Volume 7 (2024) Issue 3 No.2 Pages 75-78
Abstract
Early post-allogeneic hematopoietic stem cell transplantation (allo-HSCT) relapse in patients with acute myeloid leukemia (AML) has an almost invariably dismal prognosis. Recent studies have demonstrated that FLT3 inhibition enhances the graft-versus-leukemia effect in vitro and in vivo. Thus, FLT-3 inhibitors may be viable treatment options in this setting. Here, we report three patients with FLT3 and NPM1 mutated AML who relapsed early after allo-HSCT and were treated with gilteritinib (associated with donor lymphocyte Infusion in two patients) to achieve long-term remission without a second transplantation.
Introduction
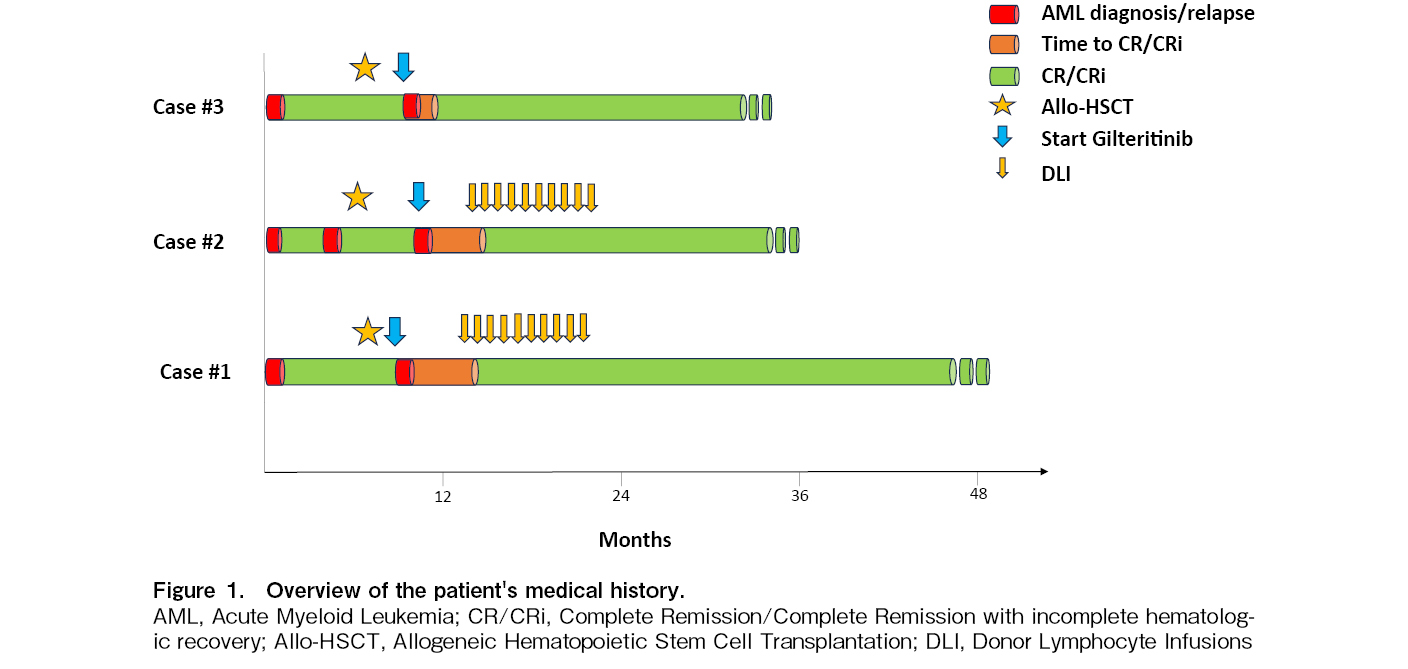
Post-allogeneic hematopoietic stem cell transplantation (allo-HSCT) relapse of acute myeloid leukemia (AML) is invariably characterized by poor outcomes. Notably, early relapses (within 6 months of allo-HSCT) are the worst scenario, with an estimated 3-year survival of 4%1. Moreover, the frequently suboptimal performance status of these patients hinders intensive treatment. In Europe, Gilteritinib is the only FLT3 inhibitor approved as monotherapy for relapsed FLT3 mutated AMl. Here, we report three patients with FLT3 and NPM1 mutated AML who experienced early relapse following Allo-HSCT and were treated with gilteritinib, achieving long-term remission without a second transplantation (Figure 1).
