Volume 8 (2025) Issue 1 No.2 Pages 147-159
Abstract
Hematopoietic stem cell transplantation [HSCT] is the only curative option for patients with myelodysplastic syndromes [MDS]. Between 1991 and 2021, 154 patients [high risk, 86; low risk, 68] including 22 children underwent HSCT with a median age of 36 years. Conditioning regimens were myeloablative [n=97] and reduced intensity [n=53]. Donors were human leucocyte antigen (HLA)-matched related donors (MRDs) in 113 and alternate donors in 41. The graft source was peripheral blood stem cells in 92%.
Engraftment occurred in 126 [81.9%] at a median of 15 days while 20 [12.9%] died before engraftment and eight [5.2%] had primary graft failure. Sinusoidal obstruction syndrome was seen in 27 [17.5%]. Grade 2-4 acute graft versus host disease [GVHD] occurred in 46.3% while Grade 3-4 GVHD was seen in 34.9% and the incidence of chronic GVHD was 69.4%. Bacterial infections occurred in 38 (24.6%) while viral infections were seen in 31 [20.1%], mainly cytomegalovirus, and invasive fungal disease in 17.5%.
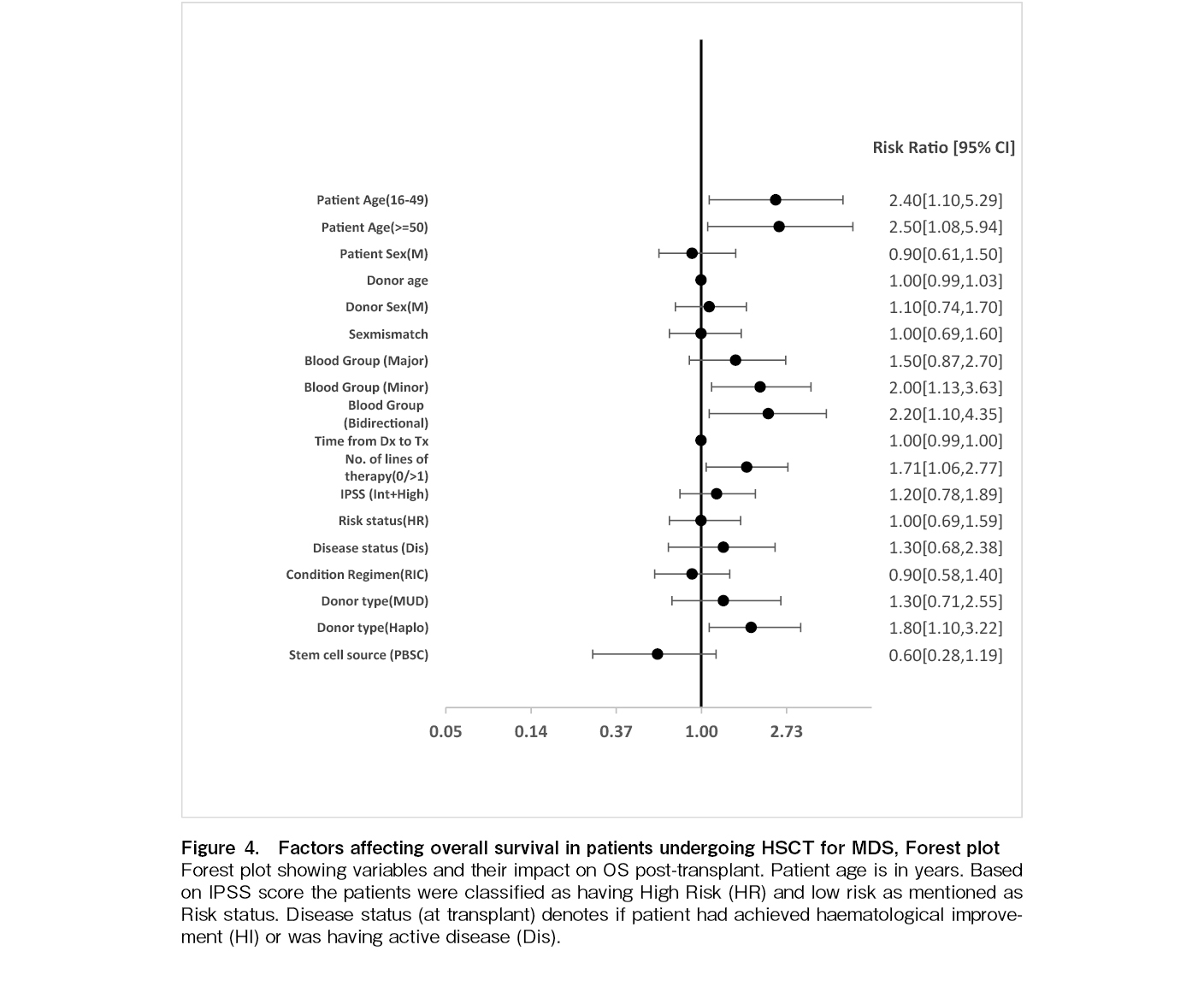
At a median of 33 months, 65 patients were alive; 14 (9.1%) had disease relapse, and 10 (6.5%) had secondary graft failure. The five-year overall survival (OS) (time from allogenic HSCT to death due to any cause) and event-free survival (time from allogenic HSCT to relapse/ progression of disease or death) were 41.69±4.2% and 40.8±4.4%, respectively. The five-year OS was significantly better in children [71%]. Outcomes were better with MRDs [45%] compared to alternate donors [29%; p=0.035]. Outcomes of HLA-MRD transplants have been improving; 44% for 1990 – 2000, 35% for 2001 – 2010, and 51% for 2011 – 2021. On multivariate analysis, age (adolescents and young adults [hazard ratio (HR) 2.7, p=0.021] and older adult age group [HR 3.6, p=0.006]), minor blood group mismatch [HR 2.0, p=0.028], bidirectional blood group mismatch [HR 2.6, p=0.010], and haploidentical stem cell donor [HR 2.2, p=0.007] were associated with poorer OS.
In conclusion, outcomes of HSCT for MDS are reasonable among matched sibling donors but outcomes in alternate donors require improvement. Strategies to reduce GVHD and infections should be explored.
Introduction
Myelodysplastic syndromes (MDS) are a group of myeloid neoplasms characterized by clonal proliferation of hematopoietic stem cells, recurrent genetic abnormalities, myelodysplasia, ineffective haematopoiesis, peripheral-blood cytopenia, and a high risk of evolution to acute myeloid leukaemia (AML)1 The treatment goals for patients with MDS are two-fold: improve peripheral blood indices (i.e., increase the hemoglobin level and reduce hemorrhage and infections) and change the natural progression of the disease2. Various prognostication systems have been used to guide risk stratification and therefore treatment options for MDS3–5. The most common are The International Prognostic scoring system [IPSS] and the Revised IPSS (R-IPSS). The IPSS-R classifies MDS into high risk (high and very high) and low risk (very low, low and intermediate) MDS. Low risk MDS is generally treated with growth factors, immune modulators or hypomethylating agents, while high risk MDS is treated with hypomethylating agents, with or without allogenic hematopoietic stem cell transplantation [HSCT]6. In patients with low risk MDS, HSCT is performed only if they have failed at least two to three lines of therapy; financial constraints to undergo allogenic stem cell transplantation are present; therefore, HSCT was only offered if the patients with low risk MDS failed at least two lines of therapy with patients arranging finance through various funds.
This dichotomy in treatment is based on the risk of progression to AML and the overall survival [OS]7–10. With the advent of next generation sequencing, newer markers for disease classifications have been discovered, which assist in prognosticating the disease and in offering tailored therapy11,12. However in countries such as India, the patients are often referred late to appropriate hematology centres for correct diagnosis and appropriate management and are often misdiagnosed as cytopenia due to acute febrile illness, aplastic anemia or immune thrombocytopenic purpurea. In addition, the median age of patients diagnosed with MDS in India is usually less than 50 years; therefore, therapeutic options including HSCT become of paramount importance13. Our experience with HSCT for MDS from a single centre in India over a period of 30 years is presented.
Patients and Methods
This retrospective study analyzed patients who underwent allogeneic HSCT at the Christian Medical College, Vellore between July 1991 to December 2021. Only matched sibling donor [MSD] transplants were performed until 2009 and only from 2010, have matched unrelated donor [MUD] and haplo-identical [Haplo] transplants been performed for patients who do not have an MSD. This retrospective study is approved by the Institutional review board, Christian Medical College Vellore, India (Approval Number- 15702). All data were collected from individual medical records and institutional databases. Informed consent was only obtained at the time of HSCT and a separate consent was not obtained for this retrospective study.
