Volume 7 (2024) Issue 3 No.3 Pages 79-86
Abstract
Introduction: Microvasculopathy and endothelial dysfunction play important roles in the development of post-transplant complications, including graft-versus-host disease (GVHD). We assessed structural microvasculopathy by employing nailfold video capillaroscopy (NFVC) and endothelial dysfunction via flow-mediated dilatation (FMD) of the brachial artery in recipients of hematopoietic stem cell transplantation.
Patients and methods: Recipients of stem cell transplantation were included in this study post day+100 and divided into two cohorts. The first cohort consisted of 35 recipients of allogeneic hematopoietic stem cell transplantation (HCT) and the second cohort was comprised of 31 recipients of autologous HCT. A third cohort included 35 healthy individuals. NFVC was conducted on the second to fifth fingers of both hands using an Optilia video capillaroscope at 200× magnification, and the images were analyzed according to the European Alliance of Associations for Rheumatology (EULAR) criteria. The following parameters were used to measure vasculopathy: (a) median capillary density, derived from the capillary density of eight fingers, (b) median capillary diameter, derived from maximum capillary apical diameters of eight fingers, and (c) significant neoangiogenesis (neoangiogenesis present in ≥2 fingers). FMD of the right brachial artery was observed by high-resolution ultrasonography using the principle of post-occlusive reactive hyperemia, and video images were analyzed using edge-detecting software.
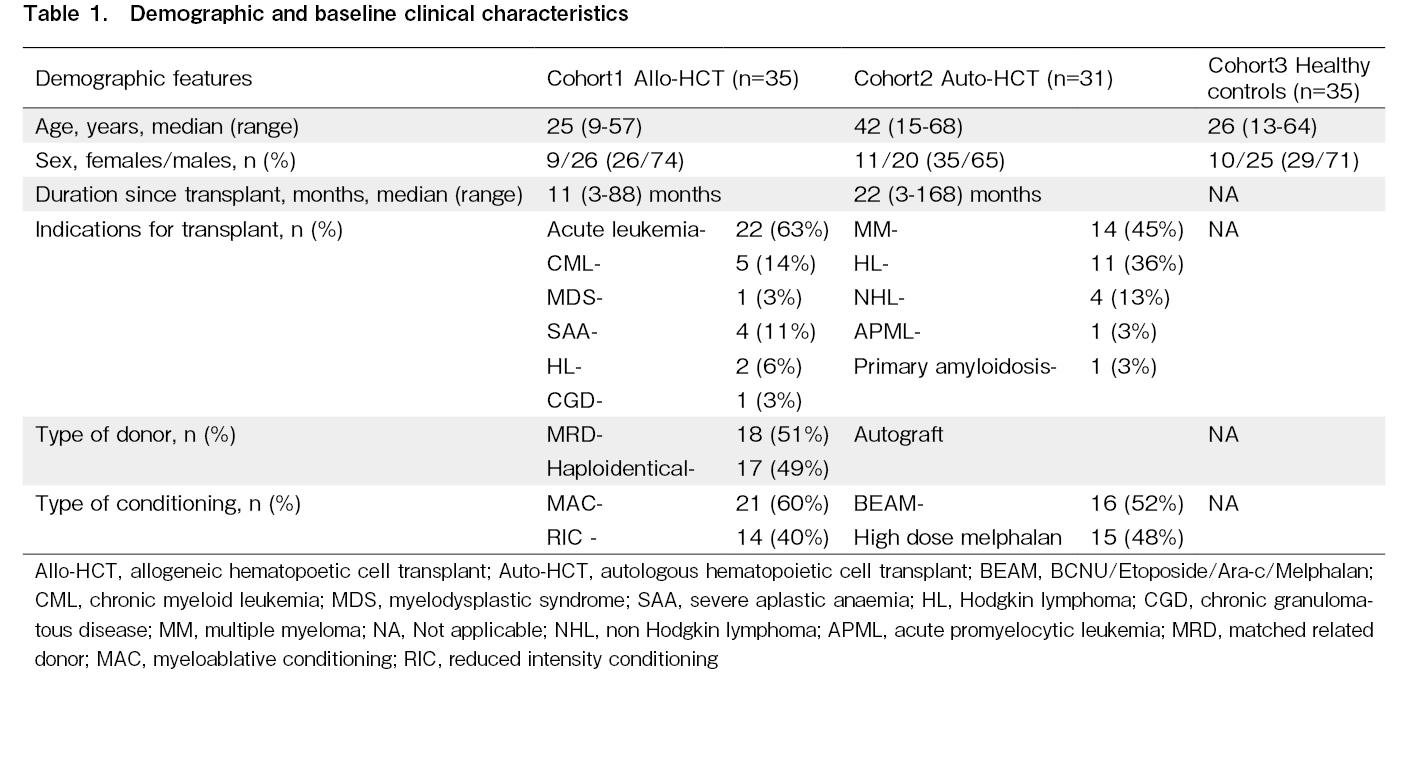
