Volume 7 (2024) Issue 3 No.5 Pages 95-100
Abstract
Background: We present comparative data of children with Fanconi anemia undergoing haploidentical hematopoietic stem cell transplantation (HSCT) with or without the addition of rabbit anti-thymocyte globulin (r-ATG) to the conditioning regimen.
Patients and methods: This retrospective study included children with Fanconi anemia aged up to 18 years who underwent haploidentical HSCT between January 2015 and December 2022. The children were included in two cohorts in this study. Cohort 1 included children who received conditioning with fludarabine/cyclophosphamide/single fraction of 2 Gy TBI. The children in cohort 2 received the same conditioning along with r-ATG. Post-transplant cyclophosphamide was administered at a dose of 25 mg/kg on day3 and day4 in both cohorts.
Results: A total of 35 children were included in the study, 25 in cohort 1 and 10 in cohort 2. Neutrophil engraftment was documented around day 14-16 post infusion in 21 children (84%) in cohort 1 and in 8 children (80%) in cohort 2. There was a significant difference in the incidence of the severity of graft versus host disease (GVHD) between the two cohorts (p = 0.003). In cohort 1, acute GVHD was documented in 17 children (68%), with grade 1/2 skin GVHD in 10 children, and grade 3/4 skin and gut GVHD in 7 children. Grade 4 gut GVHD was the cause of death in three children in cohort 1. In cohort 2, acute GVHD was documented in one child (10%) who had grade 4 skin and gut GVHD and succumbed to the above. Chronic GVHD was noted in nine (36%) children in cohort 1, and in one child (10%) in cohort 2. Cytomegalovirus reactivation was documented in 11 children (44%) in cohort 1 and three children (30%) in cohort 2. Overall survival was found to be 16/25 (64%) in cohort 1, with a median follow-up of 49 months, and 7/10 (70%) in cohort 2, with a median follow-up of 12 months.
Conclusion: Serotherapy with r-ATG significantly reduced the incidence of GVHD from 68% to 10% in children with Fanconi anemia, with an increase in overall survival from 64% to 70%, although it did not affect graft failure. Further studies should focus on decreasing graft failure rates with early HSCT before multiple transfusions.
Introduction
Fanconi anemia (FA) is an inherited bone marrow failure syndrome characterized by an underlying DNA breakage repair defect. Hematopoietic stem cell transplantation (HSCT) is an established curative option for correcting hematopoietic defects. When considering the underlying risk of long-term complications associated with regimen-related toxicity, graft-versus-host disease (GVHD), and the risk of secondary malignancies, conditioning regimens need to be tailored to ensure engraftment while reducing the risk of late effects.
Several groups have reported the efficacy of haploidentical HSCT for Fanconi anemia and the effects of various conditioning regimens1, 2. Administration of cyclophosphamide at a dose of 50 mg/kg at the 72nd hour is a strategy for haplo-HSCT with unmanipulated T-replete stem cells3, 4. It has been shown to effectively result in engraftment with limited incidence of GVHD5. To reduce exposure to alkylating agents, the recommended dose of PTCy for Fanconi anemia is 25 mg/kg on day3 and day4. However, there is a trend towards increased rates of chronic GVHD with these aforementioned doses7.
Several globulin (ATG) is effective in preventing GVHD, with a significant reduction in the rates of acute and chronic GVHD8, 9. The combination of PTCy and ATG has been shown to have synergistic effects on T lymphocyte depletion, with ATG depleting early active T lymphocytes and PTCy acting on proliferating lymphocytes10.
The authors have published outcome data on haplo-HSCT with PTCy using a regimen including fludarabine, cyclophosphamide, and single-fraction total body irradiation (TBI) of 2 Gy11. Significant rates of acute and chronic GVHD were noted, and the protocol was modified to include rabbit anti-thymocyte globulin (r-ATG). Here, we present comparative data between two cohorts based on conditioning regimens with or without r-ATG.