Transplant
Myeloablative conditioning [MAC] and reduced intensity conditioning [RIC] regimens were used in patients with MDS. MAC regimens consisted of busulfan + cyclophosphamide (Tab busulfan 1 mg/kg/dose q6h for four days; Inj cyclophosphamide 60 mg/kg/day IV for two days), fludarabine + busulfan (Inj fludarabine: 40 mg/m2 IV /day for four days; Inj busulfan: 130 mg/m2/day IV for four days and dose adjustment made as per the therapeutic drug monitoring) or fludarabine + treosulfan (Inj. fludarabine: 30 mg/m2 IV/day for five days; Inj. treosulfan: 12 g/m2 IV/day for three days). RIC regimens consisted of fludarabine + melphalan (Inj gludarabine: 30 mg/m2 IV/day for five days; melphalan: 140 mg/m2 IV for one day), fludarabine + cyclophosphamide (Inj. fludarabine: 30 mg/m2 IV/day for six days; Inj cyclophosphamide 120 mg/kg/day IV for two days) or fludarabine + busulfan for two days (Inj fludarabine: 30 mg/m2 IV/day for six days; Inj busulfan: 2.4 mg/kg/day IV for two days). The graft source was either bone marrow or peripheral blood stem cells [PBSC]. Graft versus host disease [GVHD] prophylaxis consisted of cyclosporine and short course methotrexate for MSD or MUD transplants; for MUD transplants, rabbit anti-thymocyte globulin [ATG] [4.5 mg/kg over three days] was added. Patients undergoing a Haplo HSCT received post-transplant cyclophosphamide [PTCy] 50 mg/kg on Days +3 and +4 followed by a calcineurin inhibitor [tacrolimus or cyclosporine] and mycophenolate mofetil starting Day +5. In the absence of GVHD, immunosuppression was generally tapered and stopped between Day 90-180.
Engraftment and toxicity
Neutrophil engraftment was defined as the 1st of three consecutive days with an absolute neutrophil count≥0.5×10^9/L. Platelet engraftment was defined as the 1st of seven consecutive days with a platelet count≥20×10^9/L without platelet transfusions for at least seven days. Chimerism analysis using variable number of tandem repeats was performed on Day 30 post-HSCT and repeated at 3, 6 and 12 months post-HSCT. Data on regimen-related toxicity including sinusoidal obstruction syndrome [SOS] (based on modified Seattle criteria14) and hemorrhagic cystitis was noted from medical records. Data on the presence of acute and chronic GVHD was also collected from individual medical records.
Antimicrobial prophylaxis and monitoring
All patients were nursed in HEPA filtered rooms. Fluconazole and acyclovir was started on Day +1 as antifungal and antiviral prophylaxis, respectively, but no anti-bacterial prophylaxis was given. Oral penicillin and co-trimoxazole-trimethoprim prophylaxis was added following recovery of blood counts and stable engraftment. For febrile neutropenia, antibiotics were administered as per prevalent institutional guidelines. High-resolution computed tomography scans of the chest and serum galactomannan were used to identify invasive fungal disease [IFD]. All patients were routinely monitored weekly for cytomegalovirus infection and urine was monitored every two weeks in alternate donor transplants for BK virus.
Statistical Analysis
Data was censored for analysis on 30th December 2022. OS included all patients who were alive at the final evaluation. Disease free survival [DFS] was calculated from the date of HSCT until death or relapse. For comparison of dichotomous variables, a chi-square test was performed while continuous variables were compared using either a Student's t-test or a Mann-Whitney U-test, as appropriate. The probability of OS and DFS were estimated using the Kaplan-Meier method. The prognostic relevance of clinical and biological variables was determined using univariate and multivariate Cox regression analysis. The covariates that were significant in the univariate analysis were used in the multivariate analysis and their significance was analysed using the enter method. Patients were stratified by IPSS and R-IPSS. For all tests, a two-sided p-value of 0.05 or less was considered statistically significant. Statistical analysis was performed using IBM SPSS 24.0 Software.
The primary outcome of this study was OS, and secondary endpoints included EFS, relapse incidence, non-relapse mortality, incidence of acute and chronic GVHD at any time during the follow up, incidence of documented infections (bacterial, viral, or fungal) from commencement of the conditioning regimen until the final follow up, graft failure (primary and secondary), and cause of mortality. Acute and chronic GVHD was diagnosed and graded as per established criteria15,16.
Results
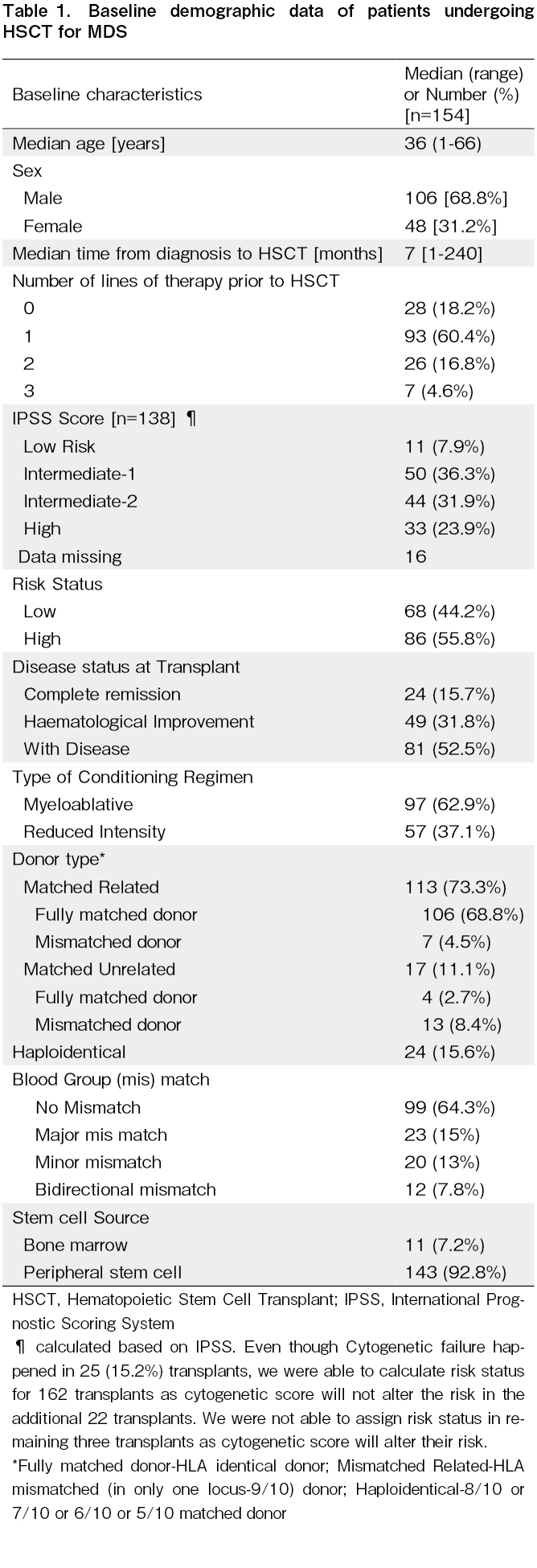
Between July 1991 and September 2021, 154 patients underwent allogeneic HSCT for MDS. Baseline characteristics are described in Table 1. Based on age, the cohort was divided into children (≤15 years of age); adolescents and young adults [AYA] (16-49 years of age)17, and older adults (≥50 years of age). The median age of the cohort was 36 years [range: one to 66 years] and included 22 children [14.2%]. The majority [68.8%] were males. The median time from diagnosis to HSCT was seven months [range: one to 240 months]. Twenty-eight patients (18.2%) did not receive specific treatment prior to HSCT while 93 (60.4%), 26 (16.8%) and seven (4.6%) received one, two, or three lines of therapy, respectively, before HSCT (prior therapies include steroid, cyclosporine, danazol, hypomethylating agents, ATG, hydroxyurea and Imids) IPSS score analysis showed that 33 patients (23.9%) had a high risk IPSS score while 44 (31.9%) had Intermediate 2, 50 (36.3%) had Intermediate 1, and 11 (7.9%) had low risk scores. Sixteen patients did not have an IPSS score due to missing cytogenetic data or failure of cytogenetics. Overall, 86 (55.8%) had high risk MDS while 68 (44.2%) had low risk MDS. At the time of HSCT, 24 (15.7) were in complete remission, 49 (31.8%) had hematological improvement, and 81 (52.5%) with active disease received HSCT.
