Volume 7 (2024) Issue 2 No.3 Pages 41-48
Abstract
Mycophenolate mofetil (MMF), in combination with a calcineurin inhibitor, is used as the prophylaxis for graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). Compared to intravenous methotrexate (MTX), MMF is associated with a lower incidence of mucositis and shorter time for hematopoietic engraftment but comparable incidence of acute GVHD, resulting in the preferred use of MMF for GVHD prophylaxis in elderly patients or those undergoing cord blood transplantation (CBT). Although several studies have evaluated the clinical impact of MTX omission due to toxicity after allogeneic HCT, the impact of oral MMF interruption for GVHD prophylaxis on transplant outcomes remains unclear. Therefore, in this study, we retrospectively analyzed the consecutive data of adult patients who underwent single-unit unrelated CBT and received oral MMF in combination with cyclosporine for GVHD prophylaxis at our hospital. Among the 53 patients, the planned dose of MMF was interrupted in 14 with a median of 19.5 d (range, 3-27 d) of CBT. In multivariate analysis, MMF interruption, which was treated as a time-dependent covariate, was significantly associated with poorer overall survival (hazard ratio [HR], 5.41; 95% confidence interval [CI], 2.03-14.43; P < 0.001) and higher non-relapse mortality (HR, 7.56; 95% CI, 1.99-28.79; P = 0.002). Further studies with larger cohorts are necessary to confirm the clinical significance of oral MMF interruption in GVHD prophylaxis.
Introduction
In combination with a calcineurin inhibitor, mycophenolate mofetil (MMF) is used for the prophylaxis of graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (HCT). Compared to intravenous methotrexate (MTX), MMF is associated with a lower incidence of mucositis and shorter time to hematopoietic engraftment but comparable incidence of acute GVHD1–3, resulting in the preferred use of MMF for GVHD prophylaxis in elderly patients4–6 or those undergoing cord blood transplantation (CBT)5–8. In contrast to other countries, MMF has only been approved as an oral formulation in Japan. However, some patients are unable to take oral MMF mainly because of regimen-related toxicity (RRT). Although several studies have evaluated the clinical impact of omitting planned MTX due to toxicity after allogeneic HCT9–15, no study has evaluated the clinical impact of interrupting planned oral MMF on GVHD prophylaxis. Therefore, in this study, we investigated the clinical influence of oral MMF interruption on CBT outcomes at our hospital.
Materials and Methods
Patients and transplant procedures
We retrospectively analyzed the consecutive data of 53 adult patients who underwent single-unit unrelated CBT and received planned oral MMF in combination with cyclosporine for GVHD prophylaxis between November, 2013 and March, 2023 at our hospital. GVHD prophylaxis consisted of intravenous cyclosporine (3 mg/kg/day from day -1) and oral MMF (30 mg/kg/day from days 0 to 27)16. Unrelated cord blood was supplied by cord blood banks in Japan. The cord blood unit, conditioning regimen, GVHD prophylaxis, and supportive care were determined by the treating physicians16–20. The Institutional Review Board of the Institute of Medical Science, the University of Tokyo approved this retrospective study (2023-33-0810) and the adoption of an opt-out consent mechanism.
Definitions
Neutrophil recovery was defined as the recovery achieved on the first three consecutive days when the absolute neutrophil count was higher than 0.5 × 109/L. Platelet recovery was defined as that achieved on the first seven consecutive days when the platelet count was higher than 20 or 50 × 109/L without platelet transfusion support. Diagnosis and grading of acute and chronic GVHD were based on the standard criteria21, 22. Overall survival (OS) was defined as the time from CBT to death, subsequent allogeneic HCT, or the date of last contact with patients who were lost to follow-up. Non-relapse mortality (NRM) was defined as the time from CBT to death without disease relapse. The number of human leukocyte antigen (HLA) disparities between the cord blood grafts and recipients was defined as low resolution for HLA-A, HLA-B, and HLA-DR in the graft-versus-host direction. HCT-specific comorbidity index (HCT-CI)23 and refined disease risk index (rDRI)24 were classified according to published criteria.