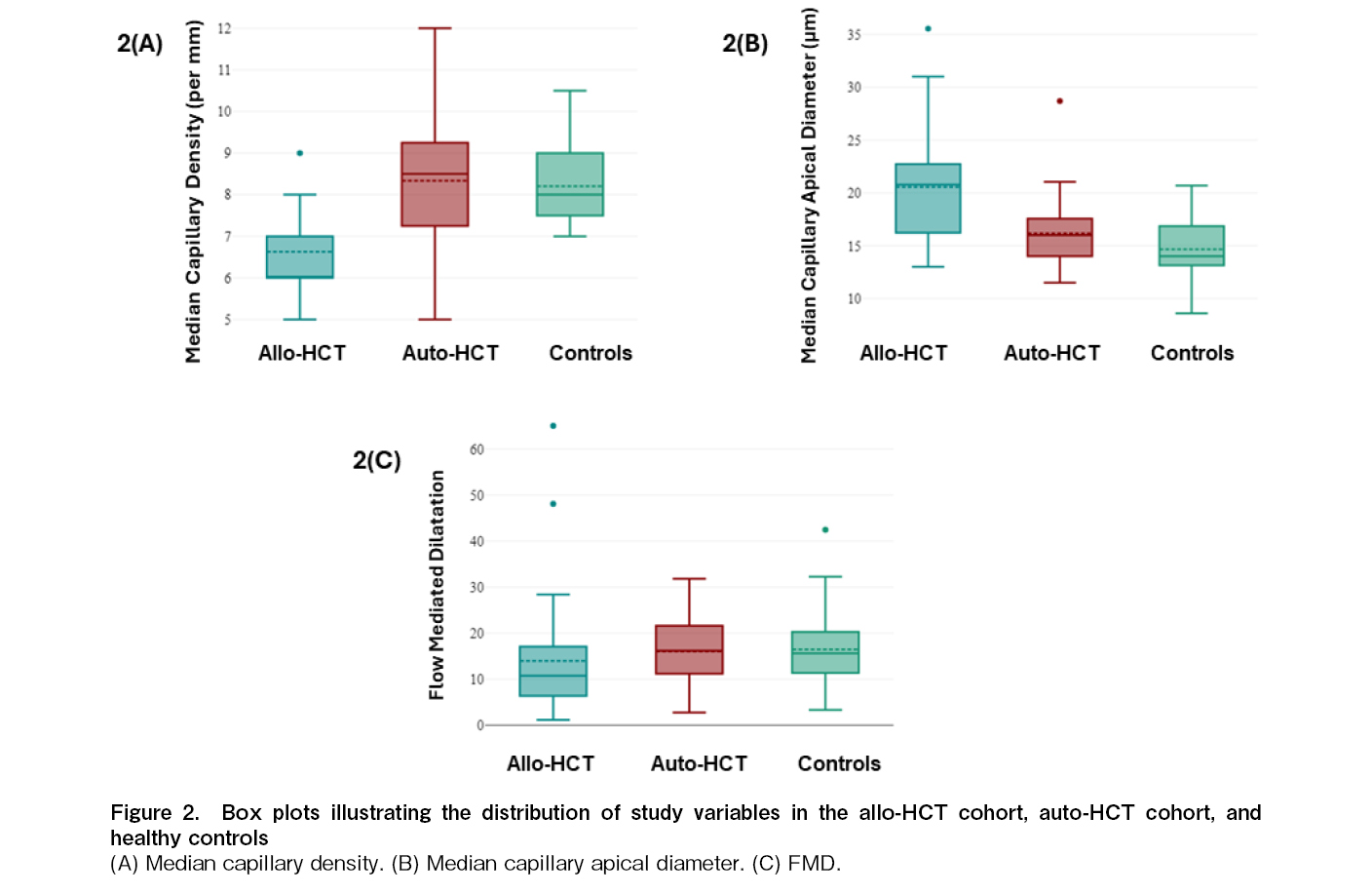
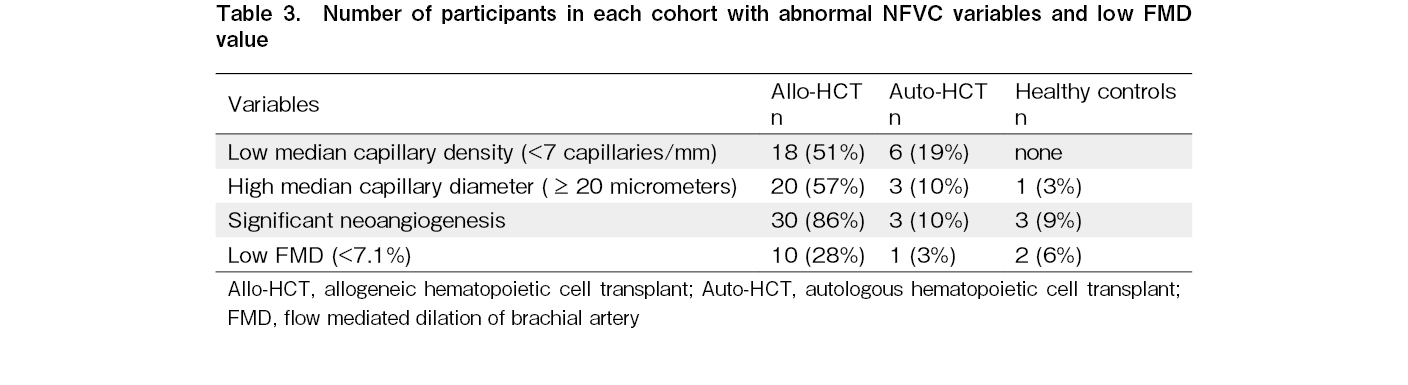
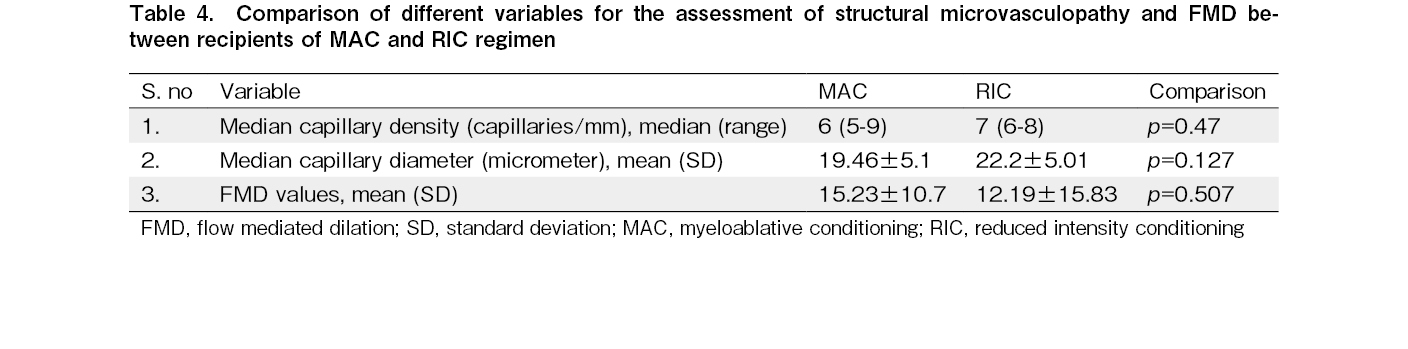
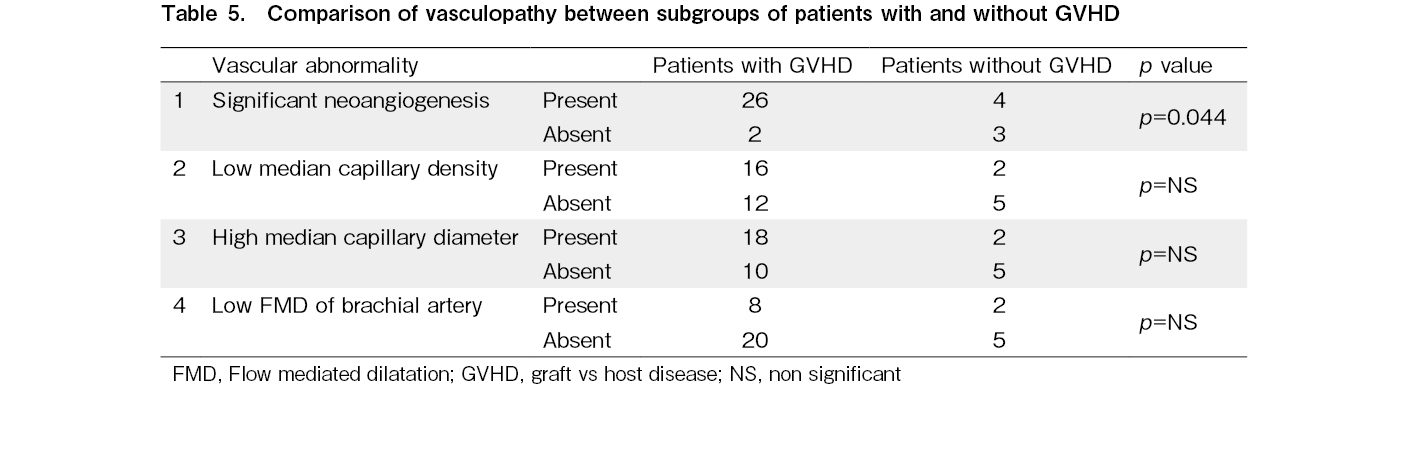
Results: The median capillary diameter was significantly higher in the allo-HCT cohort (20.56±5.17 micrometer) compared to the auto-HCT cohort (16.19±3.31 micrometer; p<0.001) and healthy controls (14.66±2.61 micrometer; p<0.001). The median capillary density was significantly lower in the allo-HCT cohort (median: 6 capillaries/mm, range: 5-9 capillaries/mm) compared to the auto-HCT cohort (median: 8.5 capillaries, range: 5-12 capillaries/mm; p<0.001) and healthy controls (median: 8 capillaries/mm, range: 7-10.5 capillaries/mm; p<0.001). The allo-HCT cohort had a higher proportion of patients with significant neoangiogenesis (86%) than the auto-HCT cohort (10%) and healthy controls (9%). The presence of significant neoangiogenesis was more frequent in the subgroup of patients with a history of GVHD (93%) compared to the subgroup of patients without any history of GVHD (57%; p=0.044). No significant differences in NFVC parameters or FMD were observed between recipients of myeloablative and reduced-intensity conditioning regimens. There was no significant difference in NFVC parameters between the auto-HCT cohort and healthy controls. There was no significant difference in FMD among the three cohorts; however, a higher proportion of patients in the allo-HCT cohort (28%) had lower FMD than those in the auto-HCT cohort (3%) and healthy controls (6%), suggesting endothelial dysfunction.
