Volume 7 (2024) Issue 4 No.4 Pages 118-120
Abstract
Immune effector cell-associated neurotoxicity syndrome usually occurs within the first four weeks of chimeric antigen receptor (CAR)-T cell therapy. In addition, prolonged cytopenia is a long-term adverse effect following the use of CAR T-cell therapies. Here, we present a case of prolonged severe cytopenia followed by fatal CAR T-cell-mediated encephalitis. A 22-year-old male patient was referred to our hospital for a second relapse of B-cell precursor acute lymphoblastic leukemia (ALL), which was diagnosed 22 months after hematopoietic stem cell transplantation from an unrelated donor. CAR T-cells (tisagenlecleucel) were infused during the third cycle of complete remission after chemotherapy. The patient developed grade 2 cytokine release syndrome requiring a single dose of tocilizumab. Cytopenia was profound from day 30 onward, but no other serious complications were observed. On day 50, the patient developed sensory impairment, disturbing behavior, and confusion. Brain magnetic resonance imaging (MRI) scan and cerebrospinal fluid (CSF) analysis revealed no pathological findings. Severe neutropenia persisted despite G-CSF treatment, and the patient's neurological symptoms rapidly progressed from day 65. Brain MRI revealed hydrocephalus. The CSF showed elevated xanthochromia, mononuclear cell counts, and protein levels. A therapeutic attempt with prednisolone for encephalitis was ineffective, and the patient died on day 77 owing to neurological toxicity. Late-onset CAR T-cell-mediated encephalitis was suspected, although the CSF was not assessed for CAR T-cells. In addition, the patient developed prolonged and severe cytopenia. To the best of our knowledge, this is the first report of prolonged severe cytopenia followed by late-onset CAR T-cell-mediated encephalitis. These unexpected long-term adverse effects may occur and should also be considered.
Introduction
Chimeric antigen receptor (CAR)-T therapy is an effective treatment modality for relapsed B-cell precursor acute lymphoblastic leukemia (ALL). The most prevalent reported adverse events include cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS), and prolonged cytopenia1–3. Severe ICANS usually occur within the first 4 weeks after CAR T-cell therapy and respond well to steroids1. Patients who experience cytopenia after CAR T-cell therapy typically recover their blood counts by 30 days, but several cases demonstrate persistent or late cytopenia beyond 30 days3. Here, we report a case of prolonged severe cytopenia followed by fatal progressive central neurotoxicity that occurs 2 months after CAR T-cell administration in a patient with relapsed B-cell precursor ALL who previously underwent allogeneic hematopoietic stem cell transplantation (HSCT).
Case Presentation
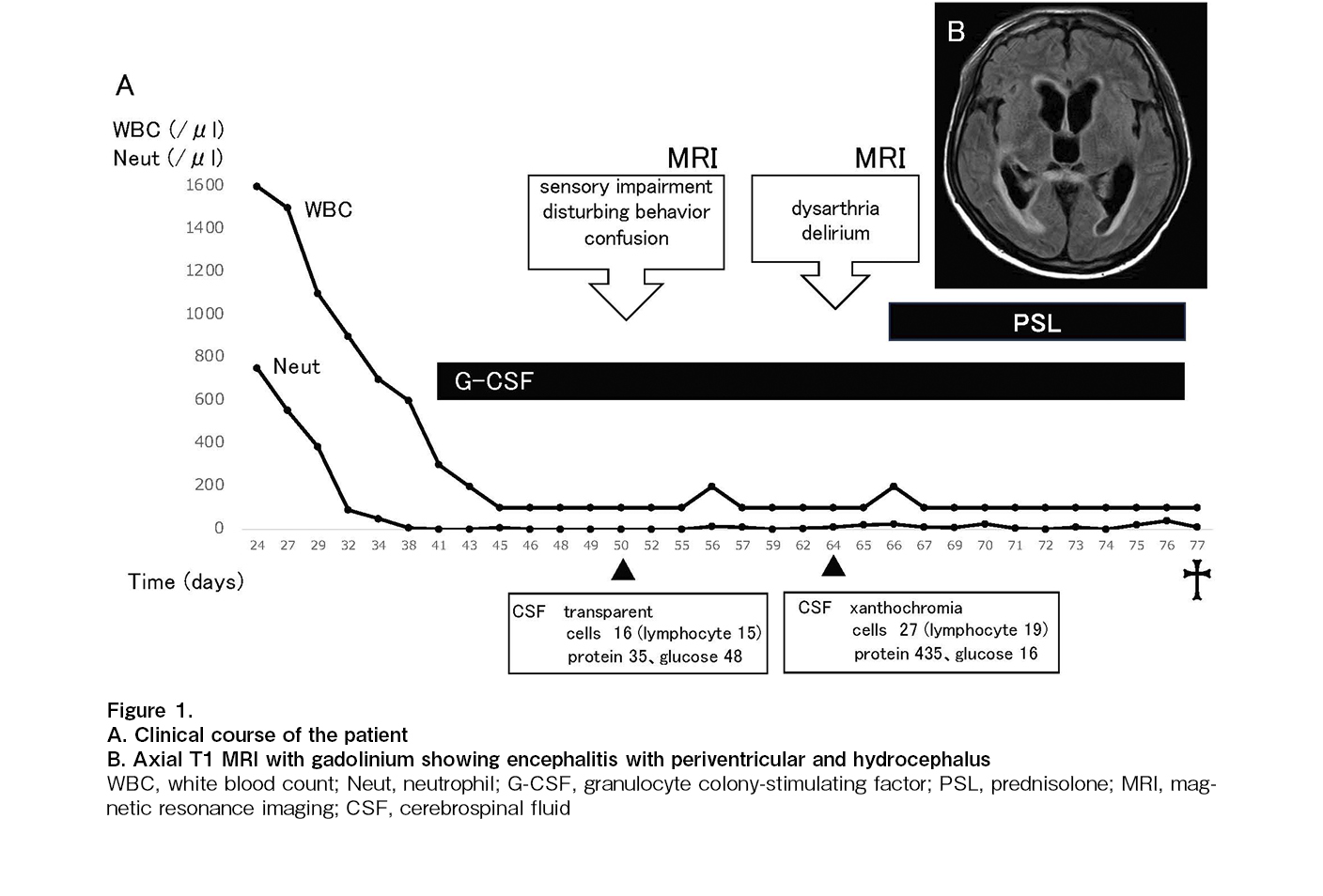
