Volume 7 (2024) Issue 4 No.2 Pages 106-110
Abstract
Background: Muscle involvement, termed polymyositis, is an uncommon manifestation of graft-versus-host disease (GvHD) in which the upper and lower limbs are commonly affected. However, respiratory failure due to diaphragmatic weakness has rarely been reported. Diagnosis is usually based on a combination of elevated muscle enzyme levels in the blood, neurophysiological studies, and muscle biopsies.
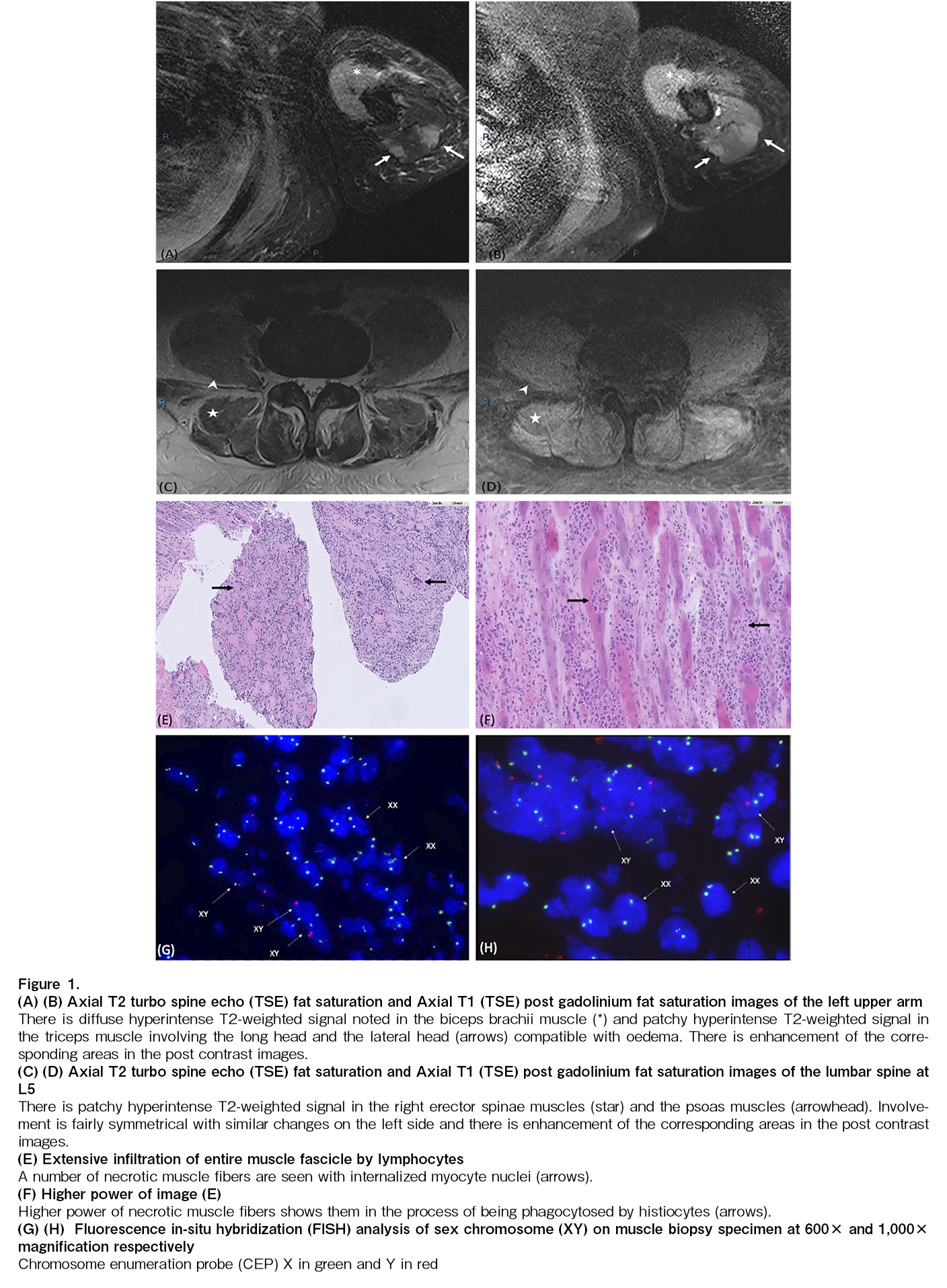
Case report: A 23-year-old man who presented with Philadelphia chromosome (Ph)-positive chronic myeloid leukemia in myeloid blast crisis, underwent HLA-matched sibling (sister) hematopoietic stem cell transplantation. Six months post-transplant, he experienced bilateral arm pain and weakness, with an inability to raise his limbs against gravity. He was also unable to sit erect, and was dyspneic and hypoxic, thus requiring oxygen supplementation. Serum muscle enzyme levels were found to be markedly elevated. Magnetic resonance imaging showed a patchy hyperintense T2-weighted signal and enhancement in the muscle groups of the limbs, as well as in the psoas and erector spinae muscles. The electromyogram results were consistent with those of inflammatory myopathy. Muscle biopsy revealed extensive necrotizing myositis with extensive lymphocyte infiltration throughout the muscle fascicle. Additionally, fluorescence in situ hybridization (FISH) analysis demonstrated that 30% of the nuclei scored were in the muscle fibers of recipient XY origin, and 70% were in T-lymphocytes of donor XX origin. GvHD polymyositis was diagnosed, and the patient responded well to corticosteroids and extracorporeal photopheresis.