Transplant characteristics
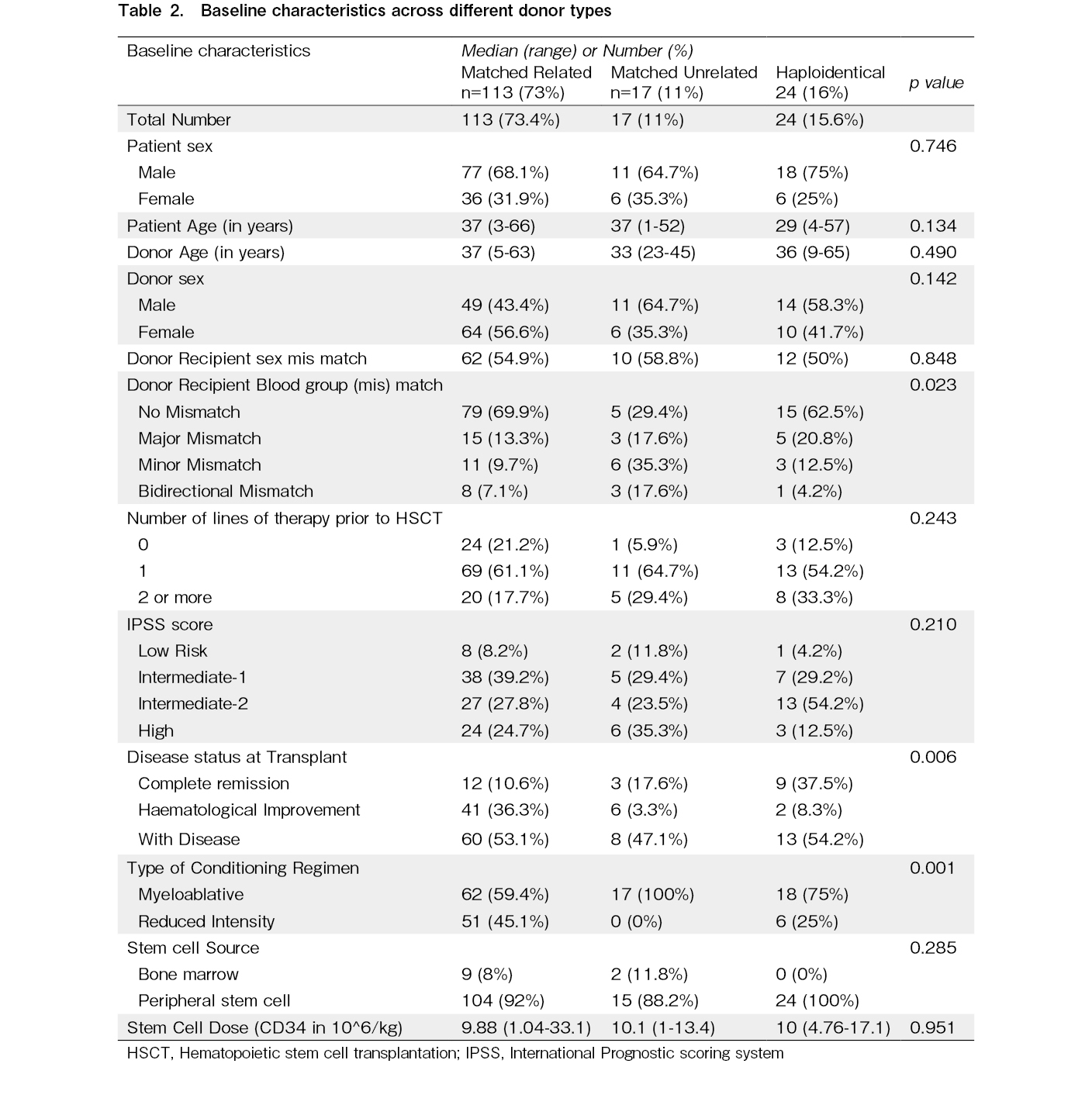
The conditioning regimen was MAC in 97 (62.9%) patients and RIC in 53 (37.1%). The stem cell donor was a human leucocyte antigen-MRD in 113 (73.3%), a MUD in 17 (11.1%), and a Haplo donor in 24 (18.8%). Mismatched MSD or MUD transplants were performed in 20 [12.9%] patients. The graft source was predominantly PBSCs in 143 (92.8%) while bone marrow was used for 11 (7.2%) transplants. The blood group was identical in donor and recipient in 99 (64.3%) instances, major mismatch in 23 (15%), minor mismatch in 20 (13%), and bidirectional mismatch in 12 (7.8%). The baseline characteristics across the different donor types are shown in Table 2.
Engraftment, toxicity and infections
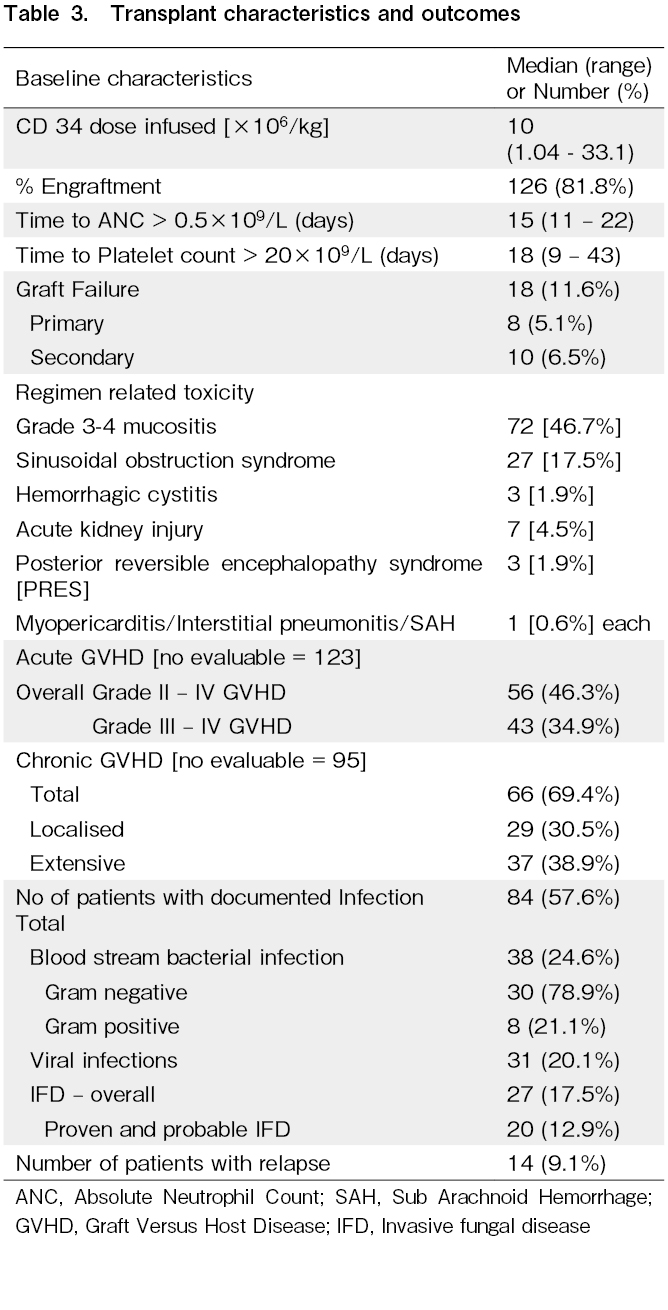
The median CD34+ dose infused was 10×106/kg (range: 1.04-33.1). One hundred and twenty-six patients [81.9%] were engrafted at a median of 15 days [range: 11-22]; 20 [12.9%] died before engraftment due to sepsis or SOS and eight [5.2%] had primary graft failure. The median time to a platelet count >20×109/L was 18 days [range: 9-43]. Grade 3-4 mucositis was seen in 72 patients [46.7%]; it was significantly higher in patients having MAC compared to RIC [56.7% vs 29.8%; p=0.001]. SOS was the most common regimen related toxicity [RRT] and it was seen in 27 patients [17.5%]. The incidence of SOS was not significantly different between MAC [16.4%] and RIC [19.2%] but there was a strikingly high incidence of the use of oral busulfan + cyclophosphamide [69.2%] compared to other regimens [fludarabine/melphalan, 19%; fludarabine/IV busulfan, 8.8%; cyclophosphamide/total body irradiation, and fludarabine/cyclophosphamide, 33%]. Other RRT included acute kidney injury in seven patients [4.5%], hemorrhagic cystitis and posterior reversible encephalopathy syndrome in three patients each [1.9%] and myo-pericarditis, subarachnoid hemorrhage and non-infective interstitial pneumonitis in one patient each.
