Volume 7 (2024) Issue 4 No.3 Pages 111-117
Abstract
Diffuse hyperpigmentation is common in patients who undergo chemotherapy or stem cell transplantation (SCT). However, only a few studies have reported the relation between skin reactions and SCT-related complications. Serum 5-S-cysteinyldopa (5SCD), a pheomelanin precursor, is elevated in individuals with hyperpigmentation. Here, we serially examined 5SCD levels during SCT to determine their association with SCT-related complications. We prospectively analyzed serum 5SCD levels in 41 patients (median age: 7.9 years; range: 0-22 years) who underwent SCT (allogeneic in 34 patients and autologous in 7 patients). The serum level of 5SCD increased on day 0, remained high on day 5, and gradually decreased to baseline levels on day 40 after SCT. An increase in 5SCD levels on day 0 was associated with the presence of viral reactivation (odds ratio [OR]: 3.32; 95% confidence interval [CI] 1.07-10.21, p = 0.002) while an increase in 5SCD levels on day 5 was associated with pre-engraftment syndrome (OR: 2.18; 95% CI 1.11-4.26, p = 0.007). In patients who underwent allogeneic SCT, the difference between the baseline level of 5SCD before SCT and the highest level after SCT was associated with acute graft-versus-host disease (GVHD) (OR for a 10 nmol/L increase in biomarker levels: 1.90; 95% CI 1.04-3.45, p = 0.015) and acute cutaneous GVHD (OR for a 10 nmol/L increase in biomarker levels: 2.34; 95%CI 1.11-4.52, p = 0.005). The conditioning regimen was not associated with serum 5SCD levels. Therefore, this study demonstrated the potential of 5SCD as a predictive biomarker for SCT-related complications, such as viral reactivation, pre-engraftment syndrome, and acute GVHD.
Introduction
Stem cell transplantation (SCT) is a widely used treatment for hematological disorders, malignant solid tumors, and primary immunodeficiencies. Complications occurring within 100 days of transplantation can be a major cause of transplant-related mortality. Therefore, the prediction and prevention of such complications are extremely important.
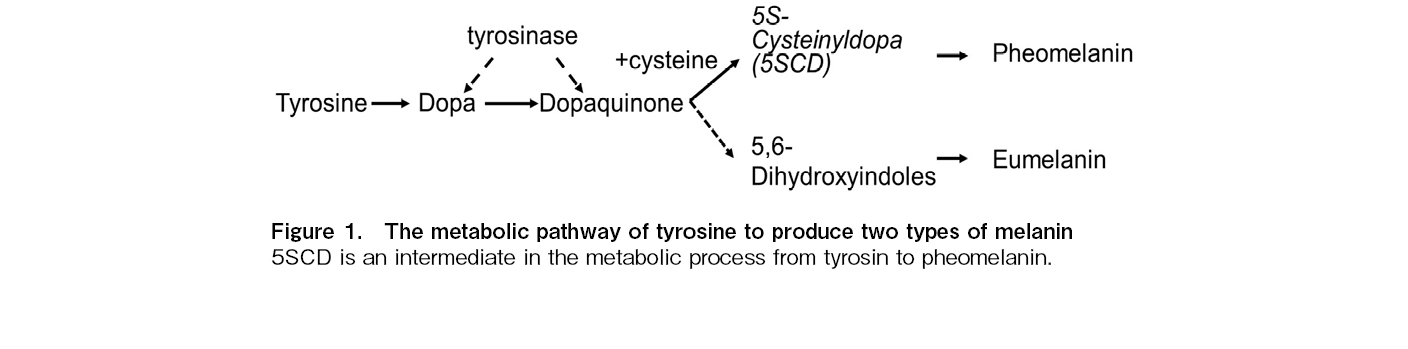
Diffuse hyperpigmentation commonly occurs after chemotherapy and SCT. Hyperpigmentation usually appears after 1-4 weeks of chemotherapy, especially with alkylating agents, such as cyclophosphamide, ifosfamide, busulfan, and thiotepa. It may be generalized or localized at sites, such as the nails and teeth1, 2. A previous study reported cutaneous symptoms, such as diffuse erythema, desquamation, and hyperpigmentation, in approximately 80% of children who underwent autologous SCT with thiotepa3. Because a variety of SCT complications manifest as early signs of skin reactions to chemotherapy, radiation, graft-versus-host disease (GVHD), and infection, skin monitoring is considered very important4. However, there have been very few reports on the relation between skin reactions and treatment-related complications, and the cause of pigmentation after chemotherapy or SCT. Skin pigmentation is thought to result from melanin accumulation in the dermal layer, caused by increased melanin synthesis and the destruction of the basement membrane due to inflammation. Melanin pigments are classified into two types: brown-to-black eumelanin and yellow-to-reddish-brown pheomelanin. The ratio of the two types of melanin, brown-to-black eumelanin and yellow-to-reddish-brown pheomelanin, determines the skin and hair color. The metabolic pathways involved in the production of these two melanin types are shown in Figure 1. After the oxidation of tyrosine by tyrosinase to dopaquinone, eumelanin is produced by the oxidation of 5,6-dihydroxyindoles as an intermediate, while pheomelanin is formed by the oxidation of 5-S-cysteinyldopa (
As 5SCD has not been reported as an SCT-related biomarker so far, we serially examined serum 5SCD levels before and after SCT to determine its association with SCT-related complications.