Conclusion: GvHD polymyositis can affect various muscle groups and results in various clinical presentations. In our case, truncal involvement resulting in an inability to sit erect was a unique presentation. Prompt diagnosis is important, and we have highlighted a comprehensive multimodal approach, including the potential use of FISH analysis, to aid in diagnosis.
Introduction
Graft-versus-host disease (GvHD) is a major complication of allogeneic hematopoietic stem cell transplantation (alloHCT)1. It is a multisystem disorder of immune dysregulation that commonly affects the skin, gastrointestinal tract and liver2. Uncommonly, muscle involvement, termed polymyositis, can occur3. Upper and lower limb muscles are usually involved, and the patients present with proximal weakness, muscle tenderness, and elevated creatine phosphokinase/aldolase enzyme levels. Respiratory failure due to diaphragmatic weakness has been reported in some cases4. Theoretically, all muscle groups can be involved; however, GvHD polymyositis affecting the truncal muscles has not been reported. We present a case of extensive GvHD polymyositis affecting both the respiratory and truncal muscles and highlight the potential role of fluorescence in situ hybridization (FISH) and chimerism analysis in supporting the diagnosis of GvHD-associated myositis.
Case presentation
A 23-year-old man with Philadelphia chromosome (Ph)-positive chronic myeloid leukemia in myeloid blast crisis underwent HLA-matched sibling (sister) hematopoietic stem cell transplantation (HCT) during his first complete cytogenetic remission. Peripheral blood stem cell transplantation was performed with ABO mismatch (Recipient B+, Donor O+) and a total CD34+ cell dose of 5.56 × 106/kg. He received busulfan and cyclophosphamide as myeloablative preparative regimens, and tacrolimus and methotrexate for graft-versus-host disease (GvHD) prophylaxis. BCR-ABL was detected by qPCR at 0.0134% on day 28 post-transplantation, which quickly increased to 0.55% on day 56 with a T315I mutation, and ponatinib 15 mg daily was initiated thereafter. Four months post-transplantation, the patient developed grade II GvHD of the skin and liver, which responded to a tapering course of oral prednisolone. All immunosuppressants were discontinued six months post-transplantation. At 7 months post-transplantation, the patient presented with fatigue, bilateral arm pain, and weakness, which quickly progressed to proximal myopathy with an inability to raise his limbs against gravity. He was also unable to maintain an erect posture and was dyspneic and hypoxic, thus requiring oxygen supplementation. Sensory and cerebellar examinations were normal. Undertaken serum investigations revealed a markedly raised creatinine phosphokinase (CPK) of 5,105 U/L (upper limit of normal (ULN): 336 U/L) and aldolase of 51.9 U/L (ULN: 6.3 U/L). Magnetic resonance imaging (MRI) of the spine and upper limbs revealed patchy hyperintense T2-weighted signals and enhancement in the psoas muscle, erector spinae muscles, bilateral gluteal muscles, and bilateral upper arm and forearm muscle groups, suggestive of polymyositis (Figure 1A-D). Arterial blood gas showed type 1 respiratory failure, likely due to diaphragmatic involvement. Electromyogram findings were consistent with those of inflammatory myopathy. Muscle biopsy showed extensive necrotizing myositis involving the entire muscle fascicle, with extensive infiltration by numerous lymphocytes and a few macrophages/histiocytes (Figure 1E and F). The lymphocytes were predominantly T-lymphocytes with an estimated CD4:CD8 ratio of 2:1. Given the context of full donor chimerism and the karyotype of the female donor origin in the blood and marrow, we employed fluorescence in situ hybridization (FISH) analysis to further differentiate the donor vs. recipient origin of the lymphocytes. FISH using chromosome centromere probe (CEP) X and CEP Y sets on immunohistochemically stained muscle tissue specimens revealed that 30% of the nuclei scored were in the muscle fibers