M, Perumal Kalayarasi J, et al. Gemcitabine, vinorelbine and dexamethasone: A safe and effective regimen for treatment of relapsed/refractory hodgkin's lymphoma. Leuk Res. 2019; 84: 106188.
9.Moskowitz AJ, Perales MA, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009; 146: 158-63.
10.Prakash G, Jain A, Sahu K, Bal A, Singh C, Basher R, et al. Bendamustine in combination with ifosfamide, etoposide, and vinorelbine (VIBE) is an effective salvage regimen for heavily pre-treated patients with relapsed or refractory Hodgkin lymphoma: a single-center experience. Blood Res. 2021; 56: 134-40.
11.Neste EVD, Casasnovas O, André M, Touati M, Senecal D, Edeline V, et al. Classical Hodgkin's lymphoma: the Lymphoma Study Association guidelines for relapsed and refractory adult patients eligible for transplant. Haematologica. 2013; 98: 1185-95.
12.Lavoie JC, Connors JM, Phillips GL, Reece DE, Barnett MJ, Forrest DL, et al. High-dose chemotherapy and autologous stem cell transplantation for primary refractory or relapsed Hodgkin lymphoma: long-term outcome in the first 100 patients treated in Vancouver. Blood. 2005; 106: 1473-8.
13.Nikolaenko L, Chen R, Herrera AF. Current strategies for salvage treatment for relapsed classical Hodgkin lymphoma. Ther Adv Hematol. 2017; 8: 293-302.
14.Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016; 128: 1562-6.
15.Radhakrishnan VS, Bajaj R, Raina V, Kumar J, Bhave SJ, Sukumaran Nair RK, et al. Relapsed Refractory Hodgkin Lymphoma and Brentuximab Vedotin-Bendamustine Combination Therapy as a Bridge to Transplantation: Real-World Evidence From a Middle-Income Setting and Literature Review. Front Oncol. 2022; 11: 796270.
16.Kumar S, Sharma A, Bakhshi S, Pushpam D, Gogia A, Sahoo RK, et al. Autologous Stem Cell Transplantation in Adult Hodgkin Lymphoma at a Tertiary Care Center in India: Analysis of Outcomes and Prognostic Factors. Indian J Hematol Blood Transfus. 2024; 40: 181-9.
17.Gobbi PG. Tumor burden in Hodgkin's lymphoma: much more than the best prognostic factor. Crit Rev Oncol Hematol. 2014; 90: 17-23.
18.Bröckelmann PJ, Müller H, Casasnovas O, Hutchings M, von Tresckow B, Jürgens M, et al. Risk factors and a prognostic score for survival after autologous stem-cell transplantation for relapsed or refractory Hodgkin lymphoma. Ann Oncol. 2017; 28: 1352-8.
19.Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin's lymphoma autografted after a first relapse. Ann Oncol. 2005; 16: 625-33.
20.Sucak GT, Çakar MK, Suyanı E, Akı Z, Altındal Ş, Acar K. Outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin lymphoma patients in a centre from Turkey. Hematology. 2013; 18: 269-76.
21.Kuruvilla J, Keating A, Crump M. How I treat relapsed and refractory Hodgkin lymphoma. Blood. 2011; 117: 4208-17.
22.Sirohi B, Cunningham D, Powles R, Murphy F, Arkenau T, Norman A, et al. Long-term outcome of autologous stem-cell transplantation in relapsed or refractory Hodgkin's lymphoma. Ann Oncol. 2008; 19: 1312-9.
23.Popat U, Hosing C, Saliba RM, Anderlini P, Besien KV, Przepiorka D, et al. Prognostic factors for disease progression after high-dose chemotherapy and autologous hematopoietic stem cell transplantation for recurrent or refractory Hodgkin's lymphoma. Bone Marrow Transplant. 2004; 33: 1015-23.
24.Senapati J, Devasia AJ, Korula A, Fouzia NA, Kulkarni U, Lakshmi KM, et al. Disease Status at Transplant has a Significant Impact on Outcomes of Autologous Transplantation (ASCT) in Patients with Hodgkin Lymphoma―A Single Center Experience. Indian J Hematol Blood Transfus. 2022; 38: 290-8.
25.Broccoli A, Zinzani PL. The role of transplantation in Hodgkin lymphoma. Br J Haematol. 2019; 184: 93-104.