A 17-year-old boy was diagnosed with B-cell precursor ALL that relapsed in August 2019 during maintenance therapy after first-line chemotherapy. Central nervous system (CNS) involvement was negative at the time of diagnosis and relapse. The patient achieved a second complete response (CR) with reinduction therapy, which consisted of clofarabine, etoposide, and cyclophosphamide, as well as an intrathecal injection (IT) of prednisolone/cytarabine/methotrexate. The patient underwent unrelated allogeneic HSCT during second CR. The conditioning regimen consisted of total body irradiation (12 Gy), cyclophosphamide (120 mg/kg), and etoposide (30 mg/kg). However, a second combined relapse (bone marrow and skin) occurred in November 2021. There was no CNS involvement even at the second relapse. Salvage treatment with commercial CAR T-cell therapy using tisagenlecleucel (Tisa-Cel) was planned. The patient underwent autologous collection and demonstrated the successful manufacture of a CD19-CAR product, Tisa-Cel, after controlling for disease status using prednisolone and vincristine. He achieved a third CR after bridging therapy with inotuzumab, ozogamicin, mini-hyper-CVD, and IT with prednisolone/cytarabine/methotrexate. Additionally, he received a single dose of Tisa-Cel (total cell dose: 1.1 × 108) 4 months after the second relapse following lymphodepletion chemotherapy with cyclophosphamide and fludarabine. The patient developed grade 2 CRS (ASTCT grading)4 on day 4 and required a single dose of tocilizumab (8 mg/kg). His symptoms were relieved after two days and white blood cell and neutrophil counts increased to 2.0 and 0.5 × 109/L, respectively, on day 15. However, pancytopenia with severe neutropenia (0.0 × 109/L) developed on day 30 (Figure 1A). Severe neutropenia (0.0 × 109/L) persisted despite granulocyte colony-stimulating factor (G-CSF) administration. The patient developed sensory impairment, disturbing behavior, and confusion on day 50; however, brain magnetic resonance imaging (MRI) scans and cerebrospinal fluid (CSF) analysis revealed no pathological findings. His neurological symptoms, such as dysarthria and delirium, rapidly progressed, and MRI resonance imaging revealed encephalitis with periventricular hydrocephalus on day 64 (Figure 1B). CSF demonstrated elevated xanthochromia, mononuclear cell counts (27/μL), and protein levels (435 mg/dL) and decreased glucose (16 mg/dL). We did not analyze the presence of CAR T-cells in the CSF because we could not suspect CAR T-cell-mediated encephalitis at this time. Subsequently, late-onset CAR T-cell-mediated encephalitis was suspected because CSF analysis also revealed no pathological findings and severe pancytopenia persisted owing to CAR T-cells. Although he received drainage and prednisolone as therapeutic treatments for encephalitis, his neurological symptoms worsened on day 77, and he died. His blood counts never recovered, and pancytopenia with severe neutropenia (0.0 × 109/L) persisted until death.
