Volume 7 (2024) Issue 3 No.4 Pages 87-94
Abstract
Background: Approximately half of allogeneic hematopoietic cell transplantation (HCT) recipients experience significant bone loss in the early post-HCT period. Only recently have international guidelines started recommending early screening. However, the guidance for intervention remains conservative. In this study, we sought to evaluate the efficacy of pre-transplant prophylactic zoledronate in preventing early bone loss in allogeneic HCT recipients.
Methods: This was an open-label, investigator-initiated, phase 2 randomized controlled trial (RCT) of prophylactic zoledronate versus observation to prevent bone loss in allogeneic HCT recipients. Recipients aged ≥ 18 years of age were included after informed consent and randomized to prophylactic zoledronate 4 mg pre-HCT or observation in a 1:1 ratio. The primary outcome of the study was bone mineral density (BMD) loss at the femoral neck (FN), total hip (TH), and lumbar spine (LS), as assessed using dual-energy X-ray absorptiometry (DXA) on day+100 post-HCT. The secondary outcomes included BMD loss on day+365 and Z scores on day+100 and day+365 at the FN, TH, and LS sites.
Results: The trial was terminated because the interim analysis showed a significant benefit in the intervention arm, with 50% planned recruitment. A total of 40 patients were randomized to the zoledronate and control arms. Both arms were matched for age, sex, diagnosis, pre-HCT steroid exposure, body mass index, human leukocyte antigen (HLA) match, and conditioning intensity. The grade 2-4 acute graft versus host disease (GVHD) incidences were comparable. The primary endpoint of BMD loss at FN and TH at day+100 was significant (5.62% vs. -6.78%, p = 0.009, -1.59 vs. -3.98, p = 0.016, respectively). There was no difference in the secondary endpoint of BMD loss on day+365 compared to that on day+100 or baseline at any BMD site. There was no difference in the Z-scores at any site on day+100 or day+365.
Conclusions: Prophylactic zoledronate prevented early bone loss on day+100. The indicated preemptive zoledronate beyond day+100 in recipients prevented further bone loss. Patients receiving prophylactic zoledronate may benefit from a supplementary dose of the indicated preemptive zoledronate.
Introduction
With a significant decrease in transplant-related mortality due to advancements in transplantation techniques and supportive care in allogeneic hematopoietic stem cell transplantation (HCT), the number of long-term survivors has greatly increased. Bone loss, in the form of osteopenia and osteoporosis, is a significant long-term complication of HCT. It can lead to a decreased quality of life due to bone pain, limited mobility, and fragility fractures. Approximately 50% of HCT survivors develop osteopenia or osteoporosis within 6 months of transplantation1–3.
Currently, there are limited guidelines for the screening, prevention, and management of post-HCT bone loss4, 5. The American Society of Transplantation and Cellular Therapy (ASTCT) and International Osteoporosis Foundation guidelines recommend early screening of all patients undergoing HCT with a dual X-ray absorptiometry (DXA) scan pre-HCT or on day+100, rather than the conventional practice of screening only patients with GVHD and steroid use6. The International Osteoporosis Foundation recommends pharmacological intervention if the T-score < -1.56. The ASTCT guidance proposes starting pharmacologic therapy in those < 40 years of age receiving prednisone equivalent dose of ≥ 7.5 mg/day for ≥ 6 months and either one of the fragility fractures or Z-score < -3.0 or 10% BMD loss over a year7. There is little consensus and limited evidence for these recommendations.
The use of prophylactic infusion of bisphosphonates such as zoledronate to prevent bone loss has been well established in patients with multiple myeloma, undergoing autologous hematopoietic stem cell transplant, and undergoing hormonal therapy for breast and prostate malignancies. However, there is very scarce data on prophylactic bisphosphonate use in allogeneic HCT settings8–12. We have previously shown that without intervention, up to half of HCT recipients from our center in North India have BMD below the expected range for age (Z-score ≤ -2) on day+100 post-HCT, and the low BMD persists on day+365 despite anti-resorptive therapy13. Therefore, the current trial was designed to investigate the role of prophylactic pre-HCT zoledronate in preventing early bone loss.