Statistical analyses
Groups were compared using the Mann-Whitney U test for continuous variables and Fisher's exact test for categorical variables. The effects of MMF interruption on OS and NRM were graphically illustrated using the Simon-Makuch plots. Multivariate analysis was conducted using the Cox proportional hazards model for overall mortality, and the Fine and Gray model for NRM, neutrophil recovery, platelet recovery, grades II-IV acute GVHD, grades III-IV acute GVHD, overall chronic GVHD, and extensive chronic GVHD. Multivariate analysis involved the following factors as covariates: MMF interruption (yes vs. no), which was treated as a time-dependent covariate, age (<65 vs. ≥65 years), gender (male vs. female), HCT-CI (<3 vs. ≥3), rDRI (low/intermediate vs. high/very high), cryopreserved cord blood total nucleated cell (TNC) dose (<2.5 × 107/kg vs. ≥2.5 × 107/kg), and low-resolution HLA disparities in the graft-versus-host direction (0, 1 vs. 2). P-values < 0.05 were considered to be statistically significant. Statistical analyses were conducted using EZR version 1.6125.
Results
Patient characteristics
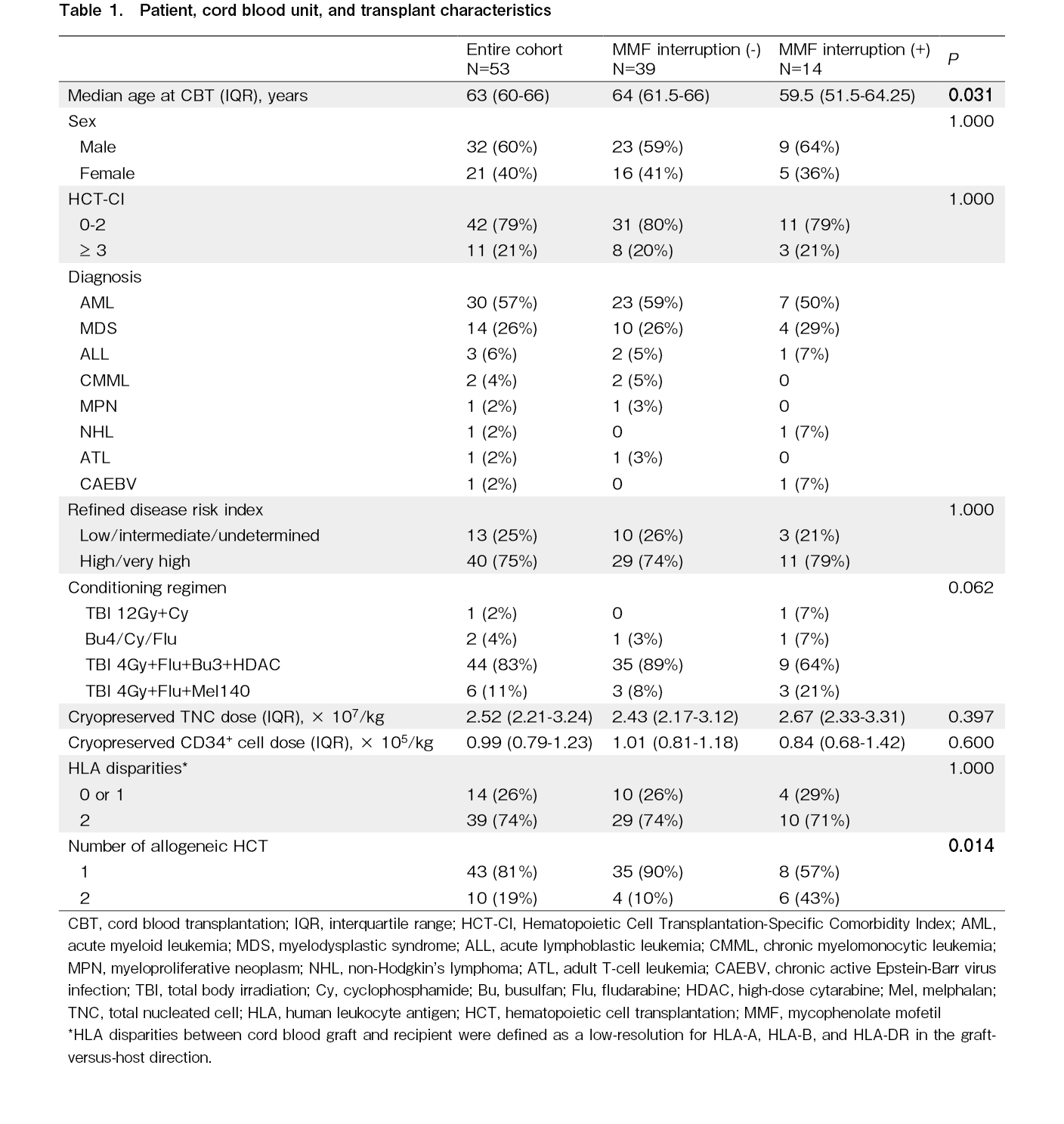
All patient and CBT characteristics are presented in Table 1. The median age of the entire cohort was 63 years (interquartile range [IQR], 60-66 years). The most common disease was acute myeloid leukemia (57%). Disease risk defined by rDRI was high or very high in 75% of the patients. The majority of conditioning regimens included 180 mg/m2 fludarabine, 9.6 mg/kg intravenous busulfan, 4 Gy total body irradiation, and 12 g/m2 high-dose cytarabine (83%)16. The median cryopreserved cord blood TNC dose was 2.52 × 107/kg (IQR, 2.21-3.24 × 107/kg), and the median cryopreserved cord blood CD34+ cell dose was 0.99 × 105/kg (IQR, 0.79-1.23 × 105/kg). Ten patients (19%) had previously undergone allogeneic HCT.