Conclusions: Our findings demonstrate the presence of structural microvasculopathy in allo-HCT recipients and suggest a possible role of alloreactivity in the pathogenesis of post-HCT microvasculopathy.
Introduction
The endothelium is a monolayer of cells that originates from the mesoderm. It lines the inner surface of blood vessels and lymphatic channels, separating the flowing blood and lymph from tissue1. From the embryonic stage onwards, hematopoietic stem cells and the endothelium share a unique relationship. During embryogenesis, a highly conserved process of endothelium-to-hematopoietic transition (EHT) occurring in endothelial cells of the dorsal aorta gives rise to the first definitive hematopoietic cells2. Endothelial cell cuddling plays an important role in the homing and engraftment of HSCs in the hematopoietic stem cell niche during development and hematopoietic stem cell transplantation (HCT)3.
Endothelial dysfunction is an important component of many post-HCT complications, including steroid-refractory graft-versus-host disease (GVHD)4,5. Post-HCT endothelial dysfunction occurs due to damage from chemotherapy, infections, immunomodulators, and allogeneic reactivity6. A murine model of acute GVHD has shown microstructural damage to the endothelium, including reduced endothelial pericyte coverage and expression of tight junction proteins. During acute intestinal GVHD, structural changes occur in the colonic vasculature. These include an increased vessel diameter and branching7.