Eighty-four patients [54.5%] had at least one documented infection (bacterial, viral, or fungal) requiring therapy. Blood stream bacterial infections occurred in 38 (24.6%) with majority of the infections [78.9%] caused by gram negative organisms. Viral infections were seen in 31 transplants [20.1%] with the majority being reactivation of cytomegalovirus. The incidence of IFD was 17.5% with a 12.5% incidence of proven/probable IFD.
Graft-Versus-Host-Disease
The incidence of Grade 2-4 acute GVHD was 46.3%; and the incidence of Grade 3-4 acute GVHD was 34.9% [Table 3]. The incidence was similar between MAC and RIC transplants [53.3% vs 58.3%, p=0.586]; however, bone marrow as the graft source was associated with a higher incidence of acute GVHD compared to PBSC [100% vs 52.1%; p=0.031], and MUD transplants had a higher incidence [84.6%] compared to MRD transplants [54.1%; p=0.036] and Haplo transplants [35.7%; p=0.009]. The overall incidence of chronic GVHD [among evaluable patients] was 69.4%, which was equally divided between localized and extensive chronic GVHD, respectively [30.5% vs 38.9%].
Survival
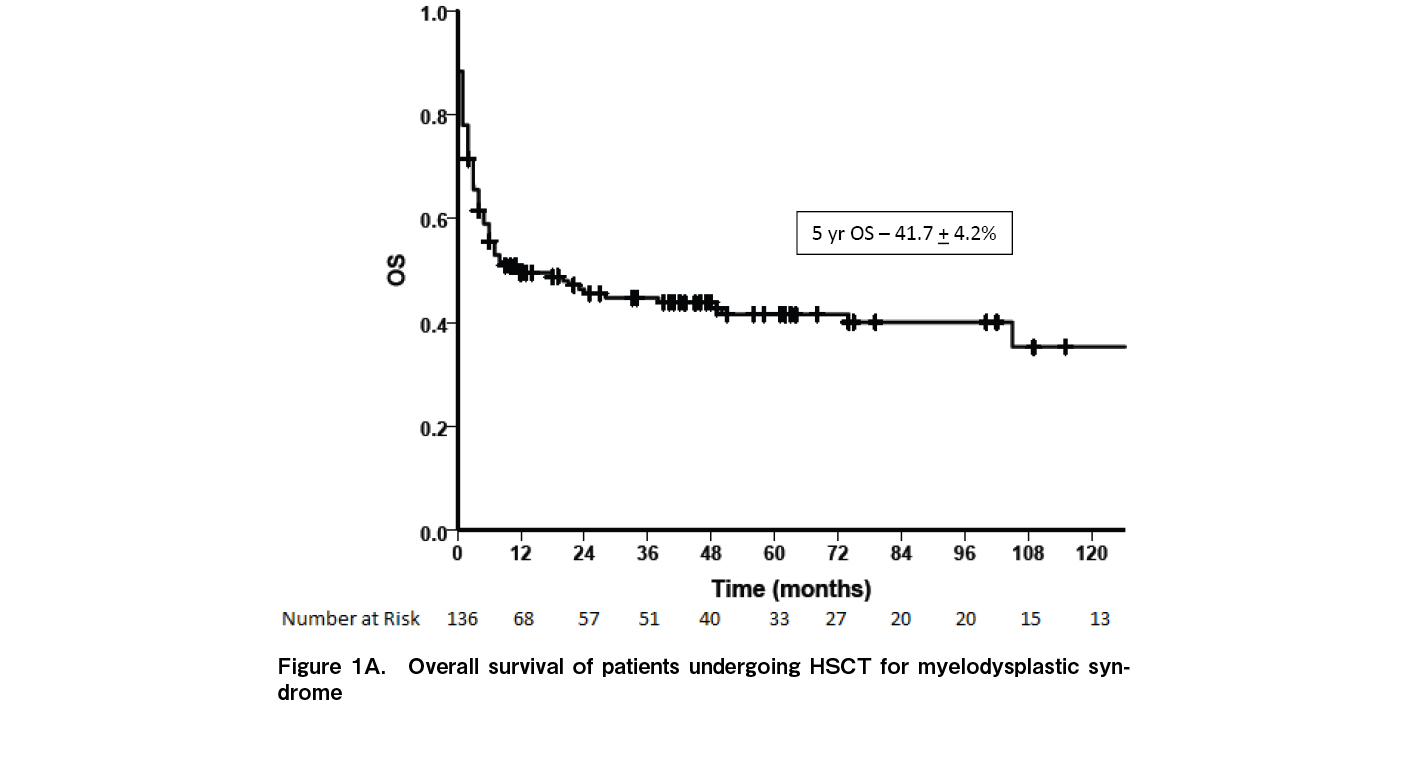
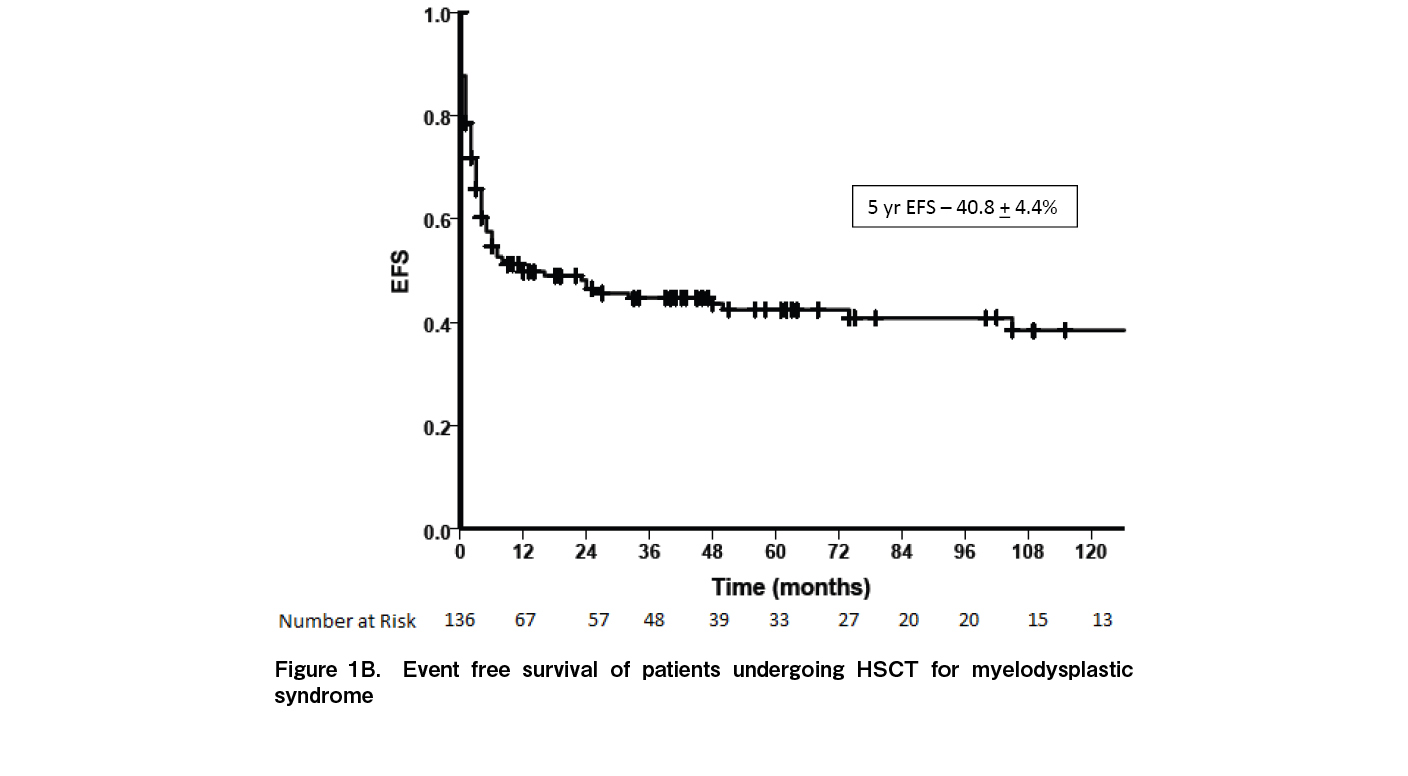
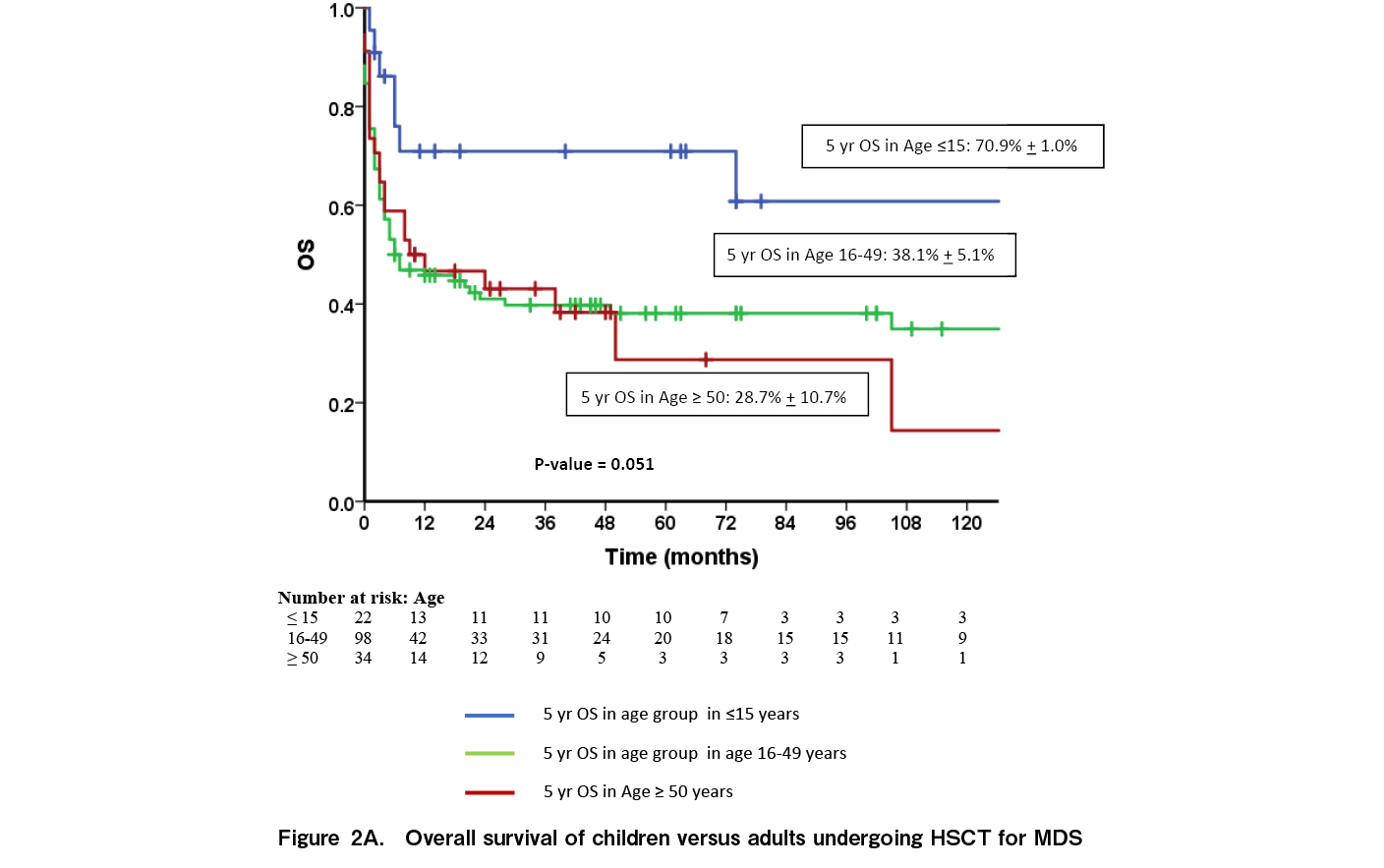
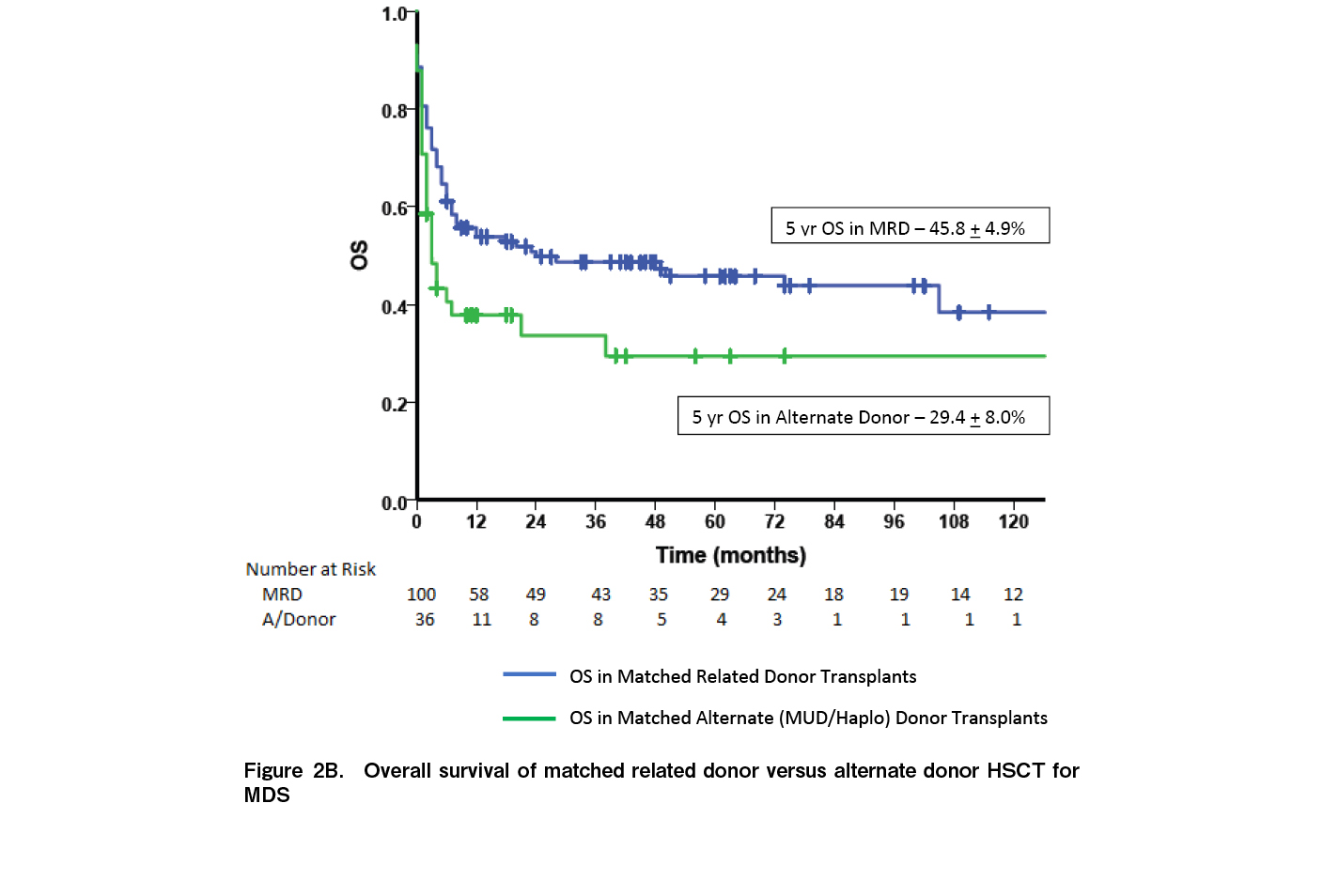
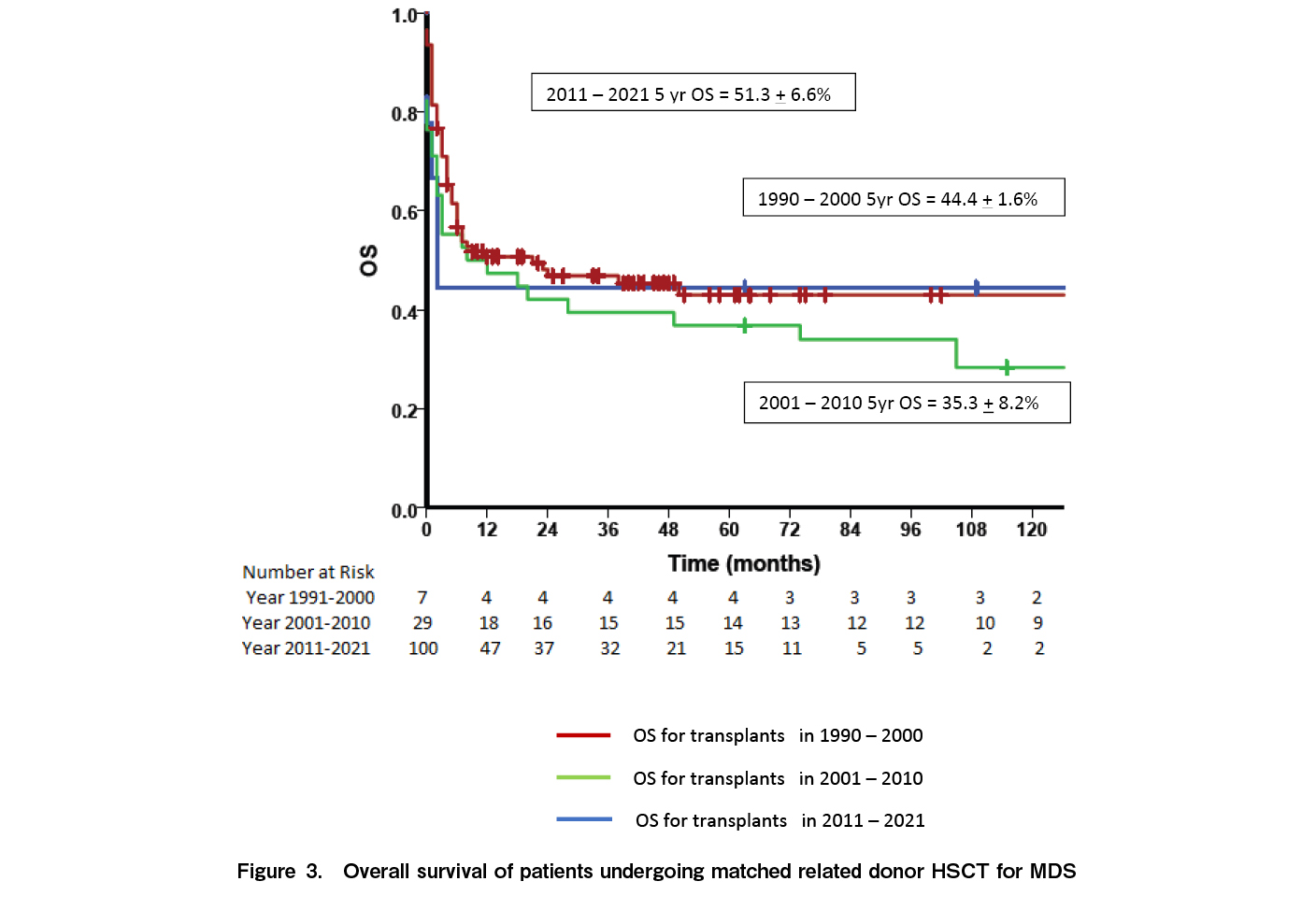
At a median follow up of 33.2 months (range: 0-270 months), 65 patients were alive while 89 died. Fourteen (9.1%) had relapse of disease while 10 (6.5%) had secondary graft failure. The median time to relapse was seven months [range: 3-195]. Relapse rates were significantly higher in patients with high risk MDS [15.1%] compared to low risk MDS [1.47%; p=0.003]. Relapses were higher in patients with persistent disease at the time of HSCT [12.3% vs 5.8%] although the difference was not statistically significant [p=0.117]. Of the 14 relapses, eight occurred as AML while six relapsed as MDS. Nine patients had a second transplant of whom only one is alive. The five-year OS for the entire cohort was 41.7±4.2% (Figure 1A) and the five-year EFS was 40.8±4.4% (Figure 1B). The five-year OS was significantly better in children [70.9±1.0] compared to AYA [38.1%±5.1%] and older adults [28.7%±10.7%] (p=0.051) (Figure 