Volume 7 (2024) Issue 4 No.1 Pages 101-105
Abstract
Introduction: The gut microbiome has an established role in allogeneic hematopoietic cell transplantation (allo-HCT), but not in an auto-HCT setting. We have hypothesized that fecal short-chain fatty acids (SCFA) and urinary 3-indoxyl sulfate (3-IS), which are metabolites derived from the action of the gut microbiome on dietary fiber, play a role in auto-HCT outcomes.
Methods: This was a single-center prospective study involving auto-HCT recipients. Baseline patient and disease details, diet diaries, and antibiotic exposure were recorded in consenting patients. Serial (pre-HCT, week two, and week four post-HCT) SCFA and urine 3-IS levels were measured using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS). HCT outcomes were correlated with these metabolites.
Results: Thirty patients (myeloma, n=13; lymphoma, n=17) were analyzed. The levels of urinary 3-IS, fecal acetate, propionate, and butyrate were found to be decreased at week two and were recovered by week four post-HCT. Those with low median nadir fecal butyrate levels at week two also had significantly lower pre-HCT and week four butyrate levels. Recipients with low butyrate levels had more grade ≥2 mucositis (80% vs. 33%, p=0.01) and low fiber intake (10.4 g vs. 13.6 g, p=0.04). They also had more carbapenem exposure (93% vs. 47%, p=0.005) and prolonged antibiotics (11 days vs. 8 days, p=0.008). There were no differences in the time to neutrophil or platelet engraftment, mortality, or disease response.
Conclusion: Low pre-HCT fecal butyrate levels tend to persist post-HCT and they are associated with mucositis, dietary fiber intake, and antibiotic exposure. The gut microbiome and its modulation may play a role in auto-HCT settings.
Introduction
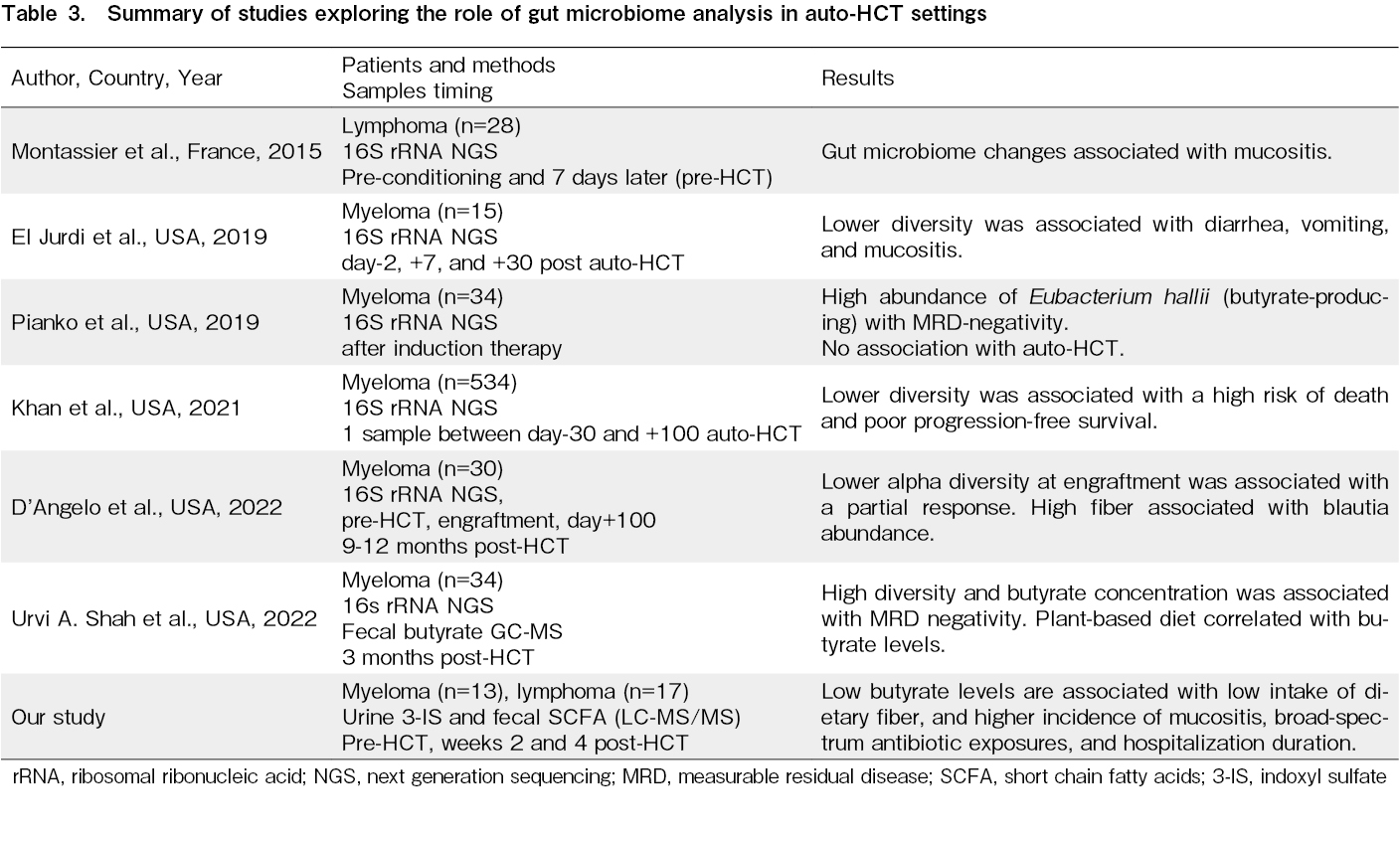
Dysbiosis of the gut microbiome is common in recipients of hematopoietic cell transplantation (HCT). This imbalance in the gut microbiome is associated with morbidity and mortality post-allogeneic HCT1. However, data on the importance of the gut microbiome in autologous HCT (auto-HCT) is still evolving. Initial exploratory studies have shown that a similar loss of gut microbial diversity in auto-HCT recipients around the engraftment period was associated with regimen-related toxicities, such as mucositis, vomiting, and diarrhea2,3. Subsequent studies have shown an association between lower diversity/particular microbe dominance, minimal residual disease (MRD), and progression-free