Characterization of post-HCT microvasculopathy and endothelial dysfunction remains incomplete due to a lack of easy-to-use, reproducible, and objective tools. In this study, we assessed structural microvasculopathy using nailfold video capillaroscopy (NFVC) and endothelial dysfunction via flow-mediated dilatation (FMD) in recipients of HCT. We also studied the relationship between vascular endothelial changes and incidence of GVHD.
Materials and Methods
Study design and oversight: This cross-sectional observational cohort study was conducted from February 2022 to June 2022. The study was reviewed by the institutional Ethics Committee and approved (Ethical Approval Number: INT/IEC/2022/592-663).
Patients: The study included three cohorts: the first cohort comprised recipients of allogeneic HCT (allo-HCT), the second cohort comprised recipients of autologous HCT (auto-HCT), and the third cohort comprised healthy controls. Thirty-five recipients of allo-HCT and 31 recipients of auto-HCT post-transplantation day+100 were included in the two study cohorts, and 35 healthy individuals were recruited as controls. Written informed consent was obtained from all the participants. Patients with established autoimmune diseases, hypertension, diabetes mellitus, coronary artery disease, cerebrovascular disease, or peripheral artery disease were excluded from the study because of the possibility of underlying endothelial dysfunction arising from their primary illnesses. Detailed histories including diagnosis, date of transplantation, interval between transplantation and assessment of microvasculopathy, conditioning regimen employed, and donor type were retrieved from the HCT case records.