Patients and Methods
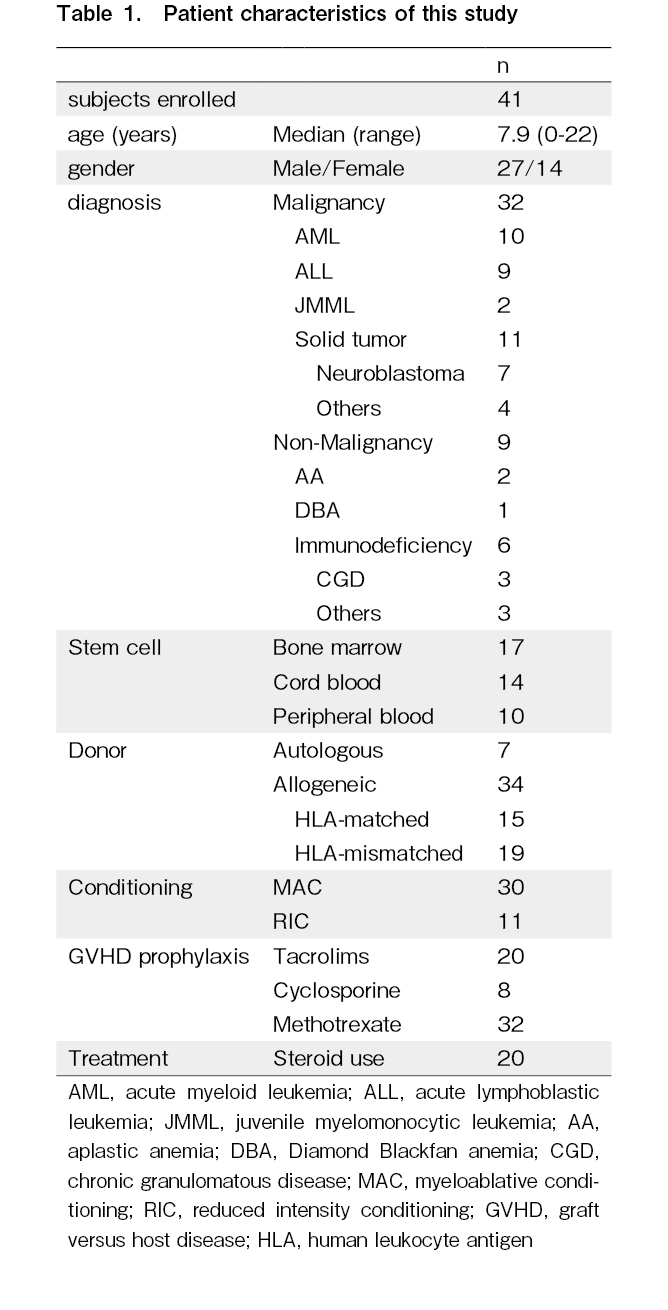
We prospectively analyzed 41 patients (27 males and 14 females) with a median age of 7.9 years who underwent SCT between May 2011 and March 2015 at Hokkaido University Hospital and provided informed consent or parental permission for enrollment in this study. This study was approved by the Institutional Review Board and Ethics Committee of Hokkaido University Hospital (Approval Number 012-0376). Serum samples were obtained from the patients using a non-invasive backflow method from a central venous catheter before conditioning therapy, on day 0 (the day of stem cell infusion), and on days 5, 10, 15, 25, and 40. Serum samples were obtained at these seven time points to monitor changes associated with conditioning regimens, engraftment, and complications, such as acute GVHD, based on the values before conditioning therapy. All blood samples were centrifuged at 3,000 rpm for 15 min and stored at -80
We analyzed the association of eight 5SCD values (at each of the six time points, the highest value, and the difference between the baseline level of 5SCD before conditioning therapy and the highest level after SCT) with the following: conditioning regimen, donor source, malignant disease (having received some cycles of chemotherapy before SCT), and the onset of SCT-related complications within 100 days after SCT, such as acute GVHD, acute cutaneous GVHD, viral reactivation, pre-engraft syndrome (PES), and death not attributed to the primary disease. We used multivariate analysis to examine the independent association between serum 5SCD levels with the items associated with SCT-related complications in the univariate analysis.