survival (PFS) in multiple myeloma4–7. All these studies examined the gut microbiome diversity using 16S rRNA sequencing. Only one previous study has additionally examined fecal short-chain fatty acid (SCFA) (butyrate) at three months post-HCT and found it to be associated with diet and negative MRD. Indigestible carbohydrates from plant-based diets are metabolized and fermented to SCFAs (acetate, propionate, and butyrate) by commensals in the colonocytes. These SCFAs play a role in maintaining the protective mucus lining of the colon, influencing regulatory T cells, and mucosal barrier homeostasis8. Similarly, dietary tryptophan is converted by the action of the intestinal microbiota to 3-indoxyl sulfate (3-IS) and has been applied in allo-HCT. Fecal SCFAs and urinary 3-IS are good surrogate markers of gut microbial diversity in allo-HCT recipients and are correlated with allo-HCT outcomes9–11. In this exploratory study, we have prospectively examined serial fecal SCFA and urinary 3-IS levels in auto-HCT recipients and their association with diet and HCT outcomes.
Material and Methods
This was a single-center prospective study in consecutive auto-HCT recipients aged ≥12 from 2021 to 2022. This study obtained approval from the Postgraduate Institute of Medical Education and Research, Chandigarh,India Institutional Ethics Committee (IEC-04/2020-1622). The procedures used in this study adhere to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Serial urinary 3-IS and fecal SCFA levels were collected prospectively at the following time points: pre-HCT and weeks two and four post-HCT. All of the samples were stored at -80℃ until further processing. Recipient demographics, diseases, and transplant details were recorded. Myeloma patients received melphalan (200 mg/m2), whereas lymphoma patients received standard (BEAM) carmustine, etoposide, cytarabine, and melphalan (140 mg/m2) as a conditioning regimen. Disease response was assessed pre-HCT and on day+100 after HCT using PET-CT. A detailed diet diary was maintained using the Indian Council of Medical Research (ICMR) Nutrify India app. Daily averages of calorie, carbohydrate, resistant starch, and dietary fiber intake were calculated. Peri-transplant use of a broad-spectrum antibiotic (carbapenem) and the incidence of drug-resistant infections were also recorded. Group comparisons were performed using 2-sided Mann-Whitney U-tests/chi-squared tests. GraphPad Prism 8.0.2 version was used for all statistical analyses.