2A). Outcomes were similar between low risk and high-risk disease [42.3±6.4% vs 41.1±5.6%; p=0.84]. Outcomes were better with the use of MRD [45.8±4.9%] compared to alternate donors [MUD + Haplo] [29.4±8.0; p=0.035] (Figure 2B). There was no difference in the outcomes between MUD and Haplo donors [31.4±1.2% vs 31.2±9.8; p=0.856]. The outcomes of MRD transplants have been gradually improving over the immediate recent years; 44.4±1.6% for 1990 to 2000, 35.3±8.2% for 2001 to 2010, and 51.3±6.6% for 2011 to 2021 [p=0.092] (Figure 3). The main causes of mortality included bacterial sepsis [n=27; 30.4%], acute GVHD [n=19; 21.4%], graft failure [n=11; 12.4%], RRT [SOS, diffuse alveolar hemorrhage, subarachnoid hemorrhage, n=9; 10.2%], IFD [n=8; 8.9%], chronic GVHD [n=8; 8.9%], and relapse [n=7; 7.8%].
On univariate analysis, several pre-transplant factors including AYA [p=0.027], older adult age [p=0.032], minor blood group mismatch [p=0.017], bidirectional blood group mismatch [p=0.024], presence of active disease at HSCT [p=0.022], number of lines of prior treatment (one versus others) [p=0.029], and use of a Haplo donor [p=0.032] were associated with poorer OS. Sex, sex mismatch, major blood group mismatch, IPSS risk score, high versus low-risk disease, time from diagnosis to transplant, MUD, conditioning regimen, graft source, stem cell dose, and year of HSCT did not affect OS. On multivariate analysis, age (AYA [hazard ratio (HR) 2.7, p=0.021] and older adult age group [HR 3.6, p=0.006]), minor blood group mismatch [HR 2.0, p=0.028], bidirectional blood group mismatch [HR 2.6, p=0.010], Haplo donor [HR 2.2, p=0.007] continued to remain significant [Table 4 and Figure 4].
Discussion
This single center study from India describes the outcomes of HSCT for patients with MDS over a 30-year period with a reported five-year survival of 42%. The outcomes were better in children [70%] compared to AYA [38%] and older adults [29%] (p=0.015) and with MRDs [45.8%] compared to alternate donors [30%; p=0.035]. Even among MRD transplants, survival has been gradually improving with 51% survival reported in the cohort that underwent HSCT between 2011 and 2021 [51%], although the differences were not