Statistical analysis
Univariate logistic regression was used to evaluate the association between each 5SCD and SCT-related complications occurring within 100 days post-SCT, conditioning regimen, donor source, and HLA disparity. These factors were further assessed using multivariate logistic regression models to analyze the relation between the 5SCD concentration and SCT-related complications. Study confounders were selected based on a literature review and change-in-estimate criteria, which were set at a value greater than 10%. Potential confounding variables considered in the analysis included age, stem cell characteristics, HLA disparity, anti-thymocyte globulin use, steroid use, and conditioning intensity. P was set at p < 0.05. All statistical analyses were performed using JMP, version 14 (SAS Institute Inc., Cary, NC, USA).
Results
Patient characteristics
The median age of the patients at transplantation was 7.9 years (range: 0-22 years). None of the patients had renal failure or were undergoing hemodialysis. The patient characteristics are shown in Table 1. Indications for SCT included malignant diseases in 32 patients (hematological malignancies in 21 patients and malignant solid tumors in 11 patients) and non-malignant diseases in nine patients. Thirty-four patients underwent allogeneic SCT, and seven patients received autologous SCT. Myeloablative conditioning was provided to 30 patients and reduced-intensity conditioning was provided to 11 patients. The conditioning regimen, defined as myeloablative conditioning, included intravenous busulfan > 7.2 mg/kg, melphalan (> 140 mg/m2), and total body irradiation (> 8 Gy). The conditioning regimens included total-body irradiation (n = 16), busulfan (n = 14), cyclophosphamide (n = 16), and melphalan (n = 22). Twenty patients were administered steroids for GVHD or PES.
SCT-related complications
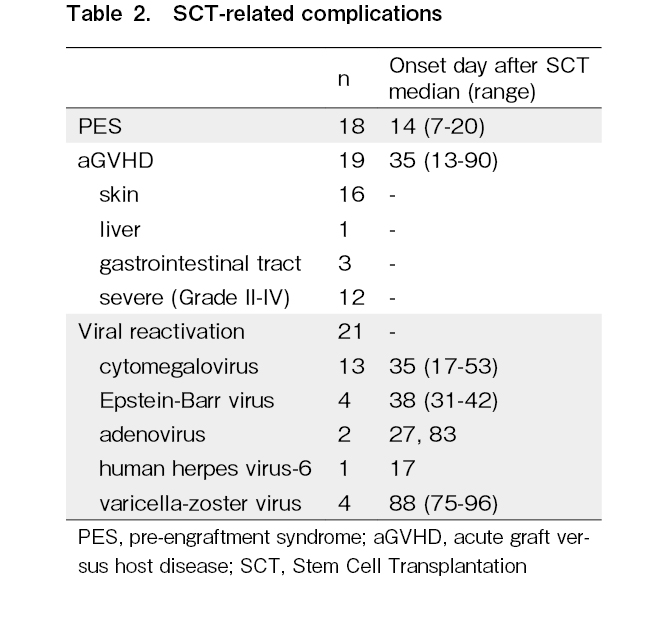
PES was observed in 18 patients. Acute GVHD was observed in 19 patients, of whom 16 had acute cutaneous GVHD and 12 had grade II-IV acute GVHD. Viral reactivation was observed in 21 patients, including that of cytomegalovirus (n = 13), Epstein-Barr virus (n = 4), varicella-zoster virus (n = 4), adenovirus (n = 2), and human herpes virus-6 (n = 1) (Table 2). Some patients showed reactivation of multiple viruses, but none showed reactivation of human herpes virus-6 or varicella-zoster virus alone. Thirteen patients relapsed or died, two of whom died of SCT-related complications within 100 days post-SCT. One patient with a solid tumor developed hepatic veno-occlusive disease and adenoviral infection after autologous SCT and died 60 days after SCT. Another patient with acute lymphoblastic leukemia had severe acute GVHD with adenoviral infection and died 87 days post-SCT.