Urinary 3-Indoxylsulfate assessment
Detection and quantification of urinary 3-IS were performed using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) as described previously12. 3-IS was eluted from the reaction mixture by reverse-phase liquid chromatography and detected by linear ion trap quadrupole LC-MS/MS (Shimadzu) in the negative-ion multiple reaction monitoring modes. Solvent A (0.1% formic acid in water) and solvent B (0.1% formic acid in acetonitrile) were used as the mobile phases, and isatin was used as the internal standard.
Fecal short-chain fatty acid assessment
As described previously, fecal SCFAs were quantified using LC-MS/MS in the negative ion mode13,14. 3-
Results
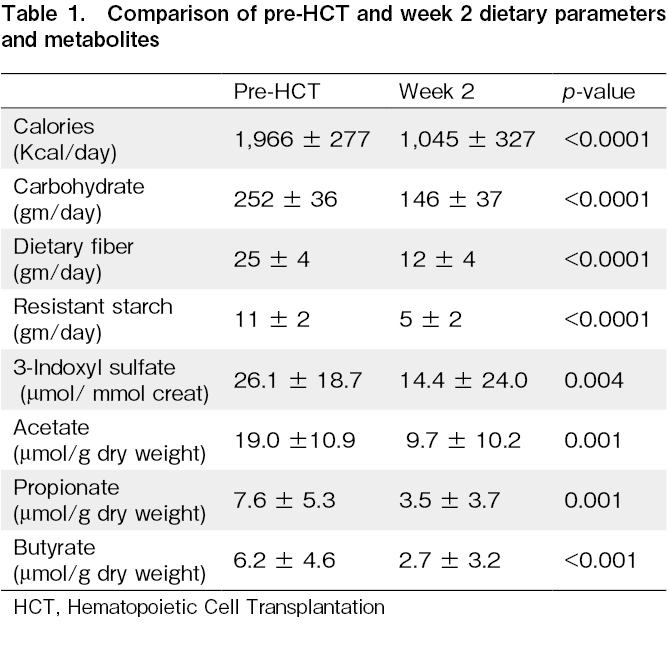
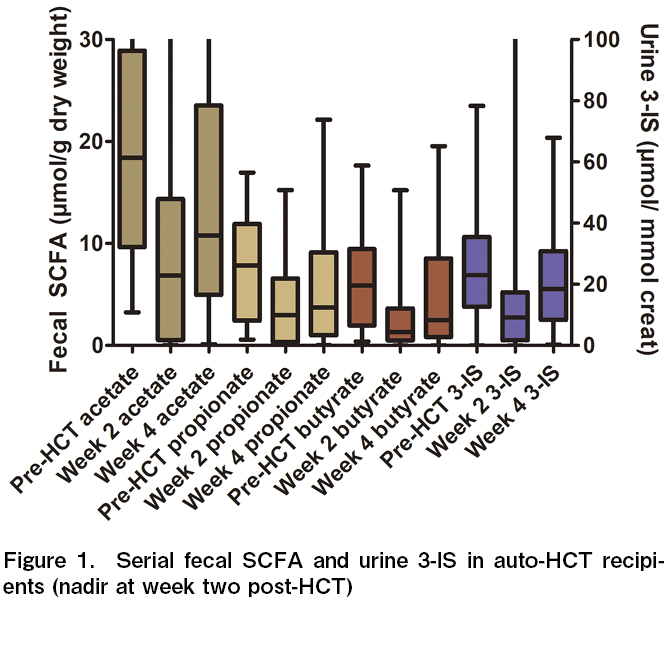
A total of 30 patients (myeloma n=13 and lymphoma n=17) were included in this study over the study period. As expected, dietary calories, carbohydrates, fiber, and resistant starch intake decreased significantly at week two after HCT when compared to pre-HCT intake (Table 1). The mean 3-IS levels were also significantly lower in week two when compared to pre-HCT levels (14.4±24.0 vs. 26.1±18.7 μmol/mmol creat, p=0.004). The 3-IS levels were found to be recovered by week four (22.6±18.6 μmol/mmol creat). A similar trend was observed for fecal SCFA. The mean acetate, propionate, and butyrate levels were significantly lower in week two compared to pre-HCT levels (19.0±10.9 vs. 9.7±10.2, p=0.001, 7.6±5.3 vs. 3.6±3.7, p=0.001, and 6.2±4.6 vs. 2.7±3.2 μmol/g dry weight, p=0.0007, respectively) (Figure 1). The SCFA levels had recovered by week four post-HCT (15.8±14.9, 6.0±6.1, and 4.9±5.5 μmol/g dry weight, respectively). There was a positive correlation between dietary fiber and fecal butyrate levels at week two post-HCT (r=0.35, p=0.05). Similarly, resistant starch intake positively correlated with fecal propionate level at week two with post-HCT (r=0.534, p=0.0023).