statistically significant. In the BMT CTN trial, with 1,102 prospectively enrolled patients with intermediate risk 2 and high risk MDS, the adjusted OS in the donor arm was 47.9%, similar to our cohort10. Our cohort is very different from most studies on HSCT in MDS since the median age of our cohort was 36 years, which is much lower compared to studies in Western countries where the median age is between 62-65 years10. Various studies from Asia have noted this age difference between cohorts of patients with MDS in Western countries and in Asia13,18,19. A higher number of patients [63%] received MAC because of the lower median age. The OS in children was 70%, similar to data reported by the EBMT14; however, the survival in younger and older adults only ranged between 35-36%. There are studies25,26 showing variable outcomes in the AYA subgroup (OS varying from 47% to 71.2%) following allogenic stem cell transplant. This may be explained by the heterogeneity in clinical behavior of MDS. A recent proposal from a US group27 highlights the requirement to implement a newer risk stratification scoring system for predicting post-transplantation outcomes in MDS. The outcomes of MSD transplants are reasonable with a 50% survival in those transplanted between 2011 and 2021; however, the outcomes of alternate donor transplants [MUD and Haplo] are low at 30%. A study from France comparing MSD and Haplo donors reported a two-year survival of 23.7% and a three-year survival of 19.8% in 48 patients undergoing Haplo transplant21. However, a study from the Adult Myelodysplastic Syndrome Working Group of the Japanese Society for Transplantation and Cellular Therapy comparing Haplo transplants with cord blood transplants have reported a two-year OS of 51%22. The Japanese data had patients who underwent cord blood transplantation along with Haplo patients and hence may not be entirely comparable with our data. Also, their study period was between 2014 and 2020. Advances in alternate donor allogenic transplantation in recent years including PTCy, better GVHD control, and advances in antimicrobial control might have contributed to the superior OS than that in our cohort, which includes patients from 1991 to 2021.