Patients and Methods
This retrospective study included children with Fanconi anemia aged up to 18 years who underwent haploidentical HSCT between January 2015 and December 2022. All children were confirmed to have Fanconi anemia using stress cytogenetics and/or whole-exome sequencing. High-resolution HLA typing was performed for all children, their siblings, and parents. If no compatible matched family donors were found, a search was performed for matched unrelated donors in registries worldwide. Families were then counseled for haploidentical HSCT, and the children were screened for the presence of anti-HLA antibodies using single-antigen bead assays. Children were considered for HSCT after donor-specific antibodies were excluded. Sibling donors were screened for the presence of underlying mutations.
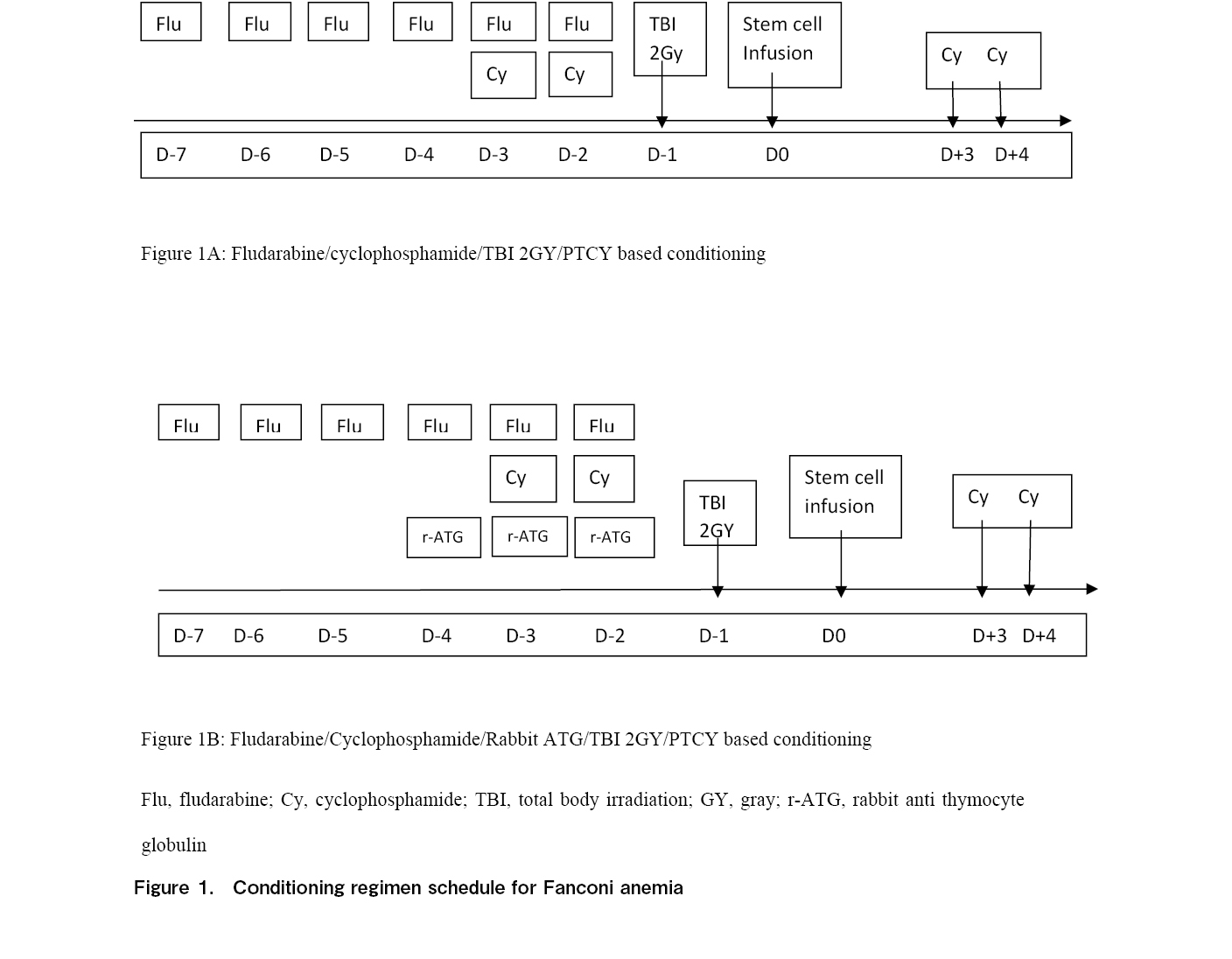
Children were included in two cohorts in this study. Cohort 1 (Figure 1A) included children who received conditioning with fludarabine 30 mg/m2/day from day-7 to day-2 for 6 days and cyclophosphamide 5 mg/kg/day on day-3 and day-2 along with a single fraction of 2 Gy TBI on day-1. Children in cohort 2 (Figure 1B) received the same conditioning regimen along with r-ATG from day-4 to day-2 for 3 days at a dose of 1.5 mg/kg/day. Post-transplant cyclophosphamide was administered at a dose of 25 mg/kg on day3 and day4 in both cohorts. In addition to PTCy, GVHD prophylaxis included calcineurin inhibitors, namely tacrolimus, and the dose was titrated based on levels to be maintained between 5-15 ng/ml.