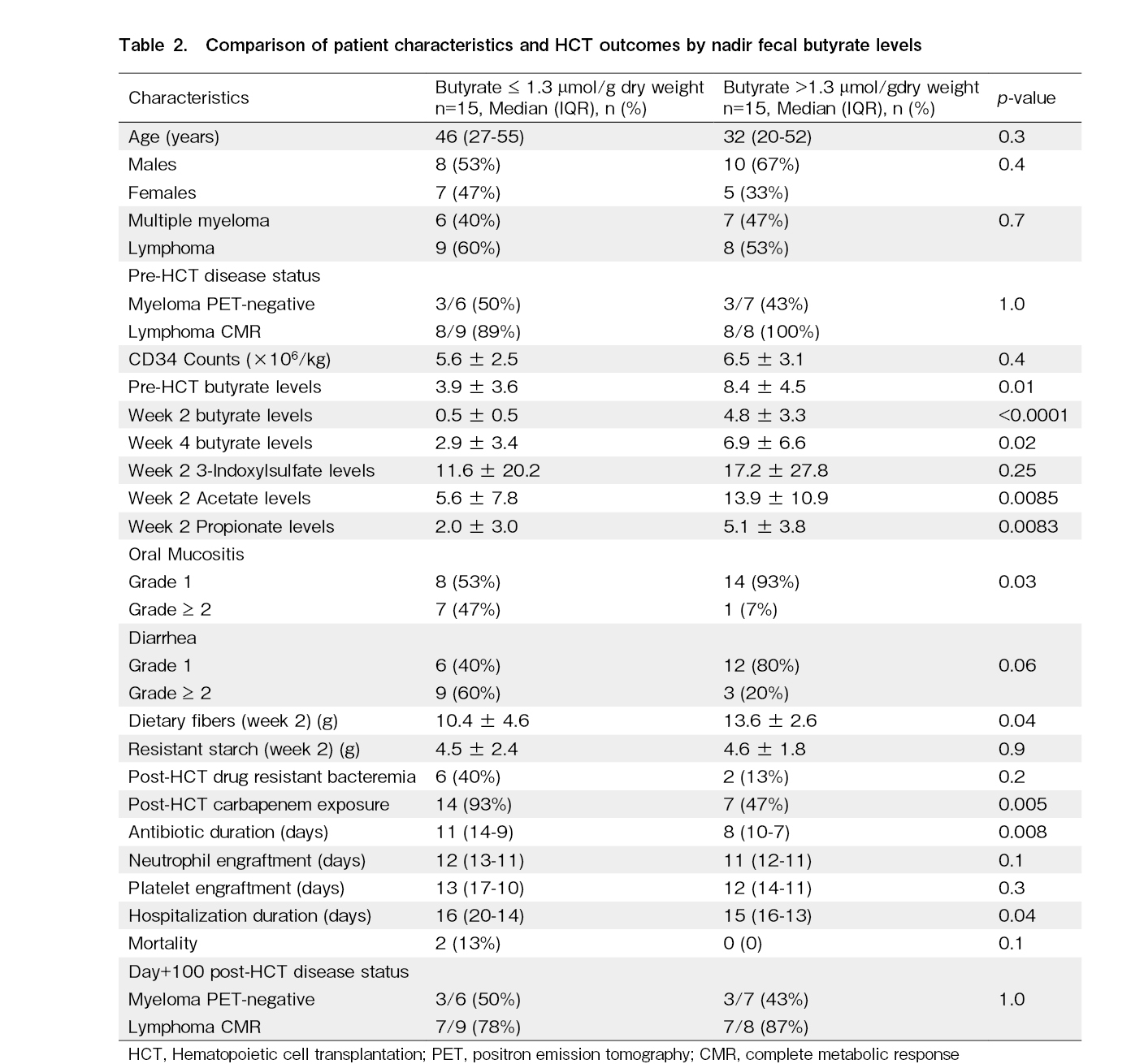
Since butyrate levels have been previously correlated with outcomes in allo-HCT recipients, we decided to compare two groups of patients separated by the median nadir (week two post-HCT) fecal butyrate levels (1.3 μmol/g dry weight). Both cohorts were matched for age, sex, diagnosis, pre-HCT disease status, and CD 34 counts (Table 2). Those with low median nadir fecal butyrate levels also had significantly lower pre-HCT and week four butyrate levels. The recipients with low butyrate levels had more grade ≥ 2 GI mucositis (80% vs. 33%, p=0.01) and low dietary fiber intake (10.4 g vs. 13.6 g, p=0.04). They also had more carbapenem exposure (93% vs. 47%, p=0.005), prolonged antibiotics (11 days vs. 8 days, p=0.008), and hospitalization duration (16 days vs. 15 days, p=0.04). However, there was no difference in the incidence of drug-resistant bacteremia, neutrophil count, or platelet engraftment days. The day+100 mortality and disease status were also not different.
Discussion
Gut microbial alterations have been associated with allo-HCT outcomes and are subjected to therapeutic interventions that modify the microbiome. A role of gut microbiome perturbation in auto-HCT outcomes has also been proposed. Although studying the gut microbiome using 16S rRNA next-generation sequencing is cumbersome and expensive, studying metabolites in urine and feces is comparatively easy and also inexpensive. This study has explored the association of these metabolites with dietary content, HCT outcomes, urinary 3-IS, and fecal SCFAs. These metabolites are directly produced by the action of gut commensals on dietary fiber and resistant starch and play a direct role in gut immune homeostasis. This study has confirmed that