Serum 5S-cysteinyldopa level
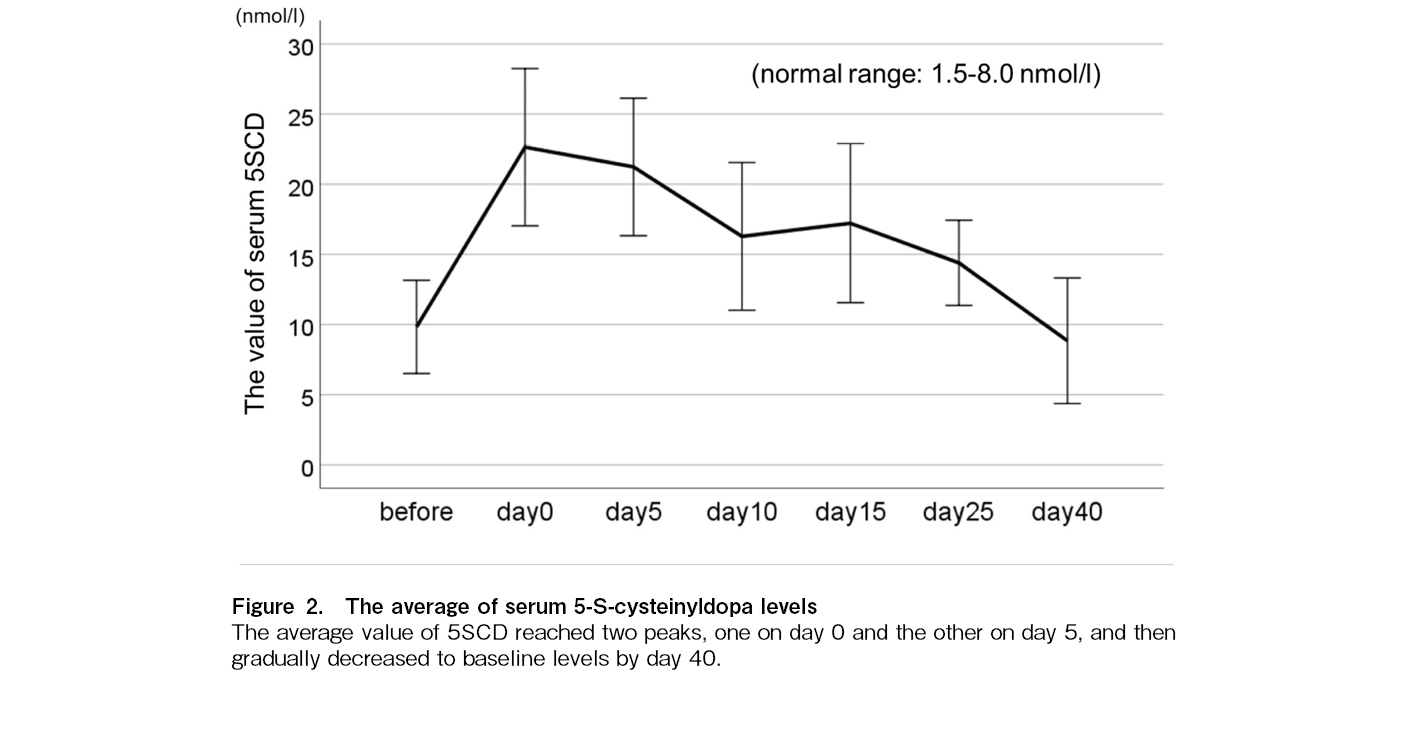
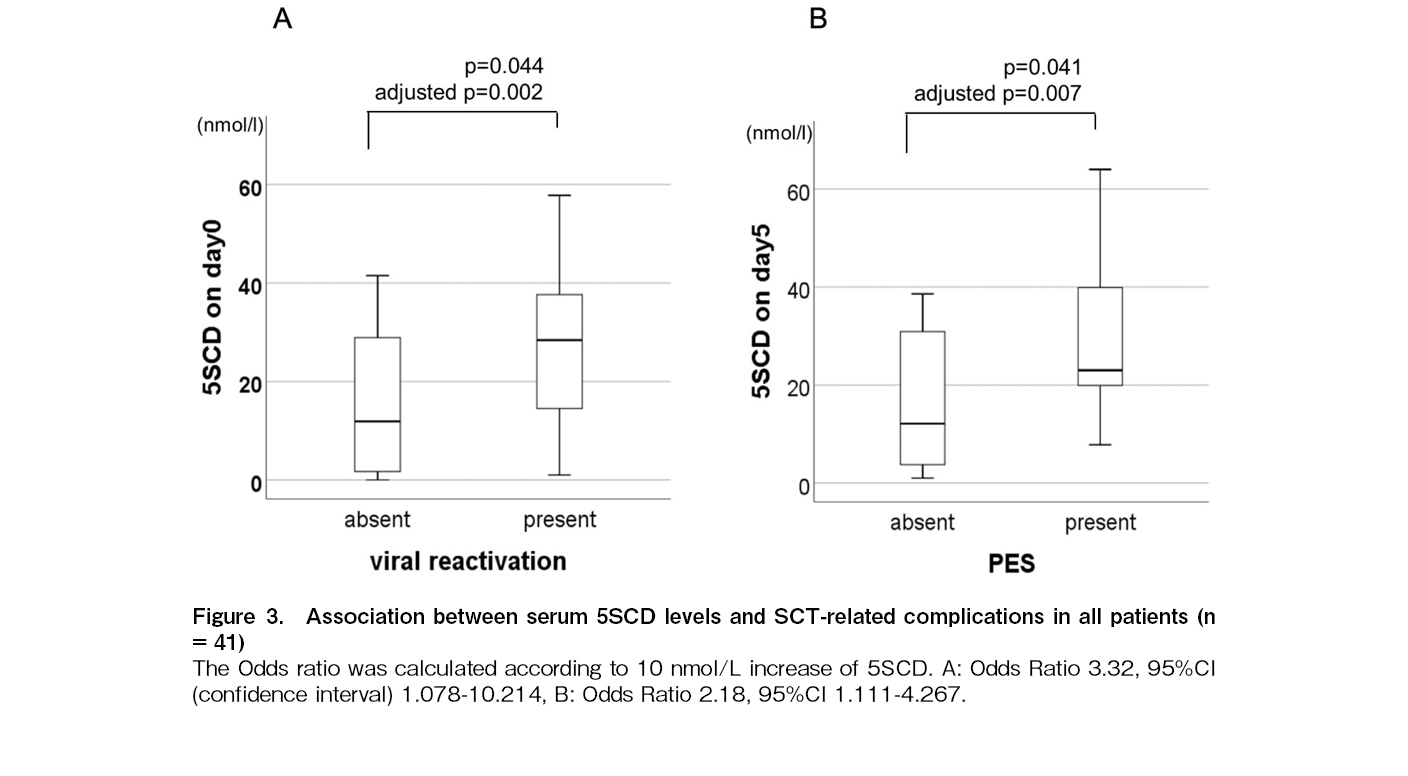
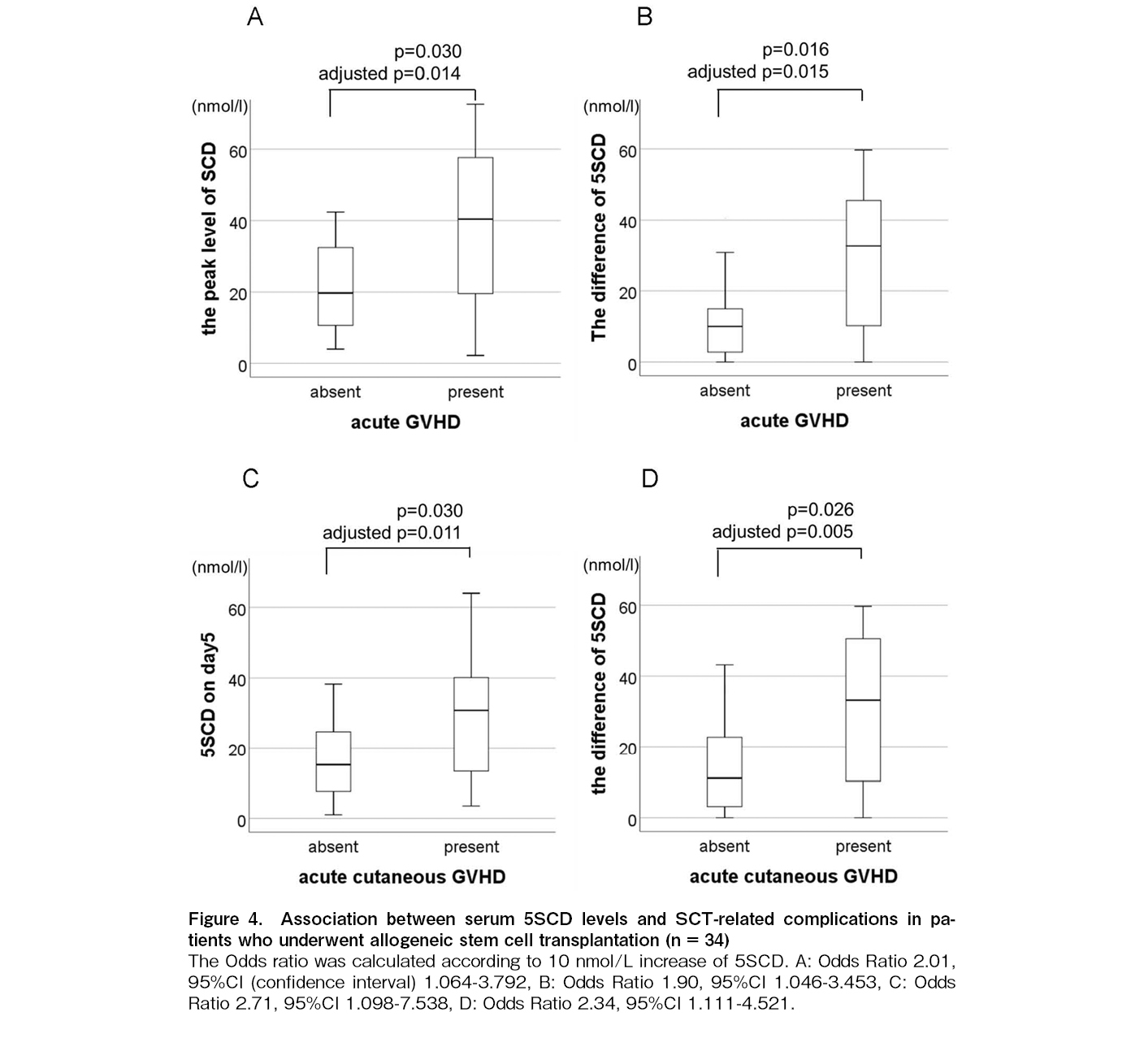
The average value of 5SCD reached two peaks, one on day 0 and the other on day 5, regardless of the stem cell source and conditioning intensity (Figure 2). Univariate analysis revealed that the 5SCD level on day 0 was associated with viral reactivation (p = 0.044) (Figure 3A), the 5SCD level on day 5 was associated with PES (p = 0.041) (Figure 3B), and the highest 5SCD level was associated with malignant disease (p = 0.038). In patients receiving allogeneic SCT (n = 34), both the peak level of 5SCD and the difference between the highest 5SCD and 5SCD level before SCT were associated with acute GVHD (p = 0.03 and 0.016, respectively) (Figure 4A and B). Both the 5SCD level on day 5 and the difference in 5SCD levels were associated with acute cutaneous GVHD (p = 0.030 and 0.026, respectively) (Figure 4C and D). There was no significant association between any serum 5SCD level and chronic GVHD, severity of acute GVHD, intensity and content of conditioning regimens, graft source, immunosuppressive agents, or death not caused by the primary disease.