Among the 53 patients, the planned dose of MMF was interrupted in 14 with a median of 19.5 d (range, 3-27 d) of CBT. The patients in whom the planned dose of MMF was interrupted were young (P=0.031) and had previously undergone allogeneic HCT (P=0.014). The main causes of MMF interruption were mucositis and vomiting due to RRT (n=8), general malaise due to organ failure or infection (n=3), alveolar hemorrhage (n=1), encephalitis (n=1), and engraftment failure (n=1). No additional immunosuppressants were administered to the patients during MMF interruption.
Association of MMF interruption with hematopoietic recovery and GVHD
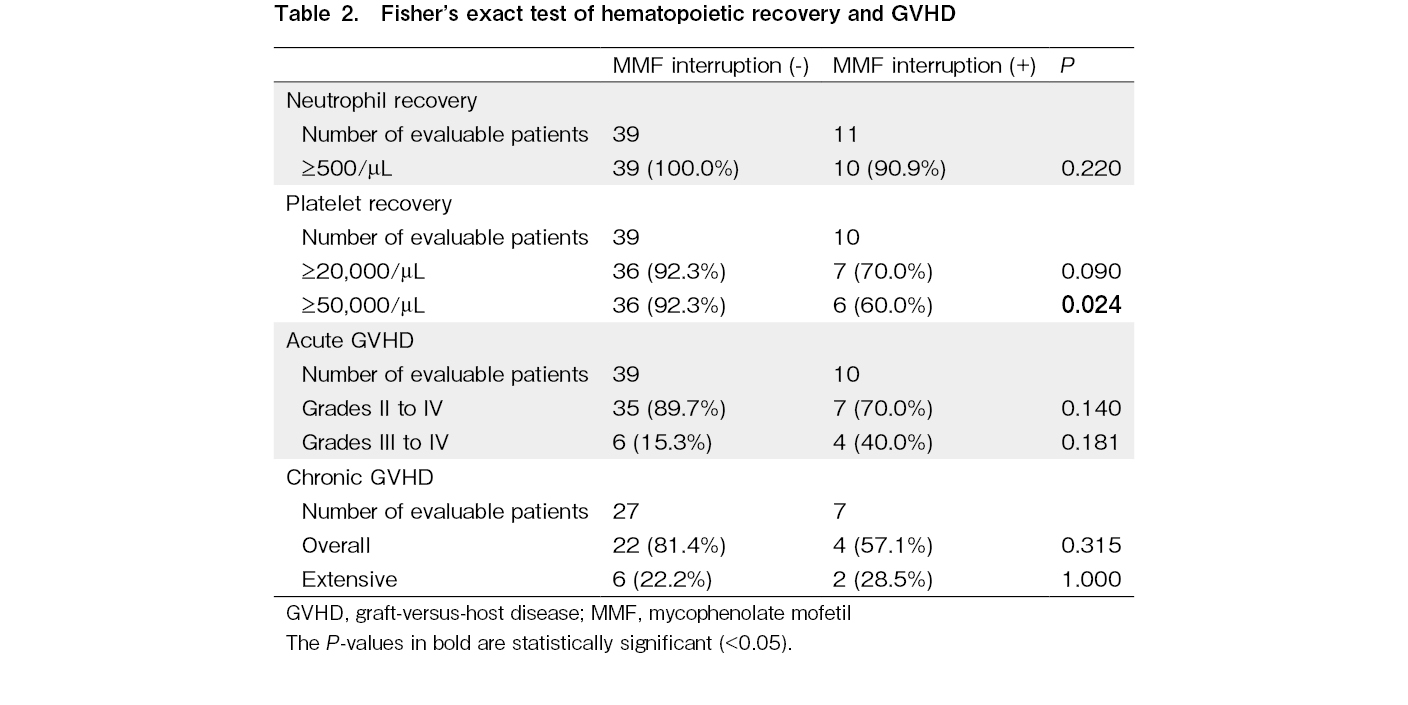
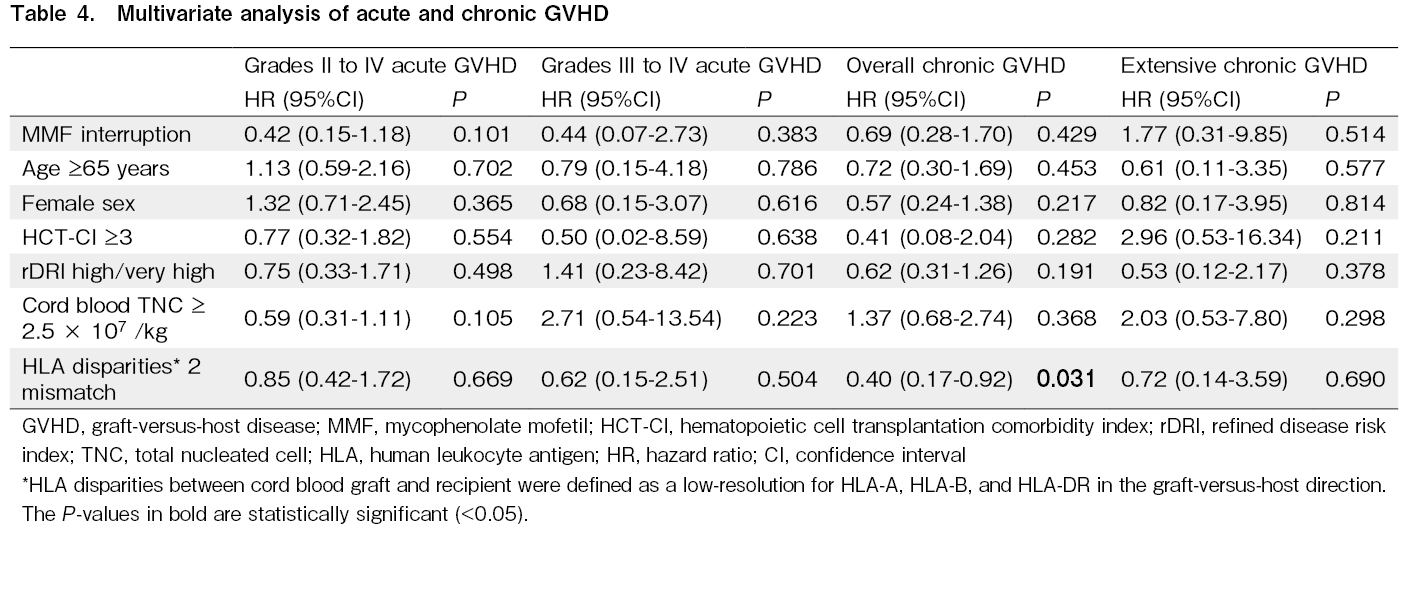