these metabolites decreased post-HCT by week two (peri-engraftment) and recovered by week four. These metabolites correlated with dietary fiber and resistant starch intake. The low fecal butyrate levels pre-HCT tended to persist after HCT. It is associated with a higher incidence of mucositis and, consequently, lower fiber intake, more broad-spectrum antibiotic exposure, longer antibiotic duration, and hospitalization duration. Its association with GI toxicity has been reported in two previous studies2,3. Although the causality of antibiotic exposure, dietary fiber, and butyrate levels is difficult to establish, our study has shown that low pre-HCT butyrate levels beget events (mucositis leading to lower fiber intake and increased use of broad-spectrum antibiotics) that lead to even lower post-HCT butyrate levels15. In a small series of patients with heterogeneous diagnoses of myeloma and lymphoma and a short follow-up of 100 days post-HCT, we could not show an association between disease status and mortality, in contrast to other previous studies4,5. This might also be due to the rapid recovery of fecal SCFAs around week four post-HCT, which is usually when auto-HCT recipients return to their regular diet and home environment.
Conclusion
The low pre-and early HCT SCFA levels associated with early HCT outcomes may suggest therapeutic intervention using specific diets rich in fiber/resistant starch and limiting broad-spectrum antibiotics. The findings of this study need to be confirmed in a larger homogeneous patient population and a longer follow-up period, with analysis to elucidate the correlation with gut microbial diversity using 16S rRNA sequencing in order to establish the role of these metabolites as biomarkers in an auto-HCT setting.