Discussion
Hematological toxicity is a known side effect of CAR T-cell therapy3. A biphasic course of cytopenia has been reported. The peripheral blood counts decreased as a consequence of lymphodepletion chemotherapy. A second decreasing phase is described, whose mechanisms remain unclear once cytopenia recovers3, 5, 6. Grade 3 or 4 neutropenia and thrombocytopenia have not resolved by day 28 in 53% and 41% of patients with ALL, respectively7, 8. Fried et al. revealed that patients who experienced high-grade CRS (grade >2) and received prior allogeneic HSCT, particularly those who received CAR T-cell therapy <1 year after HSCT, are at an increased risk of developing prolonged cytopenia3. Congruently, our patient received allogeneic HSCT before CAR T-cell therapy and experienced grade 2 CRS after CAR T-cell therapy. Sharma et al. reviewed the possible reasons for prolonged cytopenia after CAR T-cell therapy9. Interestingly, they proposed the suppressive effect of persistent CAR T-cells is one of the reasons for prolonged cytopenia. Hence, persistent CAR T-cells may have caused prolonged severe cytopenia in our case, since late-onset suggestive CAR T-cell-mediated encephalitis is complicated during cytopenia. Our patient experienced sensory impairment, disturbing behavior, and confusion for more than one month after CAR T-cell therapy, and these neurological symptoms rapidly progressed. Jung et al. described the first case of fatal late-onset CAR T-cell-mediated encephalitis after CAR T-cell therapy10. Their case initially developed painless loss of the central visual field and rapidly developed dysarthria, dysphagia, ataxia, and intermittent delirium 7 months after CAR T-cell therapy. CSF demonstrated a high CAR T-cell-specific DNA copy number. MRI resonance imaging revealed encephalitis of the brainstem and cerebellum. Therefore, the patient was diagnosed with late-onset CAR T-cell-mediated encephalitis. Intrathecal alemtuzumab administration to eliminate CSF circulating CAR T-cells was markedly effective; however, CAR T-cell DNA reappeared in the CSF after alemtuzumab discontinuation. Our patient's clinical course was consistent with that of the previous case10. CSF was not assessed for CAR T-cells because no studies have reported late-onset CAR T-cell-mediated encephalitis at that time, and this case was initially unexpected. Although CSF was assessed for CAR T-cells, late-onset CAR T-cell mediated encephalitis was suspected to be due to persistent CAR T-cells. To the best of our knowledge, this is the first report of prolonged severe cytopenia followed by late-onset CAR T-cell-mediated encephalitis. Both side effects are suggested to be related and therefore we should consider unexpected long-term side effects after CAR T-cell therapy.
Author Contributions
T.K. and S.Y. designed and performed the research and wrote the paper, and D.T., M.S., K.O., Y.S., R.K., N.K., S.F., and K.A. collected and managed the clinical data.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, et al. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol. 2021; 32: 34-48.
2.Neelapu SS. Managing the toxicities of CAR T-cell therapy. Hematol Oncol. 2019; 37 (Suppl 1): 48-52.
3.Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019; 54: 1643-50.
4.Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for CytokineRelease Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol Blood Marrow Transplant. 2019; 25: 625-38.
5.Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020; 4: 3776-87.
6.Nahas GR, Komanduri KV, Pereira D, Goodman M, Jimenez AM, Beitinjaneh A, et al. Incidence and risk factors associated with a syndrome of persistent cytopenias after CAR-T cell therapy (PTCC). Leuk Lymphoma. 2020; 61: 940-3.
7.Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017; 130: 2295-306.
8.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018; 378: 439-48.
9.Sharma N, Reagan PM, Liesveld JL. Cytopenia after CAR-T Cell Therapy-A Brief Review of a Complex Problem. Cancers. 2022; 14: 1501.
10.Jung S, Greiner J, von Harsdorf S, Popovic P, Moll R, Schittenhelm J, et al. Fatal late-onset CAR T-cell-mediated encephalitis after axicabtagene-ciloleucel in a patient with large B-cell lymphoma. Blood Adv. 2021; 5: 3789-93.
Search
News