Since 2017, all children in the group undergoing haploidentical HSCT with PTCy have received continuous infusion of N-acetylcysteine on day3 and day4 post infusion. This reduced free radical injury, regimen-related toxicity, and cardiotoxicity associated with PTCy11. Echocardiography was performed for all children after completing PTCy, and medications were instituted after discussion with a cardiologist and pediatric intensivist, if needed.
Donor chimerism was monitored by fluorescence in situ hybridization (FISH) in sex-mismatched HSCT and by polymerase chain reaction (PCR) in same-sex HSCT. Viral reactivation of cytomegalovirus, adenovirus, and Epstein Barr virus (EBV) was monitored through PCR post-engraftment in all children, once weekly up to day30, and then further based on the risk of reactivation. Acute and chronic GVHD were graded as per National Institute of Health (NIH) criteria and guidelines12, 13. The study was approved by the institutional review board and written informed consent was obtained from the parents or guardians of all children before HSCT.
The study has received full board approval from the institutional review board and the approval number is ASH-C-S-034/12-23.
Statistical Analyses
Descriptive statistics are presented as frequency (percentage) and mean (SD) for categorical and continuous factors, respectively. The median (IQR) is presented for skewed continuous factors. Normality of the data was checked using the Shapiro-Wilk test. Chi-square/Fisher's exact test was used to determine the association between factors and cohorts. Student's t-test or Mann-Whitney U test was used to determine significant changes in the follow-up time between cohorts. Kaplan-Meier survival curve analysis was performed, and survival time was compared between the two cohorts using the log-rank test. p-value<0.05 is considered as statistical significance. All analyses were performed using SPSS software (IBM, Armonk, NY, USA, version 28.0).