Methods
This open-label, investigator-initiated, phase 2 randomized controlled trial (RCT) of prophylactic zoledronate versus observation to prevent bone loss in HCT recipients (Clinical trials registry of India CTRI/2019/04/018764) was conducted in a tertiary care center in India from January 2019 to December 2022. The study was performed according to the Consolidated Standards of Reporting Trials (
Study population
Adult patients (age ≥ 18 years) who underwent HCT for any indication were included in the trial. Patients with eGFR < 30 mL/min, history of hypersensitivity to bisphosphonates, dental extraction within the past 4 weeks, and pre-existing metabolic bone disease (defined as patients with osteoporosis (T score ≤ -2.5) were excluded.
Study procedure
One author assessed the patients for study eligibility (DPL). Another author (NSK) was involved in patient randomization, study drug administration, and post-HCT follow-up. Patients were randomly assigned (1:1) to either the zoledronate or the observation arm using a computer-generated sequence.
The BMD of the patients was recorded using DXA with the HOLOGIC Discovery A machine according to the manufacturer's recommendations and the International Society for Clinical Densitometry (ISCD) guidance14. BMD was measured in the lumbar spine (LS), femoral neck (FN), and total hip (TH) at baseline before randomization and on day+100 and day+365.
Study intervention
Nutritional counselling for calcium and vitamin D intake, pharmacological vitamin D supplementation to maintain levels >30 ng/mL, and counselling for regular weight-bearing exercises three times/week were considered the standard of care and were offered to all patients in the study.