Case #1
A 60-year-old woman was diagnosed with NPM1 and FLT3-ITD mutated AML in February 2020. The karyotype of the patient was normal. She received the standard “3+7” induction regimen plus midostaurine, followed by two consolidation courses of intermediate-dose cytarabine with midostaurine to achieve complete remission (CR). In July 2020, the patient underwent allo-HSCT from an HLA-identical sibling donor with a standard myeloablative regimen and graft-versus-host disease (GVHD) prophylaxis (ATG, Methotrexate, Cyclosporine). No significant complications occurred during the first 30 days, and engraftment was easily achieved. On day+60 after allo-HSCT, bone marrow evaluation revealed AML relapse (50% myeloid blasts). Molecular assessments confirmed the persistence of the FLT3-ITD (ratio> 0.5) and NPM1 mutations. Second-line treatment with gilteritinib (120 mg/day) was initiated. Between March and October 2021, the patient received 10 monthly donor lymphocyte infusions (DLI). No GVHD occurred. Subsequent bone marrow assessments documented CR and a progressive reduction in NPM1 levels, which became undetectable in May 2021.
Following 38 months post-allo-HSCT (36 months post-relapse), the patient remained alive and in overall good health, sustaining CR with incomplete hematologic recovery attributable to residual thrombocytopenia and undetectable NPM1 levels. Donor chimerism was stable, with 97% of cases showing consistent results. The ongoing treatment with gilteritinib continues at a dosage of 120 mg/day, with the patient exhibiting excellent tolerance to the medication.
Case #2
A 32-year-old male patient with no relevant medical history was diagnosed with AML in January 2021. Molecular assessment revealed an NPM1 mutation and a high allelic ratio FLT3-ITD mutation, with a normal karyotype. The patient underwent a standard “3+7” induction chemotherapy with midostaurine, resulting in the attainment of the first CR. This remission was consolidated with two cycles of high-dose cytarabine (HDAC) plus midostaurine, with a subsequent planned allo-HSCT from a matched unrelated donor. However, a setback occurred when a bone marrow evaluation immediately before the transplant revealed disease relapse, characterized by 30% blasts and the same molecular and genetic characteristics. The patient then underwent salvage therapy with fludarabine-isarubicine-cytarabine. Upon achieving the second CR, the patient underwent allo-HSCT in July 2021, involving a standard myeloablative conditioning regimen. In December 2021 (day+140 from allo-HSCT), a second relapse was documented, with 15% bone marrow blasts observed on morphological examination. Molecular analysis confirmed the persistence of the FLT3 mutation. In response, third-line therapy with gilteritinib (120 mg/day) was initiated. Starting in February 2022, the patient received a total of 10 monthly DLI. Since May 2022 (day+256 after allo-HSCT), we have documented complete morphological and molecular remission without evidence of GVHD. At 24 months after treatment commencement (day+29 from allo-HSCT), the patient continues to receive gilteritinib, maintaining disease remission with almost complete donor chimerism (99%), normal blood count, and an excellent performance status.
Case #3
In February 2021, a 51-year-old woman was diagnosed with AML. The molecular panel revealed the presence of FLT3-TKD and NPM1 mutations, and the karyotype of the patient was normal. She underwent treatment with a standard intensive chemotherapy regimen “3+7” plus midostaurine, followed by three consolidation cycles with HDAC plus midostaurine, achieving CR. In September 2021, the patient underwent allo-HSCT with a standard myeloablative conditioning regimen and GVHD prophylaxis. However, on day+90, post-transplant disease relapse occurred, with 30% of bone marrow blasts. Molecular analysis confirmed the presence of the NPM1 mutation and the emergence of a novel FLT3-ITD mutation at a high allelic ratio, along with the loss of the TKD mutation. Second-line therapy with gilteritinib (120 mg/day) was initiated, leading to morphological remission after the first 28-day cycle. After three cycles, the NPM1 transcript became undetectable. Initially, certain treatment aspects were omitted due to chronic skin and ocular GVHD, necessitating prostacyclin and topical cyclosporine administration. After 24 months of treatment with gilteritinib (27 months after allo-HSCT), the patient remained in good general condition, maintaining a normal blood count, undetectable NPM1, and a donor chimerism of 96%. She continued to receive gilteritinib and prostacyclin treatment for chronic GVHD.
Discussion
Recently, FLT3 inhibition has been demonstrated to enhance the graft-versus-leukemia (GvL) effect both in vitro and in vivo2–5. Zhang et al. demonstrated in mice that short post-HSCT gilteritinib administration reduced co-inhibitory receptor expression on donor T cells while enhancing IL-15 expression. This suppressed leukemia expansion without affecting the incidence of GVHD2. Similar mechanisms of action have been identified for other FLT3 inhibitors, such as sorafenib3, 6.