Results
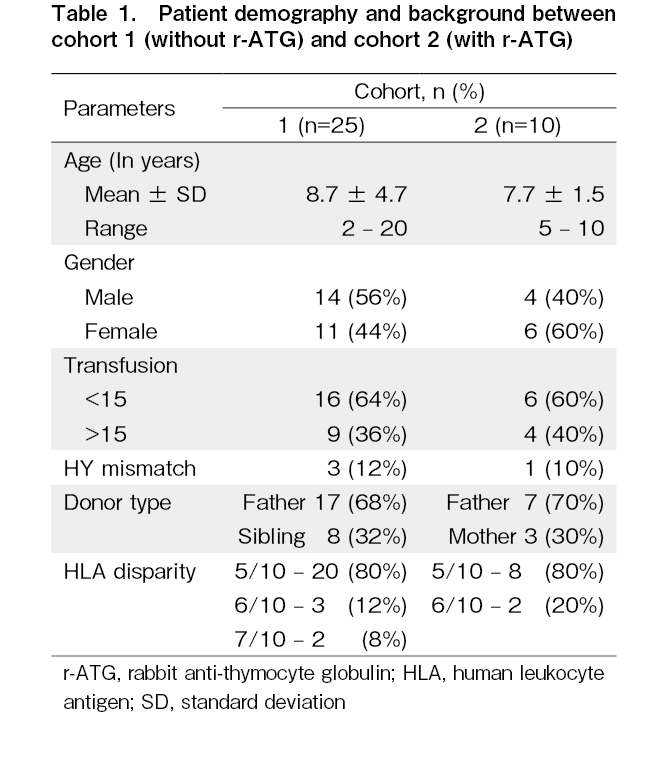
A total of 35 children were included in the study: 25 in cohort 1 and 10 in cohort 2. Next-generation sequencing data were available for 20 children and this included homozygous mutations in the FANCA gene in 12 children and in the FANCC gene in 8 children. The 19 children in cohort 1 were included in a previous study11. In cohort 1 (n=25), the median age was 8 years (range, 2-20), with 14 males and 11 females (M:F=1.2:1). Nine children (36%) received more than 15 transfusions including packed red blood cells and platelets, prior to HSCT. The donor type was haploidentical father in 17 children (68%) and haplo-matched siblings in 8 children (32%). The source of stem cells was peripheral blood stem cells (PBSC) in 21 children (84%) and bone marrow in 4 (16%). Anti-HLA antibodies were noted in one child in the first cohort, which was not donor-specific, and the range of mean fluorescence intensity (MFI) was 5,000. The mean infused CD34 cell dose was 5×106 cells/kg recipient body weight (range, 5-7.2). No cardiotoxicity was observed in this cohort.
In cohort 2 (n=10), the median age was 7 years (range, 5-10), with four males and six females (M:F=0.6:1). Four children (40%) received more than 15 transfusions before HSCT. The donor type was the father in seven children (70%) and the mother in three (30%). All the children (100%) in cohort 2 received PBSC as the stem cell source. Four children (40%) had non-donor-specific anti-HLA antibodies, with an MFI ranging from 1,000 to 8,000. The mean infused CD34 cell dose infused was 5.9×106 cells/kg recipient body weight (range, 5-7.7).