Participants randomized to the zoledronate arm received 4 mg of zoledronate (Intas Pharmaceuticals Limited. Amdavad, India) as an intravenous infusion in 100 mL of normal saline over fifteen minutes. We planned to use a standard renal dose modification for patients with reduced eGFR (60 to 30 mL/min). The participants underwent HCT according to the treating physician and departmental protocols within 30 days of randomization.
As per protocol, patients in the control arm received zoledronate infusion beyond day+100 in the follow-up period if they fulfilled the criteria of accelerated bone loss: i) ≥5% ΔBMD loss on day+100 or, ii) received systemic steroids at a dose of ≥1 mg/kg prednisolone (or equivalent) for ≥2 weeks or at a dose of ≥10 mg/day prednisolone (or equivalent) for ≥6 weeks. No additional zoledronate infusions were administered to patients in the zoledronate arm until day+365.
Outcomes
The primary outcome was the change in BMD (Δ BMD) on day+100 post-HCT at the femoral neck (FN), lumbar spine (LS), and total hip (TH). The secondary outcomes were Δ BMD on day+365 and change in Z scores on day+100 and day+365 at all three sites (TH, LS, FN).
Statistical Analysis
Estimating Δ BMD on day+100 as -6% in the control arm and -2% in the zoledronate arm based on the available literature, a sample size of 50 would provide an α of 0.05 and power of 80%. Considering an enrollment rate of 80% and attrition due to dropout/transplant-related mortality of 15%, we planned a sample size of 74 patients. The interim