Nailfold video capillaroscopy procedure: Oil immersion images of nailfold capillaries were taken under
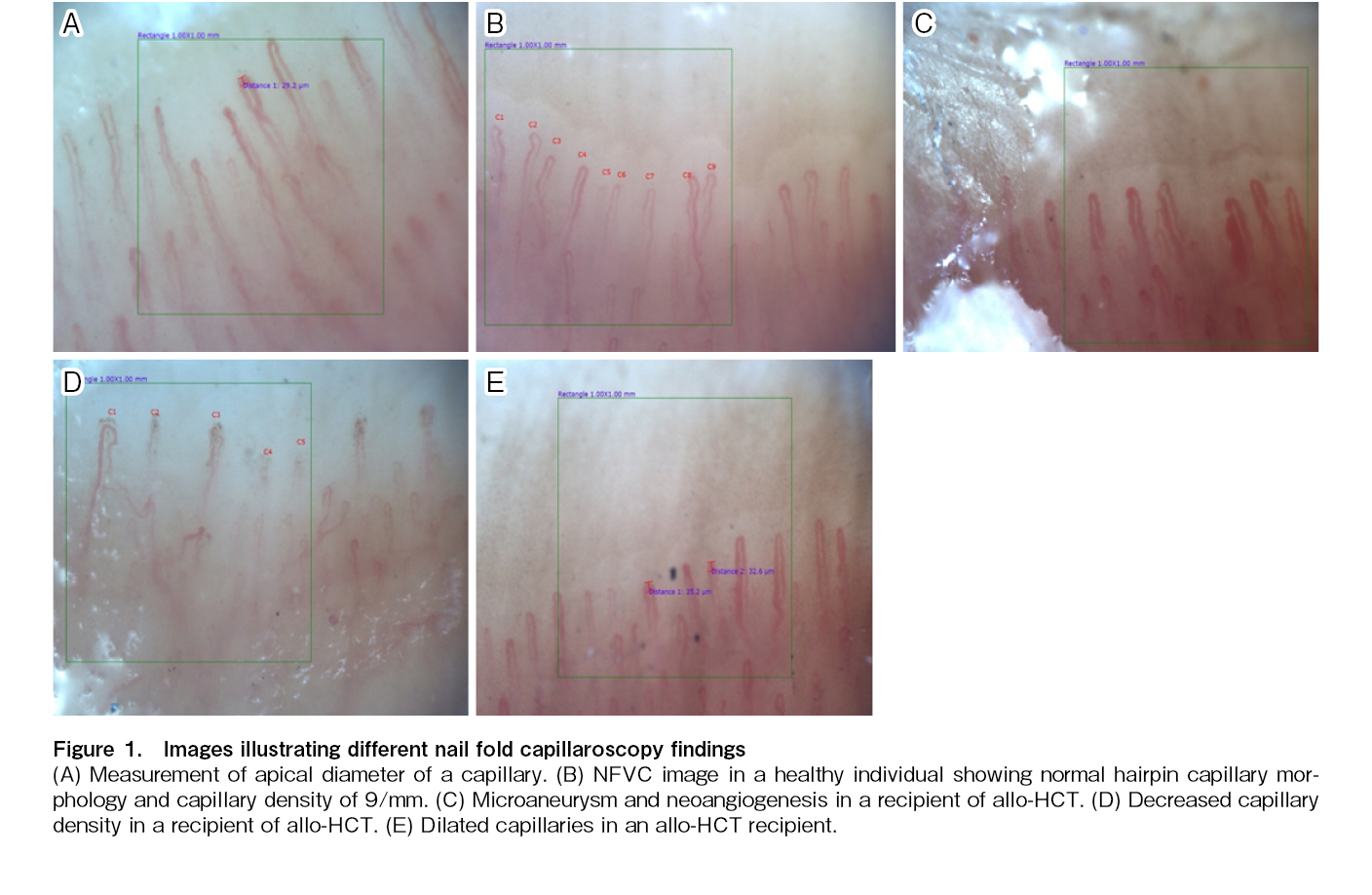
For statistical analysis, the following parameters were considered for each patient. 1. Median capillary density, derived from the capillary density of eight fingers. 2. Median capillary diameter, derived from the maximum capillary apical diameters of eight fingers. 3. Significant neoangiogenesis (presence of neoangiogenesis in greater than two fingers). During NFVC, at least two images of each finger (sparing both thumbs) were acquired for each patient. In total, 1,770 images with acceptable image quality were acquired for analysis.
Flow-mediated dilatation (FMD) of the brachial artery: FMD was measured in the right brachial artery in the supine decubitus position using high-resolution ultrasonography at a controlled room temperature after resting for 10 minutes. All images were acquired in longitudinal sections (2-3 cm above the elbow) using a 10-MHz linear array probe as follows: 1. Baseline images were acquired for 2 minutes, and the basal diameter of the brachial artery was calculated as the average of all measurements collected. 2. Subsequently, a sphygmomanometer cuff was applied distal to the site of FMD data acquisition, and a total occlusion of blood flow was achieved in the arm by inflating the cuff to 200 mmHg for 5 minutes. The cuff was then deflated and the images of flow were recorded for 5 minutes.