On comparing the outcome variables between the two groups, the median time to achieve neutrophil engraftment was day 13 (range, 12-16) post-infusion in 21 children (84%) in cohort 1 and day 12 (range, 11-14) in eight (80%) in cohort 2. Culture-positive bacteremia in the neutropenic period was documented in four children (16%; three Klebsiella pneumoniae, one Enterococcus species) in cohort 1 and in 2 children (20%) in cohort 2, with isolation of E. coli in both children. In both cohorts, the bacteria isolated were ESBL-positive, and negative cultures were documented 48 hours after antibiotic escalation, according to drug sensitivity and an infectious disease specialist review.
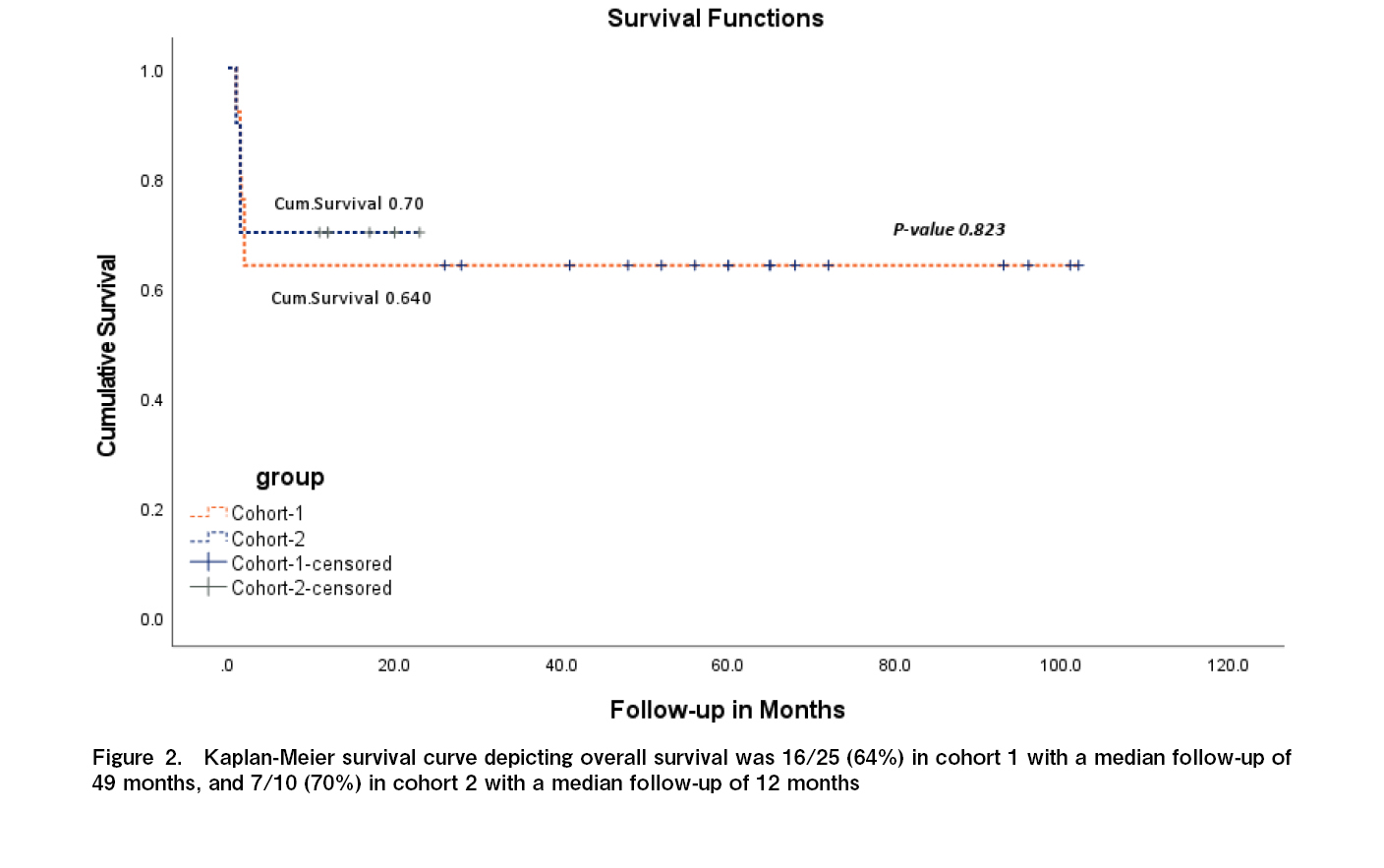
A significant difference was observed in the incidence of GVHD severity between the two cohorts (Table 1). In cohort 1, without r-ATG, acute GVHD was documented in 17 children (68%), with grade 1/2 skin GVHD in 10 children, and grade 3/4 skin and gut GVHD in 7 children. Grade 4 gut GVHD was the cause of death in three children in cohort 1. In cohort 2, acute GVHD was documented in one child (10%) who had grade 4 skin and gut GVHD and succumbed to the above. This difference was statistically significant (p=0.003). Chronic GVHD was noted in nine children (36%) in cohort 1, with chronic limited skin GVHD in five children, chronic skin, and mouth GVHD in two children, chronic genital GVHD requiring surgery for phimosis in one male child, and chronic liver GVHD in one child. In cohort 2, chronic limited skin and mouth GVHD was documented in one child (10%). Cytomegalovirus reactivation was documented in 11 children (44%) in cohort 1 and 3 children (30%) in cohort 2, with no statistically significant difference in all the children responding to ganciclovir. Overall survival was 16/25 (64%) in cohort 1, with a median follow-up of 49 months, and 7/10 (70%) in cohort 2, with a median follow-up of 12 months (Figure 2). The causes of death in cohort 1 (n=9) were primary graft failure in four, grade 4 gut GVHD in three, and bacterial sepsis in two children. In cohort 2, the causes of death (n=3) were primary graft failure in two children and grade 4 gut GVHD in one child.