analysis was preplanned after 50% recruitment. All analyses were performed using intention-to-treat analysis. Continuous variables were expressed as medians with interquartile ranges. Categorical variables were expressed as percentages. Comparisons between groups were performed using t-tests. Statistical significance was set at p < 0.05. All statistical analyses were performed using GraphPad Prism Version 9.
Results
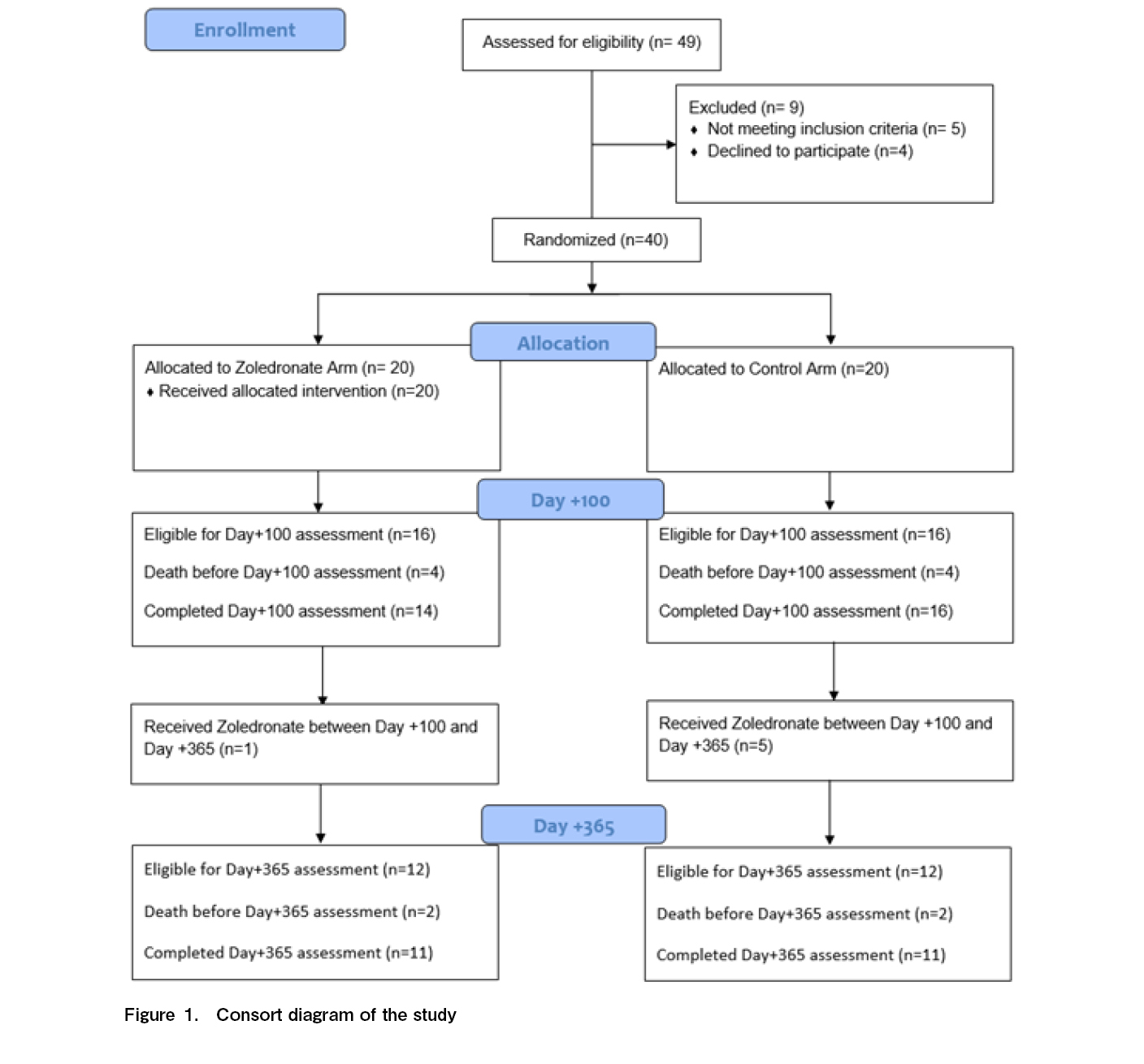
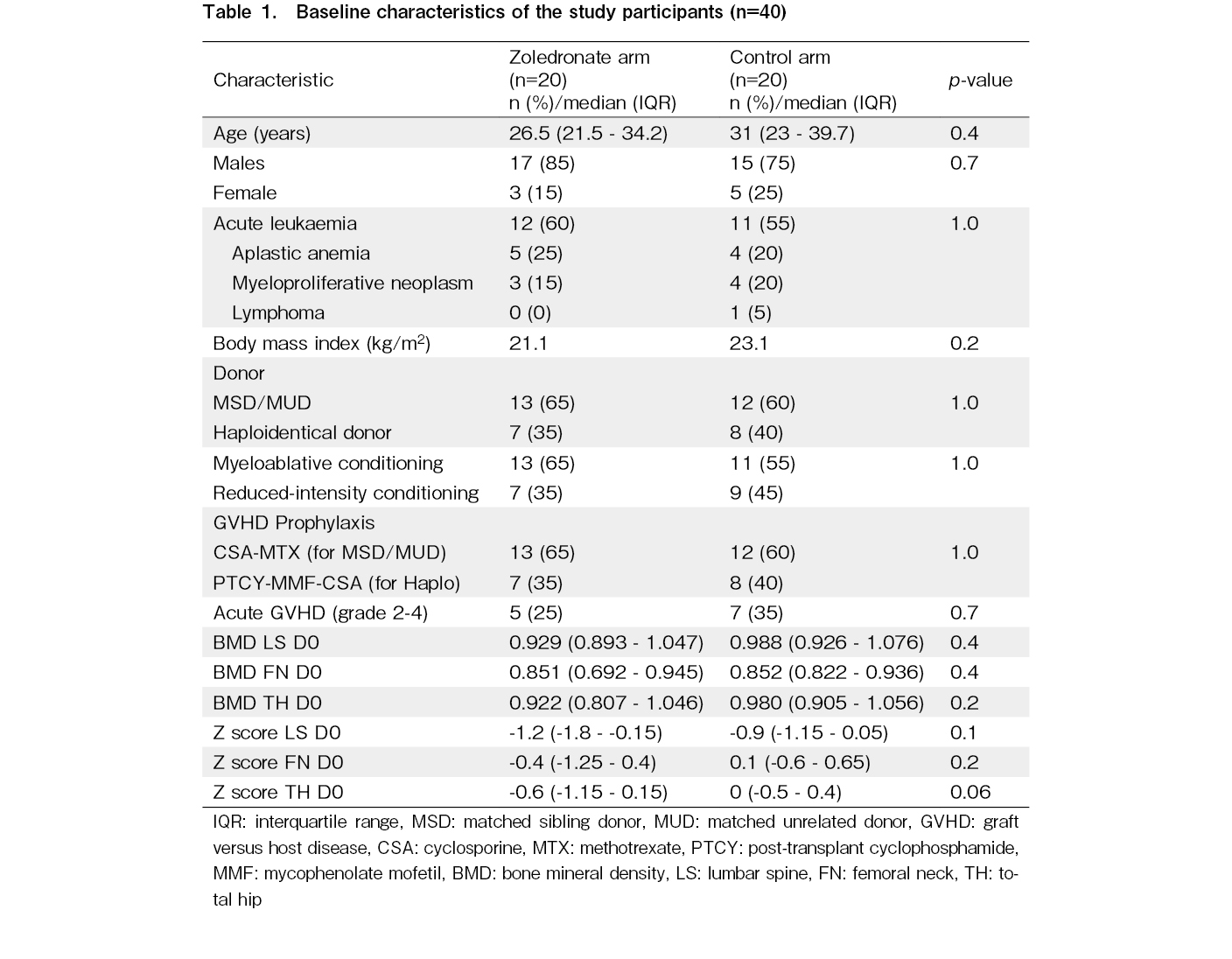
The CONSORT flow diagram for this study is shown in Figure 1. Forty-nine patients were screened for eligibility during the study period. Five patients (10.2%) were excluded because of pre-existing osteoporosis and four declined to participate. The remaining 40 patients were equally randomized into the zoledronate and control arms. The baseline characteristics of the study participants are presented in Table 1. Both arms were well-matched for demographic and transplant variables. The most common indication for HCT was acute leukemia. The grade 2-4 acute GVHD incidences were also comparable. Eight patients died or relapsed before the day+100 assessments.
Primary Outcome
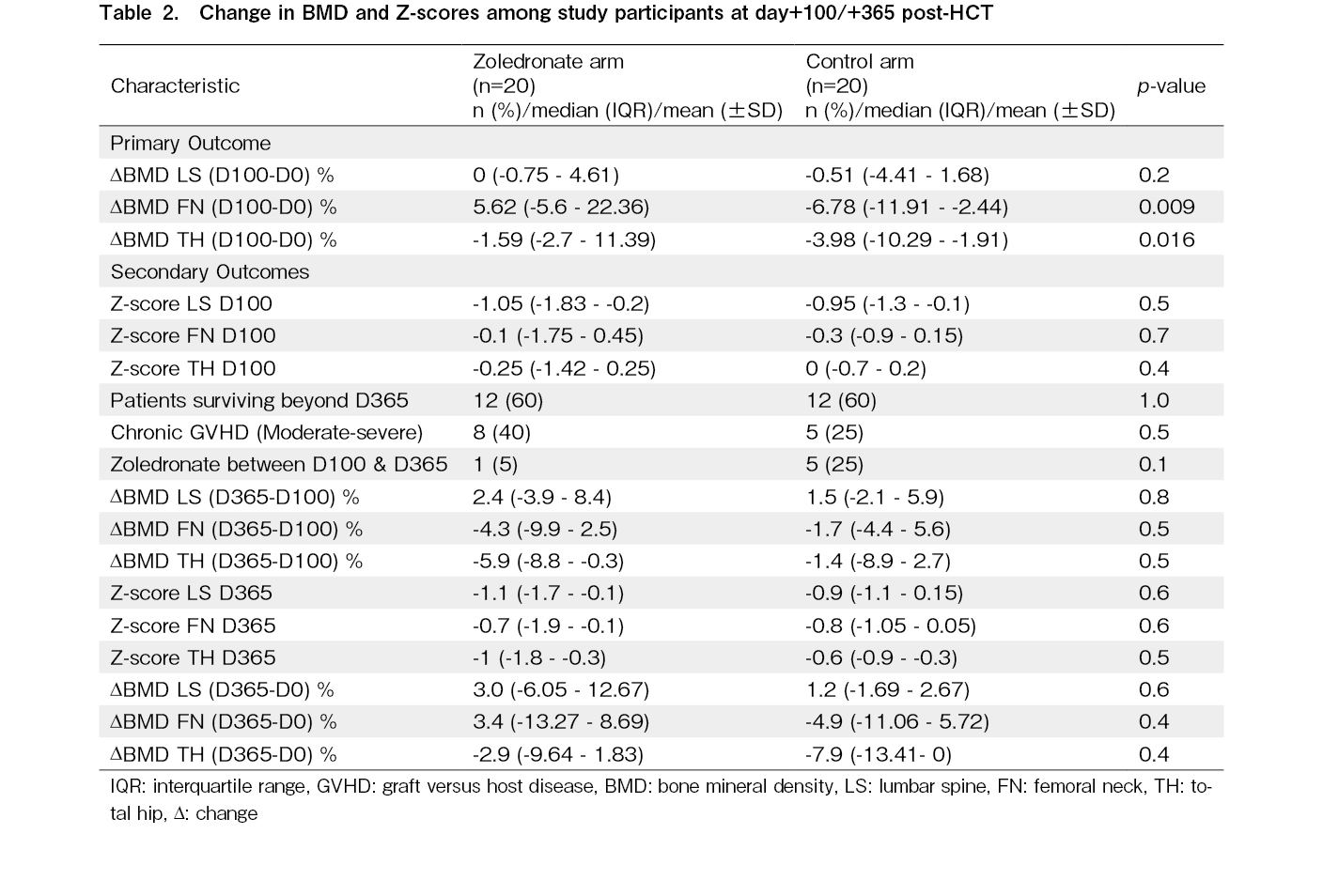
There was a significant difference in Δ BMD at FN on day+100 between the zoledronate and control arm, with a difference of 12.4% (5.62% vs. -6.78%, p = 0.009). The ΔBMD (D100-0) at TH was also significantly higher in the control arm (-1.59 vs -3.98, p = 0.016). However, the ΔBMD (D100-0) at LS was not different (0 vs -0.51, p = 0.2).
Secondary Outcomes