The images were processed by an automated edge detection FDA-approved Brachial Analyzer for Research software (Medical Imaging Applications, LLC, Coralville, IA). This software identifies a region of interest (ROI) on the image where the proximal and distal walls of the vessels are the most distinct and uses the optimal graph search-based segmentation technique to measure the vessel diameter as the distance between the intima-lumen interface of the distal and proximal walls. After obtaining the pre- and post-deflation diameters, the FMD value was calculated using the following equation: FMD = (change in arterial diameter ÷ initial baseline diameter of the artery) × 1009. An FMD value of <7.1% was considered abnormally low10. FMD was measured once for each of the 101 study participants. During FMD analysis, the readings of two study participants (one auto-HCT recipient and one healthy control) were excluded because of poor image acquisition.
Statistical analyses: Mean ± standard deviation (SD) was used as the preferred measure of central tendency for continuous variables. The median (range) was used as the preferred measure of the central tendency of discrete variables. Comparisons of the median apical capillary diameter, median capillary density, and FMD values between the allo-HCT cohort, auto-HCT cohort, and healthy controls were conducted using analysis of variance with the Bonferroni post-hoc test. Box plots were used to visualize the findings. Comparisons of abnormal findings, including significant neoangiogenesis, low median capillary density, high median capillary diameter, and low FMD, between subgroups of patients with and without GVHD was conducted using Fisher's exact test. All statistical analyses were performed using DATAtab statistics software (URL:
Results
A total of 101 participants were recruited, including 35 allogeneic HCT recipients, 31 autologous HCT recipients, and 35 healthy controls. The median age of the participants in the allo-HCT cohort was 25 years (range: 9-57 years), 42 years (range: 15-68 years) in the auto-HCT cohort, and 26 years (range: 13-64 years) in the healthy control group. The median duration from transplantation to the assessment of microvasculopathy and endothelial dysfunction was 11 months (range: 3-88 months) in the allo-HCT cohort and 22 months (range: 3-168 months) in the auto-HCT cohort.
In the allo-HCT cohort, 18 patients underwent human leukocyte antigen-matched related donor HCT, and 17 patients underwent haploidentical-related donor HCT. Peripheral blood was used as the source of stem cells in all patients. Of the 35 allo-HCT recipients, 21 (60%) received myeloablative conditioning (MAC) and 14 (40%) received reduced intensity conditioning (RIC). In the allo-HCT cohort, all recipients received cyclosporine containing GVHD prophylaxis regimen. Recipients of matched related donor (MRD) transplants received cyclosporine- and methotrexate-based GVHD prophylaxis. Haploidentical transplant recipients received cyclophosphamide on post-transplantation day
The median capillary diameter was significantly higher in the allo-HCT cohort (mean±SD: 20.56±5.17 micrometer) compared to the auto-HCT cohort (mean±SD: 16.19±3.31 micrometer; p<0.001) and healthy controls (mean±SD: 14.66±2.61 micrometer; p<0.001). There was no significant difference in the median capillary diameter between auto-HCT recipients and healthy controls. The median capillary density was significantly lower in the allo-HCT cohort (median: 6 capillaries/mm, range: 5-9 capillaries/mm) than in the auto-HCT cohort (median: 8.5 capillaries, range: 5-12 capillaries/mm; p<0.001) and healthy controls (median: 8 capillaries/mm, range: 7-10.5 capillaries/mm; p<0.001). There was no statistically significant difference in median capillary density between the auto-HCT cohort and healthy controls. There was no significant difference in FMD values between the cohorts of allogeneic and autologous transplant recipients and the healthy control group. These results are shown in Table 2 and Figure 2.
Compared to the auto-HCT cohort and healthy controls, the allo-HCT cohort had a higher proportion of patients with low median capillary density (<7 capillaries/mm), high median capillary diameter (>20 micrometer), and significant neoangiogenesis (neoangiogenesis present in greater than two fingers). In the allo-HCT cohort, 10 patients (28%) had abnormally low FMD values compared to only one patient (3%) in the auto-HCT cohort and two healthy controls (6%). As there was only one observation in the auto-HCT cohort, this variation was not statistically significant. These results are summarized in Table 3.
We compared the NFVC variables and FMD values between the subgroups of patients receiving MAC and RIC regimens in the allo-HCT cohort. The median capillary diameter and FMD values were not significantly different between the two subgroups. The results are shown in Table 4.
In our study, 28 (80%) allo-HCT recipients experienced some form of GVHD before vasculopathy assessment. We compared the distributions of low median capillary density, high median capillary diameter, significant neoangiogenesis, and low FMD between allo-HCT recipients with and without a history of GVHD using Fisher's exact test. We found significantly higher neoangiogenesis in the subgroup with a history of GVHD than in the subgroup without GVHD (p=0.044). There were no significant differences in low median capillary density, high median capillary diameter, or low FMD between the two subgroups. The results are shown in Table 5.