Discussion
This study reports comparative data of children with Fanconi anemia undergoing haplo-HSCT with PTCy, with or without the addition of r-ATG in conditioning chemotherapy. Engraftment and viral reactivation rates remained similar; however, the incidence of acute GVHD was significantly reduced after the addition of r-ATG. There was also a decrease in the incidence of chronic GVHD and an improved overall survival from 64% to 70% with the addition of r-ATG.
Several groups have investigated the combination of ATG and PTCy. Barkhordar et al. studied the addition of 40 mg/kg PTCy to a standard ATG backbone in acute leukemia and found a significantly decreased incidence of acute and chronic GVHD14. Zu et al. documented a reduced incidence of non-relapse mortality and improved overall and disease-free survival in matched unrelated donor HSCT with a combination of low-dose ATG at 6 mg/kg and low-dose PTCy at 20 mg/kg15. Similar results were reported by Li et al. in patients undergoing haplo-HSCT from maternal/collateral-related donors16.
ATG in Fanconi anemia was studied by Bonfim et al., in which the rates of severe GVHD were found to be lower and survival was better in the group that received r-ATG7. ATG has also been used to deplete host T cells, thereby decreasing the risk of graft rejection17. This is particularly relevant in developing societies, where patients are referred late after receiving multiple transfusions without leucodepletion, with significant alloimmunization, and the presence of anti-HLA antibodies.
Immune reconstitution has been shown to be delayed in haplo-HSCT with PTCy, and the addition of ATG can further reduce this delay. Close monitoring of viral reactivation is essential, and monitoring of CD4 counts after HSCT may help predict immune reconstitution18–20.
The limitations of this study include its retrospective design, small sample size in cohort 2, and short follow-up period in the second cohort. We plan to perform a multicenter national prospective study to validate these results.
Conclusion
Serotherapy with r-ATG had a significant impact on reducing GVHD from 68% to 10% in children with Fanconi anemia, with an increase in overall survival from 64% to 70%, although it did not have an impact on graft failure. Further studies should focus on decreasing graft failure rates with early HSCT before multiple transfusions.
Acknowledgments
We would like to acknowledge the contribution of the pediatric critical care team, infectious disease specialists, hematology, and apheresis team in the management of these children.
Author Contributions
RU, VVS, KG conceptualized the study and wrote the manuscript; SD, AN, VM, ARN helped with data collection; LB helped with statistics; RR helped with proof reading and editing
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Fink O, Even-Or E, Avni B, Grisariu S, Zaidman I, Schejter YD, et al. Two decades of stem cell transplantation in patients with Fanconi anemia: Analysis of factors affecting transplant outcomes. Clin Transplant. 2023; 37: e14835.
2.Cancio M, Troullioud Lucas AG, Bierings M, Klein E, de Witte MA, Smiers FJ, et al. Predictors of outcomes in hematopoietic cell transplantation for Fanconi anemia. Bone Marrow Transplant. 2024; 59: 34-40.
3.Wachsmuth LP, Patterson MT, Eckhaus MA, Venzon DJ, Kanakry CG. Optimized Timing of Post-Transplantation Cyclophosphamide in MHC-Haploidentical Murine Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2020; 26: 230-41.
4.Luznik L, Fuchs EJ. High-dose, post-transplantation cyclophosphamide to promote graft-host tolerance after allogeneic hematopoietic stem cell transplantation. Immunol Res. 2010; 47: 65-77.
5.Iwamoto M, Ikegawa S, Kondo T, Meguri Y, Nakamura M, Sando Y, et al. Post-transplantation cyclophosphamide restores early B-cell lymphogenesis that suppresses subsequent chronic graft-versus-host disease. Bone Marrow Transplant. 2021; 56: 956-9.
6.Thakar MS, Bonfim C, Walters MC, Storb R, Pasquini R, Burroughs L, et al. Dose-adapted post-transplant cyclophosphamide for HLA-haploidentical transplantation in Fanconi anemia. Bone Marrow Transplant. 2017; 52: 570-3.