of recipient XY origin, and 70% were of T-lymphocytes of donor XX origin. (Figure 1G and H). A diagnosis of immune-mediated, GVHD-associated polymyositis was made. He was started on 2 mg/kg/day (100 mg twice daily) methylprednisolone, with a response observed within 2 weeks. His muscle enzymes (creatine kinase and aldolase) normalized and he subsequently regained power in his limbs and truncal strength, allowing him to participate in intensive rehabilitation. Unfortunately, with the initiation of steroids, his BCR-ABL levels started to increase, suggesting a molecular relapse of his disease. To allow quick tapering of the steroids to harness the graft versus leukaemia effect, extracorporeal photopheresis (ECP) was initiated early as an adjunct treatment for his GvHD polymyositis. Biochemical relapse was observed during the tapering of steroids, which quickly responded to an increase in the frequency of ECP and steroid up-titration. The patient's progress is shown in Figure 2. After 2 months of intensive rehabilitation, the patient was discharged from the hospital. Unfortunately, 1 month after he was discharged, he suffered a relapse of leukemia with more than 20% blasts in his bone marrow and an increase in BCR-ABL to 54.23%. The patient was treated with salvage chemotherapy FLAG (fludarabine, high-dose cytarabine), and a stem cell top-up dose. He subsequently succumbed to the refractory disease 11 months after transplantation.
Discussion
GvHD-associated polymyositis, first described in the 1980s, is an uncommon manifestation of chronic GvHD, with an estimated frequency of 3.5% to 7.6%4. Proximal myopathy with limb pain has been invariably reported; however, in rare situations, the involvement of the respiratory muscles can cause type 1 respiratory failure5. Our case scenario highlights the unique clinical presentation of the involvement of various muscle groups including the erector spinae and respiratory muscles. Depending on the muscle group, patients can have varied clinical presentations; thus, clinicians should have a high index of suspicion for this uncommon entity. A recent review of the atypical features of chronic GvHD was reported by the 2020 National Institutes of Health Consensus Project Task force in 20226. Myositis is an atypical rather than a defined chronic manifestation of GvHD. Differentials such as autoimmune myositis and steroid myopathy can present similarly, and a comparison with chronic GvHD myositis has been well enumerated in the report. This emphasizes the need for thorough investigations, with tissue biopsy often necessary to ensure an accurate diagnosis and correct attribution of GvHD. The use of peripheral blood stem cells, female donors to male recipients, and well-known risk factors for chronic GvHD7 were the contributing factors in our patient. Ponatinib, which our patient was administered prior to his presentation until his death, has been reported to be associated with rare cases of myositis8, 9. While we were unable to definitively exclude this rare side effect of ponatinib, our patient's improvement and response to immunosuppression and ECP while continuing ponatinib make GvHD a more likely etiology. To the best of our knowledge, this case report is the first to outline a comprehensive approach that incorporates the knowledge of donor chimerism, karyotype/FISH analysis, and immunohistochemistry, together with standard imaging and histological investigations, in order to aid in the prompt diagnosis of GvHD-associated polymyositis. In 2000, Au et al. reported a case of severe skin GvHD in a female patient who underwent liver transplantation from a cadaveric male donor. Using Y chromosome-specific FISH, male lymphocytes were observed in 90% of the lymphocytes infiltrating the dermal-epidermal junction in the skin biopsy to confirm the diagnosis10. Similarly, in our patient, who received PBSCT from his sister, FISH analysis revealed female (XX) lymphocytes surrounding the patient's (XY) muscle cells, supporting the diagnosis of GvHD-associated polymyositis.