In Fisher's exact test, MMF interruption was associated with lower platelet recovery rate, which was defined as ≥ 50,000/μL (P=0.024), but not the rates of neutrophil recovery (P=0.220), grades II-IV acute GVHD (P=0.140), grades III-IV acute GVHD (P=0.181), overall chronic GVHD (P=0.315), and extensive chronic GVHD (P=1.000; Table 2). In the multivariate analysis, MMF interruption, which was treated as a time-dependent covariate, was significantly associated with lower platelet recovery, which was defined as
Impact of MMF interruption on OS and NRM
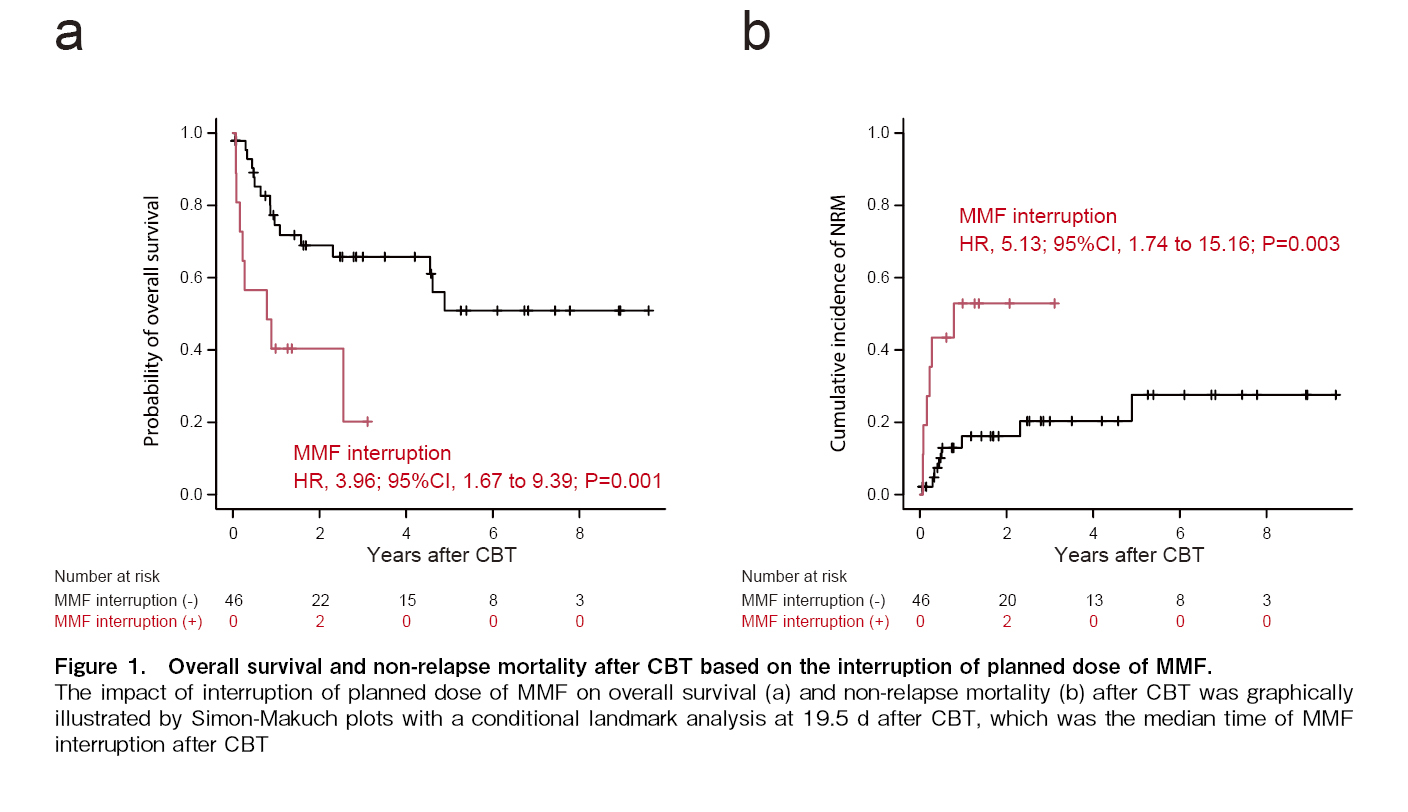
In univariate analysis, MMF interruption, which was treated as a time-dependent covariate, was significantly associated with poorer OS and higher NRM (Figure 1). Multivariate analysis also revealed that MMF interruption was significantly associated with poorer OS (HR, 5.41; 95% CI, 2.03-14.43; P < 0.001) and higher NRM (HR, 7.56; 95% CI, 1.99-28.79; P = 0.002; Table 5).