In our series, treatment with an FLT3 inhibitor was associated with DLI in two patients and was administered to a third patient with active chronic GVHD. These observations support the hypothesis of a synergistic effect between FLT3 inhibition and lymphocyte activation, enhancing the GvL effect. This consideration could provide a rationale for post-transplant maintenance therapy (MT) using FLT3 inhibitors. In this context, an overall survival benefit has been demonstrated specifically for sorafenib7, 8. Nevertheless, emerging real-life data are providing support for the safety and efficacy of other FLT3 inhibitors9–11. A clinical trial evaluating gilteritinib as post-transplant MT agent is currently underway (NCT02997202; BMT-CTN1506). The unique aspects of these cases are attributed to their distinct characteristics. Notably, an exceptionally prolonged remission was attained without the need for intensive treatment or a second allo-HSCT. Historically, the survival outlook after post-allo-HSCT relapse has been notably bleak, particularly for patients experiencing early relapse1. In the ADMIRAL trial, patients treated with gilteritinib following allo-HSCT demonstrated a median OS of 8.3 months, with seven patients surpassing a two-year survival period12, 13.
Additionally, all three patients exhibited identical disease characteristics, encompassing molecular (FLT3-ITD at a high allelic ratio and NPM1) and cytogenetic features (normal karyotype). This specific disease profile identified a subpopulation that responded well to gilteritinib, regardless of the time to relapse or prior exposure to other FLT3 inhibitors (all patients had received midostaurine previously). Unfortunately, next-generation sequencing analysis was not available for these patients. However, having such analysis could have been beneficial for a more precise definition of the molecular profile, as certain mutations are recognized to confer resistance to gilteritinib14. Finally, all patients were treated as outpatients without any significant treatment-related complications.
In conclusion, our cases provide evidence for the effectiveness and good tolerability of gilteritinib, even in patients experiencing early post-transplant AML relapse, and advocate for additional exploration of FLT3 inhibition in this context.
Author Contributions
ET and IT designed the research and wrote the paper. MS, AA, CT, GN, AB, IF, LC, AV, CS, FB and MK were involved in patient treatment. All authors contributed to the final version of the manuscript and approved it for publication.
Consent for publication
Informed consent was obtained for each patient.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of Patients with Acute Myeloid Leukemia Relapsing after Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Study. Biol Blood Marrow Transplant. 2015; 21: 454-9.
2.Zhang Z, Hasegawa Y, Hashimoto D, Senjo H, Kikuchi R, Chen X, et al. Gilteritinib enhances graft-versus-leukemia effects against FLT3-ITD mutant leukemia after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2022; 57: 775-80.
3.Mathew NR, Baumgartner F, Braun L, O'Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib promotes graft-versus-leukemia activity in mice and humans through IL-15 production in FLT3-ITD-mutant leukemia cells. Nat Med. 2018; 24: 282-91.
4.Biavasco F, Zeiser R. FLT3-inhibitor therapy for prevention and treatment of relapse after allogeneic hematopoietic cell transplantation. Int J Hematol. 2022; 116: 341-50.
5.Sorà F, Chiusolo P, Metafuni E, Bellesi S, Giammarco S, Laurenti L, et al. Sorafenib for refractory FMS-like tyrosine kinase receptor-3 (FLT3/ITD+) acute myeloid leukemia after allogenic stem cell transplantation. Leuk Res. 2011; 35: 422-3.
6.Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Götze K, et al. High activity of sorafenib in FLT3-ITD-positive acute myeloid leukemia synergizes with allo-immune effects to induce sustained responses. Leukemia. 2012; 26: 2353-9.
7.Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020; 21: 1201-12.
8.Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol. 2020; 38: 2993-3002.
9.Griffin JD, Song Y, Yang H, Freimark J, Shah MV. Post-transplant maintenance therapy in patients with FLT3-mutated acute myeloid leukemia: Real-world treatment patterns and outcomes. Eur J Haematol. 2021; 107: 553-65.
10.Blackmon A, Aldoss I, Ball BJ. FLT3 Inhibitors as Maintenance Therapy after Allogeneic Stem-Cell Transplantation. Blood Lymphat Cancer. 2022; 12: 137-47.
11.Burchert A. Maintenance therapy for FLT3-ITD-mutated acute myeloid leukemia. Haematologica. 2021; 106: 664-70.
12.Perl AE, Martinelli G, Cortes JE, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med. 2019; 381: 1728-40.
13.Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, et al. Follow-up of patients with R/R FLT3- mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood. 2022; 139: 3366-75.
14.Alotaibi AS, Yilmaz M, Kanagal-Shamanna R, Loghavi S, Kadia TM, DiNardo CD, et al. Patterns of Resistance Differ in Patients with Acute Myeloid Leukemia Treated with Type I versus Type II FLT3 Inhibitors. Blood Cancer Discov. 2021; 2: 125-34.
Search
News