There were no differences in the absolute Z-scores at the three sites on day+100 between the arms. Eight patients in the zoledronate arm and five in the control arm had chronic GVHD. Five patients in the control arm received zoledronate infusion beyond day+100 for accelerated bone loss. The median age of these five patients was 36 years, which was higher than that of the rest of the observational cohort. Three patients had an underlying diagnosis of acute lymphoblastic leukemia (ALL), one had acute myeloid leukemia (AML), and one had chronic myeloid leukemia (CML). Three patients received TBI-based myeloablative conditioning, and the other two received reduced-intensity conditioning. Table 2 highlights the BMD loss at LS, FN, and TH at day+365 (ΔBMD D365-0) and (ΔBMD D365-100) and the Z-scores at the three sites at day+365. There was no difference in the secondary endpoint of BMD loss on day+365 compared to that on day+100 or baseline at any BMD site. There were no significant differences in Z-scores on day+365 at the three sites.
Safety outcomes
Five adverse events were noted in the zoledronate arm, of which 2 were grade 1 toxicities and 3 were grade 2 toxicities. The two patients with grade 1 toxicity had mild, asymptomatic hypocalcemia in the immediate post-injection period and were managed with oral and intravenous calcium supplementation. Three patients with grade 2 toxicities developed fever, myalgia, and flu-like symptoms for 24-48 hours after zoledronate infusion. In 2 patients, conditioning had to be postponed by 3 and 4 days because of post-zoledronate fever. None of the patients in the study required any renal modification of zoledronate.