Discussion
In this study, we employed a novel approach for the non-invasive in vivo real-time assessment of structural microvasculopathy and endothelial dysfunction in post-transplantation recipients via nailfold video capillaroscopy (NFVC) and flow-mediated dilatation (FMD) that is a surrogate for endothelial nitric oxide production.
We found evidence of significant structural microvasculopathy in recipients of allogeneic HCT compared to recipients of auto-HCT and healthy controls. The median capillary diameter and number of fingers with dilated capillaries were significantly higher in allo-HCT recipients. Similarly, the number of neovessels was significantly higher in the allo-HCT cohort than in the auto-HCT cohort and healthy controls. A higher proportion of patients in the allo-HCT cohort (51%) had a reduced capillary density (< 7 capillaries/mm) than those in the auto-HCT cohort (19%) and healthy controls (0%). Our results showed no significant differences in NFVC parameters or FMD values between allo-HCT recipients receiving MAC and those receiving RIC conditioning. Patients with GVHD had significantly more neoangiogenesis than those devoid of any form of GVHD; however, the number of patients without GVHD was too small for drawing any meaningful conclusions. These findings suggest an ongoing process of pathological vascular remodeling in allo-HCT recipients and that alloreactivity and calcineurin inhibitors are probable pathogenic factors in this microvascular restructuring.
To our knowledge, this is the first study to describe the abnormalities in different NFVC parameters in HCT recipients. In this study, we detected microvascular abnormalities in transplant recipients in real-time using non-invasive methods that can be performed at the bedside or in a transplant clinic. In line with our findings, Cordes et al. have also reported microvascular abnormalities, including capillary dilatation and increased capillary branching in colonic biopsies of patients with acute GVHD7.
Microangiopathies, including dilated loops, giant loops, reduced capillary density, avascular regions, neoangiogenesis, and microhemorrhages, appear early in the course of systemic sclerosis, even before the onset of clinical manifestations11,12. Nailfold capillaroscopy is an established tool to detect these microvascular changes13, and its findings provide prognostic information that predicts the future clinical course of the disease14–16. Interestingly, the basic pathogenesis of post-allo-HCT events, such as acute and chronic GVHD, is immune mediated, similar to systemic sclerosis. Chronic GVHD shares several clinical similarities with systemic sclerosis, including sclerotic skin changes, morphea, skin depigmentation, and sicca symptoms. Even in the absence of defined GVHD, alloreactivity persisted in allo-HCT recipients. Interestingly, in this study, we demonstrated microangiopathies in allo-HCT recipients, including decreased capillary density, capillary dilatation, and neoangiogenesis. However, this comparison between allo-HCT and systemic sclerosis has some limitations. First, systemic sclerosis is an autoimmune disease, whereas allo-HCT recipients experience acute and chronic GVHD due to the alloreactivity of donor immune cells against the host. Second, there are many differences in clinical manifestations between systemic sclerosis and GVHD. Third, the kinetics of clinical progression are markedly different. Systemic sclerosis has a long preclinical stage, and microvascular changes appear long before the clinical onset of disease. However, in our study, recent recipients of allo-HCT also showed microvascular changes in NFVC. Capillary dilatation and aberrant neoangiogenesis can explain the frequent clinical observation of skin erythema in allo-HCT recipients, especially in those with chronic skin GVHD.
The overall absence of significant abnormalities in recipients of auto-HCT and the absence of significant differences in NFVC parameters and FMD values between recipients of MAC and RIC conditioning regimens in the allo-HCT cohort argue against the impact of a cytotoxic chemotherapy burden on the development of structural microvasculopathy. Another possible explanation for the lack of significant microvascular changes in auto-HCT recipients is the inclusion of cases post-transplantation day+100. It is possible that cytotoxic chemotherapy-related endothelial injury may be an acute phenomenon, and by day+100, these changes are reversed due to endothelial remodelling.
To our knowledge, this is the first study that has employed FMD to assess endothelial dysfunction in HCT recipients. We demonstrated that a higher proportion of patients in the allo-HCT cohort had abnormally low FMD values (< 7.1%); however, this was not statistically significant owing to the relatively small sample size. In a case-control study, Routhu et al. showed a significant reduction in brachial artery FMD in children with Kawasaki disease during the acute and convalescent phases compared with healthy children. This study demonstrated the utility of FMD in detecting immune-mediated endothelial dysfunction17.
Our study has several limitations, including its cross-sectional design and small sample size. Due to the small number of participants in each group, we could not assess the impact of specific disease groups, donor types, or conditioning regimens on microvasculopathy. This study was not designed to assess the effect of calcineurin inhibitors and GVHD on microvasculopathy or endothelial dysfunction.