Various immunosuppressive therapies with corticosteroids as first-line treatment have been used to treat GvHD polymyositis. The use of high-dose corticosteroids in our case was accompanied by an increasing BCR-ABL levels. ECP was initiated as an additional treatment for GvHD polymyositis in order to allow quicker steroid tapering and to reduce the suppression of graft-versus-leukemic effects. ECP has previously demonstrated good response rates in the treatment of cGvHD11; however, its use has only been reported in two patients with GvHD polymyositis, with benefits seen in one of the two patients12, 13. Our patient responded well to a combination of ECP and immunosuppression, with improvement in both limb and truncal weakness, as well as respiratory status.
In conclusion, GvHD polymyositis is an uncommon complication of allogeneic hematopoietic stem cell transplantation, with the possibility of various clinical presentations. Prompt diagnosis is important, and we highlight a comprehensive multimodal approach to aid in the diagnosis.
Author Contributions
JYT and HT drafted the original manuscript. HT contributed to the conception and design of the report. ML and LPC provided the relevant images. JYT, JKSQ, and HT contributed to patient care. All authors read and approved the final manuscript.
Conflicts of Interest
WYKH is editor of Blood Cell Therapy. He is not involved in the editorial evaluation and the decision to accept this article for publication. The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Consent for Publication
Written informed consent was obtained from the patient.
References
1.Zeiser R, Blazar BR. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med. 2017; 377: 2565-79.
2.Rozmus J. Monogenic Immune Diseases Provide Insights Into the Mechanisms and Treatment of Chronic Graft-Versus-Host Disease. Front Immunol. 2021; 11: 574569.
3.Shahzad M, Chaudhary SG, Basit A, Thellman C, Rodriguez L, Abhyankar SH, et al. Chronic graft-versus-host disease presenting as acute polymyositis: A case series and systematic review. Transpl Immunol. 2022; 70: 101520.
4.Limaye S, Limaye V. Clinical Characteristics of Myositis Associated with Graft-Versus-Host Disease. Curr Rheumatol Rep. 2021; 23: 30.
5.Stephenson AL, Mackenzie IR, Levy RD, Road J. Myositis associated graft-versus-host-disease presenting as respiratory muscle weakness. Thorax. 2001; 56: 82-4.
6.Cuvelier GDE, Schoettler M, Buxbaum NP, Pinal-Fernandez I, Schmalzing M, Distler JHW, et al. Toward a Better Understanding of the Atypical Features of Chronic Graft-Versus-Host Disease: A Report from the 2020 National Institutes of Health Consensus Project Task Force. Transplant Cell Ther. 2022; 28: 426-45.
7.Amanan I, Otoukesh S, Al Malki MM, Salhotra A. Chronic GVHD: review advances in prevention, novel endpoints, and targeted strategies. Hematology Am Soc Hematol Educ Program. 2023; 2023: 164-70.
8.Cortes JE, Kim DW, Pinilla-Ibarz J, le Coutre PD, Paquette R, Chuah C, et al. Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial. Blood. 2018; 132: 393-404.
9.Srivastava A, Singla DK. PTEN-AKT pathway attenuates apoptosis and adverse remodeling in ponatinib-induced skeletal muscle toxicity following BMP-7 treatment. Physiol Rep. 2023; 11: e15629.
10.Au WY, Ma SK, Kwong YL, Ng IO, Hawkins BR, Wan TS, et al. Graft-versus-host disease after liver transplantation: documentation by fluorescent in situ hybridisation and human leucocyte antigen typing. Clin Transplant. 2000; 14: 174-7.
11.Choi SW, Levine JE, Ferrara JL. Pathogenesis and management of graft-versus-host disease. Immunol Allergy Clin North Am. 2010; 30: 75-101.
12.Golec S, Rabinovich E, Cohen M, Baer L, Chamoun K, Lima Md. Refractory inflammatory myopathy in hematopoietic stem cell transplant patients with chronic graft-versus-host disease: report of two cases. Hematol Transfus Cell Ther. 2019; 41: 268-71.
13.Leano AM, Miller K, White AC. Chronic graft-versus-host disease-related polymyositis as a cause of respiratory failure following allogeneic bone marrow transplant. Bone Marrow Transplant. 2000; 26: 1117-20.
Search
News