Discussion
Loss of BMD and its consequences of fragility fractures and poor quality of life are well-known late consequences of HCT. The cause of this complication is multifactorial and includes the effects of preexisting malignancy and chemotherapy, direct effects of conditioning on osteoblasts, deranged calcium and vitamin D metabolism, malabsorption, use of corticosteroids, and hormonal deficiencies as the key causes15, 16. This late effect of transplantation is especially important in the Indian subcontinent, which has a high prevalence of poor nutrition, vitamin D deficiency, and poor bone health. We have previously shown that almost one-third of all patients undergoing HCT in our center have BMD below the expected range for age (Z-score ≤2), which further rises to 50% of day100 survivors13. Despite this, there is no consensus regarding the optimal screening and management strategy or prophylactic strategy for preserving bone health in patients undergoing HCT. We designed this trial to investigate the efficacy of prophylactic zoledronate in preventing early bone loss in transplant recipients.
An important finding of our study was the significant prevalence of low BMD even before transplantation. Five of the 49 patients (10.2%) screened had pre-transplant T-scores less than -2.5, at the FN and LS and had to be excluded from the trial.
Most longitudinal studies evaluating long-term bone loss post-HCT suggest that a steep decline in BMD occurs in the initial 6-12 months, followed by a slow, gradual, but frequently incomplete recovery1, 8, 17. Nonspecific interventions such as calcium and vitamin D supplementation and sex hormone replacement are inadequate preventive measures against post-HCT bone loss9, 18, 19. Bisphosphonates are analogs of pyrophosphate that can chelate divalent cations, concentrate at sites of active bone remodeling, and prevent bone resorption by decreasing the dissolution of hydroxyapatite in the bone and inducing apoptosis in activated osteoclasts. They are approved for the treatment and prevention of osteoporosis, tumor-induced osteolysis, and hypercalcemia of malignancy and for reducing the incidence of skeletal-related events in multiple myeloma20–25. With extensive clinical experience in multiple settings for bone health improvement, bisphosphonates are the drugs most often studied to prevent HCT-related bone loss6, 26.
There have been several prospective trials, including seven randomized controlled trials, on the use of bisphosphonates in allogeneic HCT, which have been reviewed in detail elsewhere6, 26. The studies typically involve a post-transplant pre-emptive strategy, with a few studies8, 11 including an additional prophylactic pretransplant bisphosphonate in addition to post-transplant bisphosphonate. Initial trials examined pamidronate pre- and post-HCT at 1-3-month intervals and reported benefits in preventing bone loss in patients with lumbar spine LS9, 10. Later studies investigated the effect of zoledronate, as it has well-established pharmacological superiority over pamidronate27. The first prospective single-arm study using 3-monthly zoledronate post-HCT reported a benefit in femoral neck bone loss at 12 months post-HCT12. Grigg et al. conducted a trial using a pre-HCT zoledronate dose in addition to several post-transplant doses in a single-arm trial design. They showed an improvement in BMD compared to the historical control cohorts11. The pre-transplant strategy was investigated by Hari et al. in an RCT using both pre- and post-HCT zoledronate. This study was terminated prematurely due to slow recruitment8. However, whether prophylactic single-dose pre-HCT zoledronate can prevent early bone loss (3 months) had remained unanswered.
In the current study, among the patients included in the trial, pre-HCT zoledronate significantly improved BMD at the FN and prevented bone loss at the TH on day+100. The initial gain in bone density at the FN and TH in the intervention arm was nullified by an increased loss of bone density at either site between day+100 and day+365. As shown in Table 2, the median loss of bone density at the FN and TH was greater in the intervention arm than in the control arm. This further emphasized the need for additional post-HCT zoledronate doses to prevent continued post-HCT bone loss.
Our observation that the pattern of bone loss predominantly affects the FN and TH, with relative sparing of the LS site, conforms to prior observations28, 29. Longitudinal studies on transplantation have shown that bone loss after transplantation predominantly affects the proximal femur, an effect that is most pronounced early (D100)30, 31 after transplantation, but can persist even later (D365)32. It has been thought that the younger age of patients and probable effects of underlying diseases and transplant procedures cause this differential pattern of bone loss as compared to postmenopausal bone loss that predominantly affects the spine17.
Although our study successfully demonstrated the role of zoledronate in preventing early bone loss in patients with HCT, it has many limitations. The prevailing COVID-19-related pandemic slowed study enrolment, and the study procedures had to be modified to include a pre-planned interim analysis due to the futility of the continuation of the study. Another limitation of the study protocol was the lack of additional zoledronate in the intervention arm, despite the indications. This strategy was adopted to avoid the confounding effects of prophylactic pre-HCT versus preemptive post-HCT zoledronate on secondary outcomes. The data on bone turnover markers and steroid dosages in both arms were lacking. Due to inherent logistical limitations requiring several years of follow-up, it was not possible to measure clinically relevant endpoints, such as cross-sectional imaging for fragility fractures, measurement of loss of vertebral spine height, or quantification of bone health-related quality of life parameters.