Author Contributions
SK, AP, PM, and DL conceived the study, and all authors contributed to patient recruitment and sample collection. SK, AP, PM, and DL processed, analyzed the data, and wrote the manuscript. SK and AP contributed equally to this study.
Funding Source
Part of this work was sponsored by the Indian Council of Medical Research (ICMR) IRIS ID 2020-0664 grant to DPL. SK did this work on a Senior Research Fellowship from the Department of Biotechnology, Government of India.
Conflicts of Interest
The authors have no competing interests to declare relevant to this article's content. Disclosure forms provided by the authors are available on the website.
Informed consent was obtained from all individual participants included in the study.
References
1.Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med. 2020; 382: 822-34.
2.El Jurdi N, Filali-Mouhim A, Salem I, Retuerto M, Dambrosio NM, Baer L, et al. Gastrointestinal Microbiome and Mycobiome Changes during Autologous Transplantation for Multiple Myeloma: Results of a Prospective Pilot Study. Biol Blood Marrow Transplant. 2019; 25: 1511-9.
3.Montassier E, Gastinne T, Vangay P, Al-Ghalith GA, Bruley des Varannes S, Massart S, et al. Chemotherapy-driven dysbiosis in the intestinal microbiome. Aliment Pharmacol Ther. 2015; 42: 515-28.
4.Khan N, Lindner S, Gomes ALC, Devlin SM, Shah GL, Sung AD, et al. Fecal Microbiota Diversity Disruption and Clinical Outcomes after Auto-HCT: A Multicenter Observational Study. Blood. 2021; 137: 1527-37.
5.Shah UA, Maclachlan KH, Derkach A, Salcedo M, Barnett K, Caple J, et al. Sustained Minimal Residual Disease Negativity in Multiple Myeloma is Associated with Stool Butyrate and Healthier Plant-Based Diets. Clin Cancer Res. 2022; 28: 5149-55.
6.Pianko MJ, Devlin SM, Littmann ER, Chansakul A, Mastey D, Salcedo M, et al. Minimal residual disease negativity in multiple myeloma is associated with intestinal microbiota composition. Blood Adv. 2019; 3: 2040-4.
7.D'Angelo C, Sudakaran S, Asimakopoulos F, Hematti P, El-Gamal D, Safdar N, et al. Perturbation of the gut microbiome and association with outcomes following autologous stem cell transplantation in patients with multiple myeloma. Leuk Lymphoma. 2023; 64: 87-97.
8.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013; 504: 451-5.
9.Masetti R, Zama D, Leardini D, Muratore E, Turroni S, Brigidi P, et al. Microbiome-derived metabolites in allogeneic hematopoietic stem cell transplantation. Int J Mol Sci. 2021; 22: 1197.
10.Shono Y, Docampo MD, Peled JU, Perobelli SM, Velardi E, Tsai JJ, et al. Increased GVHD-related mortality with broad-spectrum antibiotic use after allogeneic hematopoietic stem cell transplantation in human patients and mice. Sci Transl Med. 2016; 8: 339ra71.
11.Weber D, Oefner PJ, Hiergeist A, Koestler J, Gessner A, Weber M, et al. Low urinary indoxyl sulfate levels early after transplantation reflect a disrupted microbiome and are associated with poor outcome. Blood. 2015; 126: 1723-8.
12.Zhu W, Stevens AP, Dettmer K, Gottfried E, Hoves S, Kreutz M, et al. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011; 401: 3249-61.
13.Liebisch G, Ecker J, Roth S, Schweizer S, Öttl V, Schött HF, et al. Quantification of fecal short chain fatty acids by liquid chromatography tandem mass spectrometry―investigation of pre-analytic stability. Biomolecules. 2019; 9: 121.
14.Han J, Lin K, Sequeira C, Borchers CH. An isotope-labeled chemical derivatization method for the quantitation of short-chain fatty acids in human feces by liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2015; 854: 86-94.
15.Pianko MJ. Impact of diet and antibiotics on gut microbiota and outcomes in patients with multiple myeloma treated with autologous hematopoietic stem cell transplantation. Leuk Lymphoma. 2023; 64: 3-4.
Search
News