The common causes of mortality included acute GVHD, bacterial infections and RRT along with relapse and graft failure. The OS in Grade I acute GVHD is 37.9±14.1%; Grade II acute GVHD, 33.7±24.8%; Grade III acute GVHD, 17.3±8%; and Grade IV acute GVHD, 15.4±10%. The rate of acute GVHD reported in this series was high at 55% with a 34% incidence of Grade 3-4 acute GVHD. Similar rates of 40 – 60% have been reported in other studies with the use of MAC and PBSC10,22,23. In addition, acute GVHD was one of the main reasons for the reduced OS; therefore, it is important to reduce this incidence although few studies have found that acute GVHD of any grade has no impact on OS31,32. Grade III-IV acute GVHD is associated with a poorer OS than with Grade I-II acute GVHD [p=0.001; HR, 3.02 (95% confidence interval: 1.58-5.78)]. However, we found no survival advantage for Grade I acute GVHD compared with patients who had Grade II-IV acute GVHD (p=0.106). Multiple studies have shown that the addition of ATG to the conditioning regimen in patients undergoing MRD or MUD HSCT for MDS has been associated with low rates of GVHD, although it had no impact on OS28–30,33. A few studies have explored the use of PTCy as GVHD prophylaxis in the setting of MSD and MUD transplants with low rates of GVHD34,35.
Bacterial infections continue to remain a major problem among allogeneic transplant recipients in India and is one of the risk factors identified with poorer OS in patients undergoing HSCT for MDS36. Early initiation of antibiotics in patients at high risk of bacteremia due to multidrug resistant organisms [MDRO] has been one of the strategies that have been employed in these patients for the past three to four years and a detailed analysis is ongoing to understand if this strategy has been successful in reducing mortality due to MDRO sepsis.
Since we have a young cohort of patients with MDS, MAC has been commonly used. Initially busulfan + cyclophosphamide was used but it was associated with high rates of SOS and subsequent mortality. Since 2010, we have been using fludarabine + busulfan as the standard conditioning protocol with a reduced incidence of SOS. The ideal conditioning regimen for young and old patients has not yet been defined. Few studies have shown that the use of treosulfan instead of busulfan was associated with lower toxicity but similar survival37. We have recently shown that total marrow lymphoid irradiation is associated with low toxicity in patients with acute lymphoblastic leukemia undergoing allogeneic HSCT and we plan to further explore if this can be used in MDS, based on data that is available in myeloid malignancies that are refractory and in remission38–41.
There has been conflicting data regarding the impact on OS of blood group mismatch between a donor and the recipient42–44. In the current study we found that major blood group mismatch had no impact on OS but minor and bidirectional mismatch had worse impacts on OS.
Approximately 44% of our patients had lower risk MDS, and at our centre an allogeneic transplant was considered if at least two lines of treatment had failed. However, it is important to note that many of the drugs that are currently available for low-risk MDS are, unfortunately, unavailable in India or are too costly for routine use, and also, because of the relatively younger age of our patients, many prefer to choose a curative procedure such as an allogeneic transplant. The OS in this group was 42%, which is lower than the reported rates of 50 to 58% by different groups, including the European Society for Blood and Marrow Transplantation (EBMT)45–47. Changes in conditioning regimens and the addition of ATG to the conditioning should be considered to improve outcomes in this group of patients28,33,48.
In summary, this analysis shows that HSCT in MDS is associated with improving outcomes among MSDs; however, outcomes in Haplo and MUD transplants are poor and require improvement. GVHD and infections are the major causes of mortality, and strategies to reduce both should be incorporated into conditioning protocols to improve the outcomes of HSCT for MDS.
Author Contributions
SK is involved in collecting the data. BG, SK and UPK are involved in conceptualisation and coordination of this work. BG, KML and SK did the statistical analysis. All authors reviewed the manuscript for important intellectual content, approved the final manuscript, and agreed to be accountable for all aspects of this work.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website. AS is one of the editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
References
1.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of myelodysplastic syndromes. Br J Haematol. 1982; 51: 189-99.
2.Platzbecker U, Kubasch AS, Homer-Bouthiette C, Prebet T. Current challenges and unmet medical needs in myelodysplastic syndromes. Leukemia. 2021; 35: 2182-98.
3.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89: 2079-88.
4.Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, Bennett JM, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012; 120: 2454-65.
5.Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthankur G, Wierda WG, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008; 22: 538-43.
6.Robin M, Porcher R, Adès L, Raffoux E, Michallet M, François S, Cahn JY, et al. HLA-matched allogeneic stem cell transplantation improves outcome of higher risk myelodysplastic syndrome A prospective study on behalf of SFGM-TC and GFM. Leukemia. 2015; 29: 1496-501.
7.Cutler CS, Lee SJ, Greenberg P, Deeg HJ, Pérez WS, Anasetti C, Anasetti C, et al. A decision analysis of allogeneic bone marrow transplantation for the myelodysplastic syndromes: delayed transplantation for low-risk myelodysplasia is associated with improved outcome. Blood. 2004; 104: 579-85.
8.Koreth J, Pidala J, Perez WS, Deeg HJ, Garcia-Manero G, Malcovati L, et al. Role of reduced-intensity conditioning allogeneic hematopoietic stem-cell transplantation in older patients with de novo myelodysplastic syndromes: an international collaborative decision analysis. J Clin Oncol. 2013; 31: 2662-70.
9.Alessandrino EP, Della Porta MG, Malcovati L, Jackson CH, Pascutto C, Bacigalupo A, et al. Optimal timing of allogeneic hematopoietic stem cell transplantation in patients with myelodysplastic syndrome. Am J Hematol. 2013; 88: 581-88.
10.Nakamura R, Saber W, Martens MJ, Ramirez A, Scott B, Oran B, et al. Biological Assignment Trial of Reduced -Intensity Hematopoietic Cell Transplantation Based on Donor Availability in Patients 50-75 Years of Age With Advanced Myelodysplastic Syndrome. J Clin Oncol. 2021; 39: 3328-39.
11.Barnard E, Tuechler H, Greenberg PL, Hasserjian RP, Arango Ossa JE, Nannya Y, et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022; 1.
12.Gu S, Xia J, Tian Y, Zi J, Ge Z. A novel scoring system integrating molecular abnormalities with IPSS-R can improve the risk stratification in patients with MDS. BMC Cancer. 2021; 21: 134.
13.Chaubey R, Sazawal S, Mahapatra M, Chhikara S, Saxena R. Does Indian Myelodysplastic Syndrome Have a Biology Different from That in the West?. Asian Pac J Cancer Prev. 2016; 17: 2341-2.
14.McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993; 118: 255-67.
15.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15: 825-8.
16.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11: 945-56.
17.Konishi T, Harada Y, Sadato D, Otsuka Y, Konuma R, Adachi H, et al. Clinical and Genetic Characteristics of Adolescent and Young Adult Patients with Myelodysplastic Syndromes. Blood. 2019; 134 (Suppl 1): 3336.
18.Chen B, Zhao WL, Jin J, Xue YQ, Cheng X, Chen XT, et al. Clinical and cytogenetic features of 508 Chinese patients with myelodysplastic syndrome and comparison with those in Western countries. Leukemia. 2005; 19: 767-75.
19.Lee JJ, Kim HJ, Chung IJ, Kim JS, Sohn SK, Kim BS, et al. Comparisons of prognostic scoring systems for myelodysplastic syndromes: a Korean multicenter study. Leuk Res. 1999; 23: 425-32.
20.Starý J, Locatelli F, Niemeyer CM; European Working Group on Myelodysplastic Syndrome (EWOG-MDS) and Pediatric Diseases Working Party of the EBMT. Stem cell transplantation for aplastic anemia and myelodysplastic syndrome. Bone Marrow Transplant. 2005; 35 (Suppl 1): S13-6.
21.Michel C, Robin M, Morisset S, Blaise D, Maertens J, Chevalier P, et al. Outcome after allogeneic stem cell transplantation with haploidentical versus HLA-matched donors in patients with higher-risk MDS. Bone Marrow Transplant. 2023; 58: 534-43.