In conclusion, the administration of a single dose of 4 mg zoledronate before HCT effectively prevented early bone loss by day+100 post-HCT. Indicated pre-emptive zoledronate beyond day+100 in recipients for steroid exposure in chronic GVHD or ≥ 5% BMD loss prevents further losses. Those receiving prophylactic zoledronate may receive additional benefits from a supplementary dose of the indicated preemptive zoledronate. Whether this translates into clinically meaningful outcomes, such as a reduction in the incidence of osteoporosis or fragility fractures on long-term follow-up, needs to be determined; however, it may be challenging to study these as part of clinical trials.
Acknowledgments
Intas Private Limited, Amdavad, India, provided the study drug zoledronate. However, the company was not involved in designing the study, data collection, analysis, and writing the manuscript in any form.
Author Contributions
NK and UB contributed equally to this manuscript and are co-first authors. NK, SB, AS, and DL were involved in the study conception and design. All authors contributed to patient care. Data was collected and analyzed, and a draft manuscript was written by UB, NK, and DL. All authors revised the manuscript and approved the final version. DL, NK, and UB had full access to all the data in the study and the final responsibility to submit the paper for publication.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Tauchmanovà L, Colao A, Lombardi G, Rotoli B, Selleri C. Bone loss and its management in long-term survivors from allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2007; 92: 4536-45.
2.Yao S, McCarthy PL, Dunford LM, Roy DM, Brown K, Paplham P, et al. High prevalence of early-onset osteopenia/osteoporosis after allogeneic stem cell transplantation and improvement after bisphosphonate therapy. Bone Marrow Transplant. 2008; 41: 393-8.
3.Yao S, Smiley SL, West K, Lamonica D, Battiwalla M, McCarthy PL Jr, et al. Accelerated bone mineral density loss occurs with similar incidence and severity, but with different risk factors, after autologous versus allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010; 16: 1130-7.
4.Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, Center for International Blood and Marrow Transplant Research, and the American Society for Blood and Marrow Transplantation (EBMT/CIBMTR/ASBMT). Bone marrow transplantation. 2006; 37: 249-61.
5.Majhail NS, Rizzo JD, Lee SJ, Aljurf M, Atsuta Y, Bonfim C, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012; 18: 348-71.
6.Kendler DL, Body JJ, Brandi ML, Broady R, Cannata-Andia J, Cannata-Ortiz MJ, et al. Bone management in hematologic stem cell transplant recipients. Osteoporos Int. 2018; 29: 2597-610.
7.Bar M, Ott SM, Lewiecki EM, Sarafoglou K, Wu JY, Thompson MJ, et al. Bone Health Management After Hematopoietic Cell Transplantation: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2020; 26: 1784-802.
8.Hari P, DeFor TE, Vesole DH, Bredeson CN, Burns LJ. Intermittent zoledronic Acid prevents bone loss in adults after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013; 19: 1361-7.
9.Kananen K, Volin L, Laitinen K, Alfthan H, Ruutu T, Välimäki MJ. Prevention of bone loss after allogeneic stem cell transplantation by calcium, vitamin D, and sex hormone replacement with or without pamidronate. J Clin Endocrinol Metab. 2005; 90: 3877-85.
10.Grigg AP, Shuttleworth P, Reynolds J, Schwarer AP, Szer J, Bradstock K, et al. Pamidronate reduces bone loss after allogeneic stem cell transplantation. J Clin Endocrinol Metab. 2006; 91: 3835-43.
11.Grigg A, Butcher B, Khodr B, Bajel A, Hertzberg M, Patil S, et al. An individualised risk-adapted protocol of pre- and post transplant zoledronic acid reduces bone loss after allogeneic stem cell transplantation: results of a phase II prospective trial. Bone Marrow Transplant. 2017; 52: 1288-93.
12.Chae YS, Kim JG, Moon JH, Kim SN, Lee SJ, Kim YJ, et al. Pilot study on the use of zoledronic acid to prevent bone loss in allo-SCT recipients. Bone Marrow Transplant. 2009; 44: 35-41.