26.Abuelgasim KA, Ghazi S, Alahmari B, Alhejazi A, Alaskar A, Alzahrani M, et al. Promising remissions in relapsed refractory classical Hodgkin lymphoma patients requiring multiple salvage regimens before transplantation in the brentuximab vedotin era. Leuk Res Rep. 2021; 16: 100276.
27.Constans M, Sureda A, Terol MJ, Arranz R, Caballero MD, Iriondo A, et al. Autologous stem cell transplantation for primary refractory Hodgkin's disease: results and clinical variables affecting outcome. Ann Oncol. 2003; 14: 745-51.
28.Villa D, Seshadri T, Puig N, Massey C, Tsang R, Keating A, et al. Second-line salvage chemotherapy for transplant-eligible patients with Hodgkin's lymphoma resistant to platinum-containing first-line salvage chemotherapy. Haematologica. 2012; 97: 751-7.
29.Arantes Ade M, Teixeira FS, Ribaie TM, Duartec LL, Silva CR, Bariani C. Autologous stem-cell transplantation in Hodgkin's lymphoma: analysis of a therapeutic option. Einstein (São Paulo). 2011; 9: 124-9.
30.Majhail NS, Weisdorf DJ, Defor TE, Miller JS, McGlave PB, Slungaard A, et al. Long-term results of autologous stem cell transplantation for primary refractory or relapsed Hodgkin's lymphoma. Biol Blood Marrow Transplant. 2006; 12: 1065-72.
31.Perz JB, Giles C, Szydlo R, O'Shea D, Sanz J, Chaidos A, et al. LACE-conditioned autologous stem cell transplantation for relapsed or refractory Hodgkin's lymphoma: treatment outcome and risk factor analysis in 67 patients from a single centre. Bone Marrow Transplant. 2007; 39: 41-7.
32.Nademanee A, O'Donnell MR, Snyder DS, Schmidt GM, Parker PM, Stein AS, et al. High-Dose Chemotherapy With or Without Total Body Irradiation Followed by Autologous Bone Marrow and/or Peripheral Blood Stem Cell Transplantation for Patients With Relapsed and Refractory Hodgkin's Disease: Results in 85 Patients With Analysis of Prognostic Factors. Blood. 1995; 85: 1381-90.
33.Gupta A, Gokarn A, Rajamanickam D, Punatar S, Thippeswamy R, Mathew L, et al. Lomustine, cytarabine, cyclophosphamide, etoposide – An effective conditioning regimen in autologous hematopoietic stem cell transplant for primary refractory or relapsed lymphoma: Analysis of toxicity, long-term outcome, and prognostic factors. J Cancer Res Ther. 2018; 14: 926-33.
34.Krishnamoorthi N, Prakash B, Km D, Pani CK, Ram M, Rajesh K, et al. Outcome of CBV (Carmustine, Cyclophosphamide, Etoposide) Conditioning Regimen for Autologous Stem Cell Transplant in Lymphoma: A Retrospective Study from a Tertiary Cancer Center in South India. Indian J Med Paediatr Oncol. 2022.
35.Kulkarni V, Sapkota S, Badarkhe GV, Srinivas BJ, Naik R. Analysis of Relapsed/Refractory Hodgkin Lymphoma Treated with Autologous Transplantation: A Single-Center Experience. Indian J Med Paediatr Oncol. 2020; 41: 23-8.
36.Kumar S, Sharma A, Pramanik R, Pathak N, Gogia A, Kumar A, et al. Long-Term Outcomes and Safety Trends of Autologous Stem-Cell Transplantation in Non-Hodgkin Lymphoma: A Report From A Tertiary Care Center in India. JCO Glob Oncol. 2022; 8: e2100383.
37.Sweetenham JW, Walewski J, Nadamanee A, Masszi T, Agura E, Holowiecki J, et al. Updated Efficacy and Safety Data from the AETHERA Trial of Consolidation with Brentuximab Vedotin after Autologous Stem Cell Transplant (ASCT) in Hodgkin Lymphoma Patients at High Risk of Relapse. Biol Blood Marrow Transplant. 2016; 22: S36-7.
38.Armand P, Zinzani PL, Lee HJ, Johnson NA, Brice P, Radford J, et al. Five-year follow-up of KEYNOTE-087: pembrolizumab monotherapy for relapsed/refractory classical Hodgkin lymphoma. Blood. 2023; 142: 878-86.
39.D'Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2020; 26: e177-82.
Search
News