Subsequently, we conducted multivariate analysis to examine the independent association between viral reactivation, acute GVHD, acute cutaneous GVHD, and serum 5SCD levels (Table 2). The 5SCD level on day 0 was independently associated with viral reactivation (odds ratio [OR], 3.32; 95% confidence interval [CI] 1.07-10.21, p = 0.002) (Figure 3A). Furthermore, the
We found that both the highest level of 5SCD and the difference between the highest level of 5SCD after SCT and the baseline level of 5SCD before SCT were independent biomarkers for acute GVHD (OR: 2.01; 95% CI 1.06-3.79, p = 0.014 and OR: 1.90; 95% CI 1.04-3.45, p = 0.015, respectively) (Figure 4A and B). The 5SCD level on day 5 and difference of 5SCD levels were independent biomarkers for acute cutaneous GVHD (OR: 2.71; 95% CI 1.09-7.53, p = 0.011 and OR: 2.34; 95% CI 1.11-4.52, p = 0.005, respectively) (Figure 4C and D).
Discussion
SCT is used to treat and cure many diseases, including childhood diseases. Predicting and controlling SCT-related complications are extremely important to prevent transplant-related mortality. We found that serum
GVHD and PES are immune-mediated reactions and major complications after allogeneic SCT, respectively. Acute cutaneous GVHD is characterized by erythematous macular and papular rashes12, while PES is associated with skin rashes and capillary leak syndrome during the early engraftment phase13. Both GVHD and PES are accompanied by immunological skin inflammation. We speculate that this inflammation promotes pheomelanin production, resulting in elevated serum 5SCD levels. Further pathological examination of melanin pigments in patients with GVHD and PES is required to confirm our hypothesis.
Although the risk factors for viral reactivation include the use of anti-thymocyte globulin, steroids, and cord blood transplantation14, we did not find an association between serum 5SCD and these risk factors. Viral reactivation was associated with the levels of serum
Various biomarkers have been reported to indicate disease severity and serve as prognostic factors for SCT-related complications. Soluble ST2 (also known as interleukin-33 receptor), interleukin-2 receptor α-chain, and tumor necrosis factor receptor-1 have been identified as prognostic markers for acute GVHD, and cutaneous elafin expression has been identified as a prognostic marker for acute cutaneous GVHD16–20. MxA levels correlate with viral infections21. Shah et al. reported that elevated procalcitonin levels may be a biomarker of PES22. Compared with these biomarkers, 5SCD is widely used in several clinical settings, and its serum levels may be useful in predicting multiple SCT-related complications.
The current study has some limitations. First, it is difficult to clearly demonstrate the relation between the extent of pigmentation and SCT-related complications. Murakami et al. examined the degree of hyperpigmentation in patients undergoing hemodialysis by measuring melanin and erythema indices, revealing a significant correlation with serum 5SCD levels, suggesting the accumulation of pheomelanin in the skin8. Further studies to determine whether the degree of pigmentation can be an indicator of SCT-related complications would be useful for preventing transplant-related mortality. Secondly, the sample size was small. Therefore, further investigations with larger sample sizes, including adults, could validate our findings. Finally, it was difficult to identify the activation site in the melanin synthesis pathway leading to an elevation in 5SCD levels, as the precursor of eumelanin could not be measured in our study.
In conclusion, serum 5SCD levels may be predictive biomarkers for SCT-related complications, such as acute GVHD and viral reactivation. Pheomelanin production is presumed to be induced by inflammatory or immunological processes during SCT. However, further prospective studies are required to confirm these findings.
Acknowledgments
We would like to show our greatest appreciation to Professor Emeritus Tadashi Ariga, who gave valuable comments and suggestions to our study. We also thank Jimei Zhao for assisting submission of the manuscript.
Author Contributions
YT and AI designed the study; HG and IY performed statistical analysis; YT, AI, MS, YC, and AM analyzed and interpreted the data; YT and AM wrote the manuscript.
Conflicts of Interest
IY received research fund from Nihon Medi-Physics and honoraria for lectures from Chugai Pharmaceucial Co., AstraZeneca, and Pfizer. AM received honoraria for lectures from Novartis, Ohara, and Servier.
References
1.Reyes-Habito CM, Roh EK. Cutaneous reactions to chemotherapeutic drugs and targeted therapies for cancer: part1. Conventional chemotherapeutic drugs. J Am Acad Dermatol. 2014; 71: 203.e1-12.