In conclusion, our results show that structural microvasculopathy, including capillary dilatation, neoangiogenesis, and reduced capillary density, are frequent in recipients of allo-HCT. This observation indicates that alloreactivity plays a pathophysiological role in vascular damage as well as pathological angiogenesis that occur in parallel and result in the restructuring of the microvasculature. NFVC is a non-invasive in vivo real-time assessment tool for microvasculopathy in allo-HCT recipients. Our results may serve as a foundation for the use of NFVC and FMD in future studies to explore the association and temporal relationships between different microvascular abnormalities and post-transplant complications of endothelial origin, including veno-occlusive disease and GVHD.
Acknowledgments
The authors acknowledge all the participants who actively participated in the experiments.
Author Contributions
SSR, GP, and MS contributed to the concept and design of the study; SSR, GP, RS, AS, and MS performed the experiments and gathered the readings.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
The authors acknowledge all the participants who actively participated in the experiments.
Ethical Approval
The study was reviewed by the Institute Ethics Committee and approved (letter no: INT/IEC/2022/592-663).
Acknowledgments
The authors acknowledge all the participants who actively participated in the experiments.
Informed Consent
A written informed consent was obtained from each patient.
References
1.Florey L. The endothelial cell. Br Med J. 1966; 2: 487-90.
2.Ottersbach K. Endothelial-to-haematopoietic transition: an update on the process of making blood. Biochem Soc Trans. 2019; 47: 591-601.
3.Perlin JR, Sporrij A, Zon LI. Blood on the tracks: hematopoietic stem cell endothelial cell interactions in homing and engraftment. J Mol Med (Berl). 2017; 95: 809-19.
4.Varma A, Rondon G, Srour SA, Chen J, Ledesma C, Champlin RE, et al. Endothelial activation and stress index (EASIX) at admission predicts fluid overload in recipients of allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2020; 26: 1013-20.
5.Dai H, Penack O, Radujkovic A, Schult D, Majer-Lauterbach J, Blau IW, et al. Early Bilirubinemia after allogeneic stem cell transplantation―an endothelial complication. Bone Marrow Transplant. 2021; 56: 1573-83.
6.Palomo M, Diaz-Ricart M, Carbo C, Rovira M, Fernandez-Aviles F, Martine C, et al. Endothelial dysfunction after hematopoietic stem cell transplantation: role of the conditioning regimen and the type of transplantation. Biol Blood Marrow Transplant. 2010; 16: 985-93.
7.Cordes S, Mokhtari Z, Bartosova M, Mertlitz S, Riesner K, Shi Y, et al. Endothelial damage and dysfunction in acute graft-versus-host disease. Haematologica. 2021; 106: 2147-60.
8.Smith V., Vanhaecke A., Herrick A.L., Distler O., Guerra M.G., Denton C.P., et al. Fast track algorithm: How to differentiate a “scleroderma pattern” from a “non-scleroderma pattern”. Autoimmun Rev. 2019; 18: 102394.
9.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: A report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002; 39: 257-65.
10.Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, et al. Endothelial Dysfunction, Increased Arterial Stiffness, and Cardiovascular Risk Prediction in Patients With Coronary Artery Disease: FMD-J (Flow-Mediated Dilation Japan) Study A. J Am Heart Assoc. 2018; 7: e008588.
11.Bredemeier M, Xavier RM, Capobianco KG, Restelli VG, Rohde LE, Pinotti AF, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol. 2004; 31: 286-94.
12.Grassi W, Medico PD, Izzo F, Cervini C. Microvascular involvement in systemic sclerosis: capillaroscopic findings. Semin Arthritis Rheum. 2001; 30: 397-402.
13.Tiev KP, Cabane J. Digestive tract involvement in systemic sclerosis. Autoimmun Rev. 2011; 11: 68-73.
14.Caramaschi P, Canestrini S, Martinelli N, Volpe A, Pieropan S, Ferrari M, et al. Scleroderma patients nailfold videocapillaroscopic patterns are associated with disease subset and disease severity. Rheumatology (Oxford). 2007; 46: 1566-9.
15.Bredemeier M, Xavier RM, Capobianco KG, Restelli VG, Rohde LE, Pinotti AF, et al. Nailfold capillary microscopy can suggest pulmonary disease activity in systemic sclerosis. J Rheumatol. 2004; 31: 286-94.
16.Chen ZY, Silver RM, Ainsworth SK, Dobson RL, Rust P, Maricq HR. Association between fluorescent antinuclear antibodies, capillary patterns, and clinical features in scleroderma spectrum disorders. Am J Med. 1984; 77: 812-22.
17.Routhu SK, Singhal M, Jindal AK, Kumar V, Yadav AK, Singh S. Assessment of Endothelial Dysfunction in Acute and Convalescent Phases of Kawasaki Disease Using Automated Edge Detection Software: A Preliminary Study From North India. J Clin Rheumatol. 2021; 27: 143-9.
Search
News