7.Bonfim C, Ribeiro L, Nichele S, Loth G, Bitencourt M, Koliski A, et al. Haploidentical Bone Marrow Transplantation with Post-Transplant Cyclophosphamide for Children and Adolescents with Fanconi Anemia. Biol Blood Marrow Transplant. 2017; 23: 310-7.
8.Mohty M. Mechanisms of action of anti-thymocyte globulin: T-cell depletion and beyond. Leukemia. 2007; 21: 1387-94.
9.Yang X, Li D, Xie Y. Anti-Thymocyte Globulin Prophylaxis in Patients With Hematological Malignancies Undergoing Allogeneic Hematopoietic Stem Cell Transplantation: An Updated Meta-Analysis. Front Oncol. 2021; 11: 717678.
10.Zu Y, Li Z, Gui R, Liu Y, Zhang Y, Yu F, et al. Low-dose post-transplant cyclophosphamide with low-dose antithymocyte globulin for prevention of graft-versus-host disease in first complete remission undergoing 10/10 HLA-matched unrelated donor peripheral blood stem cell transplants: a multicentre, randomized controlled trial. Bone Marrow Transplant. 2022; 57: 1573-80.
11.Uppuluri R, Swaminathan VV, Ramanan KM, Meena S, Varla H, Ramakrishnan B, et al. Haploidentical Stem Cell Transplantation with Post-Transplant Cyclophosphamide in Fanconi Anemia: Improving Outcomes with Improved Supportive Care in India. Biol Blood Marrow Transplant. 2020; 26: 2292-8.
12.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016; 22: 4-10.
13.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21: 389-401.e1.
14.Barkhordar M, Kasaeian A, Janbabai G, Kamranzadeh Fumani H, Tavakoli S, Rashidi AA, et al. Modified combination of anti-thymocyte globulin (ATG) and post-transplant cyclophosphamide (PTCy) as compared with standard ATG protocol in haploidentical peripheral blood stem cell transplantation for acute leukemia. Front Immunol. 2022; 13: 921293.
15.Zu Y, Gui R, Li Z, Wang J, Zhang Y, Yu F, et al. Low-dose PTCy plus low-dose ATG as GVHD prophylaxis after UD-PBSCT for hematologic malignancies: a prospective, multicenter, randomized controlled trial. Blood Cancer J. 2023; 13: 10.
16.Li T, He Q, Yang J, Cai Y, Huang C, Xu X, et al. Low-Dose Anti-Thymocyte Globulin Plus Low-Dose Posttransplant Cyclophosphamide as an Effective Regimen for Prophylaxis of Graft Versus Host Disease After Haploidentical Peripheral Blood Stem Cell Transplantation With Maternal/Collateral Related Donors. Cell Transplant. 2022; 31: 9636897221139103.
17.Pascal L, Mohty M, Ruggeri A, Tucunduva L, Milpied N, Chevallier P, et al. Impact of rabbit ATG-containing myeloablative conditioning regimens on the outcome of patients undergoing unrelated single-unit cord blood transplantation for hematological malignancies. Bone Marrow Transplant. 2015; 50: 45-50.
18.de Koning C, Admiraal R, Nierkens S, Boelens JJ. Immune reconstitution and outcomes after conditioning with anti-thymocyte-globulin in unrelated cord blood transplantation; the good, the bad, and the ugly. Stem Cell Investig. 2017; 4: 38.
19.Massoud R, Klyuchnikov E, Gagelmann N, Zabelina T, Wolschke C, Ayuk F, et al. Impact of Anti-T-lymphocyte globulin dosing on GVHD and Immune reconstitution in matched unrelated myeloablative peripheral blood stem cell transplantation. Bone Marrow Transplant. 2022; 57: 1548-55.
20.Wang H, Wang N, Wang L, Du J, Li F, Shao Y, et al. Targeted dosing of anti-thymocyte globulin in adult unmanipulated haploidentical peripheral blood stem cell transplantation: A single-arm, phase 2 trial. Am J Hematol. 2023; 98: 1732-41.
Search
News