2.Susser WS, Whitaker-Worth DL, Grant-Kels JM. Mucocutaneous reactions to chemotherapy. J Am Acad Dermatol. 1999; 40: 367-98.
3.Rosman IS, LIoyd BM, Hayashi RJ, Bayliss SJ. Cutaneous effects of thiotepa in pediatric patients receiving high-dose chemotherapy with autologous stem cell transplantation. J Am Acad Dermatol. 2008; 58: 575-8.
4.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012; 8: 1150-63.
5.Ito S, Wakamatsu K. Chemistry of mixed melanogenesis: pivotal roles of dopaquinone. Photochem Photobiol. 2008; 84: 582-92.
6.Horikoshi T, Ito S, Wakamatsu K, Onodera H, Eguchi H. Evaluation of melanin-related metabolites as markers of melanoma progression. Cancer. 1994; 73: 629-36.
7.Murakami K, Wakamatsu K, Nakanishi Y, Takahashi H, Sugiyama S, Ito S. Serum levels of pigmentation markers are elevated in patients undergoing hemodialysis. Blood Purif. 2007; 25: 483-9.
8.Murakami K, Nakanishi Y, Wakamatsu K, Yamamoto K, Kohriyama N, Hasegawa M, et al. Serum levels of 5-S-Cysteinyldopa are correlated with skin colors in hemodialysis patients but not in peritoneal dialysis patients. Blood Purif. 2009; 28: 209-15.
9.Brochez L, Naeyaert JM. Serological markers for melanoma. Br j Dermatol. 2000; 143: 256-68.
10.Bánfalvi T, Glide K, Gergye M, Boldizsár M, Kremmer T, Ottó S. Use of serum 5-S-CD and S-100B protein levels to monitor the clinical course of malignant melanoma. Eur J Cancer. 2003; 39: 164-9.
11.Uemura H, Yamasaki O, Kaji T, Otsuka M, Asagoe K, Takata M, et al. Usefulness of serum 5-S-cysteinyl-dopa as a biomarker for predicting prognosis and detecting relapse in patients with advanced stage malignant melanoma. J Dermatol. 2017; 44: 449-54.
12.Bacigalupo A, Bullen K, Rizzo D, Giralts S, Lazarus H, Ho V, et al. Defining the intensity conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009; 15: 1628-33.
13.Cornell RF, Hari P, Drobyski WR. Engraftment Syndrome after Autologous Stem Cell Transplantation: An Update Unifying the Definition and Management Approach. Biol Blood Marrow Transplant. 2015; 21: 2061-8.
14.Boeckh M, Nichols WG. The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood. 2004; 103: 2003-8.
15.Locksley RM, Flournoy N, Sullivan KM, Meyers JD. Infection with varicella-zoster virus after marrow transplantation. J Infect Dis. 1985; 152: 1172-81.
16.Brüggen MC, Petzelbauer P, Greinix H, Contassot E, Jankovic D, French L, et al. Epidermal elafin expression is an indicator of poor prognosis in cutaneous graft-versus-host disease. J Invest Dermatol. 2015; 135: 999-1006.
17.Paczesny S. Biomarkers for posttransplantation outcomes. Blood. 2018; 131: 2193-204.
18.Paczesny S, Krijanovski OI, Braun TM, Choi SW, Clouthier SG, Kuick R, et al. A biomarker panel for acute graft-versus-host disease. Blood. 2009; 113: 273-8.
19.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of the therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013; 369: 529-39.
20.McDonald GB, Tabellini L, Storer BE, Lawler RL, Martin PJ, Hansen JA. Plasma biomarkers of acute GVHD and nonrelapse mortality: predictive valueu of measurements before GVHD onset and treatment. Blood. 2015; 126: 113-20.
21.Yoshimasu T, Manabe A, Ebihara Y, Tanaka R, Ooi J, Iseki T, et al. MxA expression in patients with viral infection after allogeneic stem cell transplantation. Bone Marrow Transplantation. 2003; 32: 313-6.
22.Shah NN, Watson TM, Yates B, Liewehr DJ, Steinberg SM, Jacobsohn D, et al. Procalcitonin and cytokine profiles in engraftment syndrome in pediatric stem cell transplantation. Pediatr Blood Cancer. 2017; 64: e26273.
Search
News