22.Konuma T, Shimomura Y, Ishiyama K, Ara T, Nakamae H, Hiramoto N, et al. Haploidentical transplantation with post-transplant cyclophosphamide versus single cord blood transplantation for myelodysplastic syndrome: A retrospective study from the Adult Myelodysplastic Syndrome Working Group of the Japanese Society for Transplantation and Cellular Therapy (JSTCT). Am J Hematol. 2022; 97: E447-50.
23.Kurosawa S, Shimomura Y, Itonaga H, Najima Y, Kobayashi T, Ozawa Y, et al. Myeloablative versus reduced intensity conditioning with fludarabine/busulfan for myelodysplastic syndrome: a propensity score-matched analysis. Transplant Cell Ther. 2022; 28: 323.e1-9.
24.Konuma T, Itonaga H, Ishiyama K, Doki N, Uchida N, Sawa M, et al. Should a matched sibling donor still be considered the primary option for allogeneic hematopoietic cell transplantation in patients over 50 years of age with myelodysplastic syndrome? Bone Marrow Transplant. 2023; 58: 893-906.
25.Grabska J, Shah B, Reed D, Al Ali N, Padron E, Ramadan H, et al. Myelodysplastic Syndromes in Adolescent Young Adults: One Institution's Experience. Clin Lymphoma Myeloma Leuk. 2016; 16 (Suppl): S53-6.
26.Shimomura Y, Hara M, Konuma T, Itonaga H, Doki N, Ozawa Y, et al. Allogeneic hematopoietic stem cell transplantation for myelodysplastic syndrome in adolescent and young adult patients. Bone Marrow Transplant. 2021; 56: 2510-17.
27.Gooptu M, Koreth J. A post-transplant optimized transplant-specific risk score in myelodysplastic syndromes. Haematologica. 2019; 104: 859-61.
28.Arcuri LJ, Kerbauy MN, Kerbauy LN, Santos FPS, Ribeiro AAF, Hamerschlak N. ATG in HLA-Matched, Peripheral Blood, Hematopoietic Cell Transplantation in Acute Myeloid Leukemia and Myelodysplastic Syndrome: A Secondary Analysis of a CIBMTR Database. Transplant Cell Ther. 2023; 29: 40.e1-4.
29.Forcade E, Chevret S, Finke J, Ehninger G, Ayuk F, Beelen D, et al. Impact of in vivo T-cell depletion in patients with myelodysplastic syndromes undergoing allogeneic hematopoietic stem cell transplant: a registry study from Chronic Malignancies Working Party of the EBMT. Bone Marrow Transplant. 2022; 57: 768-74.
30.Fuji S, Hirakawa T, Takano K, Doki N, Sawa M, Kanda Y, et al. Disease-specific impact of anti-thymocyte globulin in allogeneic hematopoietic cell transplantation: a nationwide retrospective study on behalf of the JSTCT, transplant complications working group. Bone Marrow Transplant. 2022; 57: 479-86.
31.Konuma T, Ishiyama K, Igarashi A, Uchida N, Ozawa Y, Fukuda T, et al. Effects of Acute and Chronic Graft-versus-myelodysplastic Syndrome on Long-term Outcomes Following Allogeneic Hematopoietic Cell Transplantation. Clin Cancer Res. 2020; 26: 6483-93.
32.Ishiyama K, Aoki J, Itonaga H, Uchida N, Takahashi S, Ohno Y, et al. Graft-versus-MDS effect after unrelated cord blood transplantation: a retrospective analysis of 752 patients registered at the Japanese Data Center for Hematopoietic Cell Transplantation. Blood Cancer J. 2019; 9: 31.
33.Yahng SA, Min GJ, Park SS, Park S, Jeon YW, Yoon JH, et al. Role of pretransplant anti-thymocyte globulin in matched sibling donor stem cell transplantation after reduced intensity conditioning for myelodysplastic syndrome. Eur J Haematol. 2020; 104: 459-68.
34.Modi D, Kondrat K, Kim S, Deol A, Ayash L, Ratanatharathorn V, et al. Post-transplant Cyclophosphamide Versus Thymoglobulin in HLA-Mismatched Unrelated Donor Transplant for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Transplant Cell Ther. 2021; 27: 760-7.
35.Alanazi W, Chen S, Lipton JH, Kim DD, Viswabandya A, Kumar R, et al. Post-Transplant Cyclophosphamide Combined with Anti-Thymocyte Globulin as Graft-versus-Host Disease Prophylaxis for Allogeneic Hematopoietic Cell Transplantation in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Acta Haematol. 2021; 144: 66-73.
36.Korula A, Perumalla S, Devasia AJ, Abubacker FN, Lakshmi KM, Abraham A, et al. Drug-resistant organisms are common in fecal surveillance cultures, predict bacteremia and correlate with poorer outcomes in patients undergoing allogeneic stem cell transplants. Transpl Infect Dis. 2020; 22: e13273.
37.Beelen DW, Stelljes M, Reményi P, Wagner-Drouet EM, Dreger P, Bethge W, et al. Treosulfan compared with reduced-intensity busulfan improves allogeneic hematopoietic cell transplantation outcomes of older acute myeloid leukemia and myelodysplastic syndrome patients: Final analysis of a prospective randomized trial. Am J Hematol. 2022; 97: 1023-34.
38.George B, Balakrishnan R, Patricia S, Lionel S, Selvarajan S, Devasia AJ, et al. Total marrow lymphoid irradiation and cyclophosphamide is associated with low toxicity and good outcomes in patients undergoing hematopoietic stem cell transplantation for acute lymphoblastic leukemia and chronic myeloid leukemia in lymphoid blast crises – A phase I study. Clin Transplant. 2023; 37: e15010.
39.Al Malki MM, Palmer J, Tsai NC, Mokhtari S, Hui S, Tsai W, et al. Total marrow and lymphoid irradiation as conditioning in haploidentical transplant with post-transplant cyclophosphamide. Blood Adv. 2022; 6: 4098-106.
40.Stein A, Palmer J, Tsai NC, Al Malki MM, Aldoss I, Ali H, et al. Phase I trial of Total Mrrow and Lymphoid Irradiation Transplantation Conditioning in Patients with Relapsed/Refractory Acute Leukemia. Biol Blood Marrow Transplant. 2017; 23: 618-24.
41.Stein AS, Al Malki MM, Yang D, Palmer JM, Tsai NC, Aldoss I, et al. Total Marrow and lymphoid Irradiation with Post-Transplantation Cyclophosphamide for Patients with AML in Remission. Transplant Cell Ther. 2022; 28: 368.e1-7.
42.Kimura F, Sato K, Kobayashi S, Ikeda T, Sao H, Okamoto S, et al. Impact of ABO-blood group incompatibility on the outcome of recipients of bone marrow transplants from unrelated donors in the Japan Marrow Donor Program. Haematologica. 2008; 93: 1686-93.
43.Erker CG, Steins MB, Fischer RJ, Kienast J, Berdel WE, Sibrowski W, et al. The influence of blood group differences in allogeneic hematopoietic peripheral blood progenitor cell transplantation. Transfusion. 2005; 45: 1382-90.
44.Stussi G, Muntwyler J, Passweg JR, Seebach L, Schanz U, Gmür J, et al. Consequences of ABO incompatibility in allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002; 30: 87-93.
45.Robin M, Porcher R, Zinke-Cerwenka W, van Biezen A, Volin L, Mufti G, et al. Allogeneic haematopoietic stem cell transplant in patients with lower risk myelodysplastic syndrome: a retrospective analysis on behalf of the Chronic Malignancy Working Party of the EBMT. Bone Marrow Transplant. 2017; 52: 209-15.
46.Lee SE, Kim YJ, Yahng SA, Cho BS, Eom KS, Lee S, et al. Survival benefits from reduced-intensity conditioning in allogeneic stem cell transplantation for young lower-risk MDS patients without significant comorbidities. Eur J Haematol. 2011; 87: 510-20.
47.Díez Campelo M, Sanchez-Barba M, de Soria VG, Martino R, Sanz G, Insunza A, et al. Results of allogeneic stem cell transplantation in the Spanish MDS registry: prognostic factors for low risk patients. Leuk Res. 2014; 38: 1199-206.
48.Park SS, Jeon YW, Min GJ, Park S, Yahng SA, Yoon JH, et al. Graft-versus-Host Disease-Free, Relapse-Free Survival after Allogeneic Stem Cell Transplantation for Myelodysplastic Syndrome. Biol Blood Marrow Transplant. 2019; 25: 63-72.
Search
News