13.Khaire NS, Dinesan A, Sinha A, Bhadada S, Malhotra P, Khadwal A, et al. Early bone loss in Indian patients undergoing allogeneic hematopoietic cell transplantation. Blood Cell Ther. 2021; 4: 48-53.
14.Shuhart CR, Yeap SS, Anderson PA, Jankowski LG, Lewiecki EM, Morse LR, et al. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J Clin Densitom. 2019; 22: 453-71.
15.Khan Z, Agarwal NB, Bhurani D, Khan MA. Risk Factors for Hematopoietic Stem Cell Transplantation-Associated Bone Loss. Transplant Cell Ther. 2021; 27: 212-21.
16.Miglietta F, Iamartino L, Palmini G, Giusti F, Marini F, Iantomasi T, et al. Endocrine sequelae of hematopoietic stem cell transplantation: Effects on mineral homeostasis and bone metabolism. Front Endocrinol (Lausanne). 2023; 13: 1085315.
17.Ria R, Scarponi AM, Falzetti F, Ballanti S, Di Ianni M, Sportoletti P, et al. Loss of bone mineral density and secondary hyperparathyroidism are complications of autologous stem cell transplantation. Leuk Lymphoma. 2007; 48: 923-30.
18.Välimäki MJ, Kinnunen K, Volin L, Tähtelä R, Löyttyniemi E, Laitinen K, et al. A prospective study of bone loss and turnover after allogeneic bone marrow transplantation: effect of calcium supplementation with or without calcitonin. Bone Marrow Transplant. 1999; 23: 355-61.
19.Tauchmanovà L, De Simone G, Musella T, Orio F, Ricci P, Nappi C, et al. Effects of various antireabsorptive treatments on bone mineral density in hypogonadal young women after allogeneic stem cell transplantation. Bone Marrow Transplant. 2006; 37: 81-8.
20.Li EC, Davis LE. Zoledronic acid: a new parenteral bisphosphonate. Clin Ther. 2003; 25: 2669-708.
21.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007; 356: 1809-22.
22.McClung M, Miller P, Recknor C, Mesenbrink P, Bucci-Rechtweg C, Benhamou CL. Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass: a randomized controlled trial. Obstet Gynecol. 2009; 114: 999-1007.
23.Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des. 2010; 16: 1262-71.
24.Corral-Gudino L, Tan AJ, Del Pino-Montes J, Ralston SH. Bisphosphonates for Paget's disease of bone in adults. Cochrane Database Syst Rev. 2017; 12: CD004956.
25.Richardson PG, Laubach JP, Schlossman RL, Ghobrial IM, Mitsiades CS, Rosenblatt J, et al. The Medical Research Council Myeloma IX trial: the impact on treatment paradigms. Eur J Haematol. 2012; 88: 1-7.
26.Pundole X, Cheema HI, Petitto GS, Lopez-Olivo MA, Suarez-Almazor ME, Lu H. Prevention and treatment of bone loss and fractures in patients undergoing a hematopoietic stem cell transplant: a systematic review and meta-analysis. Bone Marrow transplant. 2017; 52: 663-70.
27.Rosen LS, Gordon D, Kaminski M, Howell A, Belch A, Mackey J, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer. 2003; 98: 1735-44.
28.Stern JM, Sullivan KM, Ott SM, Seidel K, Fink JC, Longton G, et al. Bone density loss after allogeneic hematopoietic stem cell transplantation: a prospective study. Biol Blood Marrow Transplant. 2001; 7: 257-64.
29.Schulte C, Beelen DW, Schaefer UW, Mann K. Bone loss in long-term survivors after transplantation of hematopoietic stem cells: a prospective study. Osteoporos Int. 2000; 11: 344-53.
30.Pawlowska M, Yang Q, Hamata B, Kendler DL, Broady R. Early changes in bone mineral density and trabecular bone score following allogeneic stem cell transplant. Bone marrow transplantation. 2016; 51: 738-40.
31.Tauchmanova L, Selleri C, Esposito M, Di Somma C, Orio F Jr, Bifulco G, et al. Beneficial treatment with risedronate in long-term survivors after allogeneic stem cell transplantation for hematological malignancies. Osteoporos Int. 2003; 14: 1013-9.
32.Lee WY, Baek KH, Rhee EJ, Tae HJ, Oh KW, Kang MI, et al. Impact of circulating bone-resorbing cytokines on the subsequent bone loss following bone marrow transplantation. Bone marrow transplantation. 2004; 34: 89-94.
Search
News