Among the 14 patients in whom the planned dose of MMF was discontinued, 10 died during the last follow-up. The causes of death were pneumonia in 3 patients, relapse in 2, alveolar hemorrhage in 1, gastrointestinal hemorrhage in 1, acute GVHD in 1, multiple organ failure in 1, and sepsis in 1.
Discussion
Previous studies have demonstrated the clinical impact of MTX omission day 11 due to toxicity after allogeneic HCT; however, their results are controversial, mainly because of the small sample size9–15. A recent meta-analysis by Kharfan-Dabaja et al. demonstrated that day 11 omission of MTX was associated with poor OS, but not NRM, in acute or chronic GVHD15. For GVHD prophylaxis, MMF is started at 15-45 mg/kg orally or intravenously twice or thrice a day starting on day 0 and continued for 27-40 d until termination or tapered down through days 96-18026–30. However, the ideal MMF concentration, dosage schedule, and treatment duration for GVHD prevention remain unclear31, 32. Our study is the first to evaluate the clinical impact of interrupting planned oral MMF treatment on transplant outcomes. We found that MMF interruption led to poor platelet recovery, poor OS, and high NRM after CBT, but did not affect the incidence of acute and chronic GVHD. However, our results should be interpreted cautiously as most patients with interrupted MMF did not take any other oral drugs or diet. Poor oral intake alters the microbiota diversity and composition, resulting in a high incidence of gastrointestinal GVHD and poor clinical outcomes33, 34. Therefore, although intravenous MMF is safe and effective for GVHD prophylaxis35, 36, whether the use of intravenous MMF overcomes the negative effects of oral MMF interruption remains unclear.
Here, our data showed that MMF interruption did not affect the incidence of acute or chronic GVHD, which is consistent with a meta-analysis evaluating the clinical impact of day 11 MTX omission due to toxicity after allogeneic HCT15. Only one patient in whom the planned dose of MMF was interrupted died due to acute GVHD. Interruption of planned oral MMF for GVHD prophylaxis may be associated with the development of severe RRT. Indeed, most patients in whom the planned dose of oral MMF was administered exhibited significant organ damage, which may have contributed to a higher NRM apart from GVHD. Previous studies have shown the significant impact of early complications on subsequent complications after allogeneic HCT37, 38. Grade 2 or higher gastrointestinal RRT is frequently observed with the most common conditioning regimen16. Early severe gastrointestinal RRT may be associated with the interruption of planned oral MMF for GVHD prophylaxis, possible contributing to subsequent complications and poor outcomes after CBT.
Here, we found that the interruption of oral MMF was significantly associated with poor platelet recovery after CBT. The exact mechanisms underlying the association between the interruption of oral MMF and lower platelet recovery remain unknown. However, a previous study reported that tacrolimus combined with MMF is superior to tacrolimus alone in neutrophil engraftment after CBT, but not in platelet recovery39. Addition of MMF may promote engraftment by suppressing hyperimmune reactions in cord blood cells39. These effects may be responsible for the poor platelet recovery in patients in whom the planned dose of MMF was interrupted. Several studies have shown that delayed platelet recovery is associated with poor outcomes after HCT40, 41, which is consistent with our CBT data. Overall, our findings suggest an association between MMF interruption and poor platelet recovery and mortality after CBT.
In summary, we found that MMF interruption was significantly associated with poor OS and high NRM after CBT. However, further studies with larger cohorts are required to confirm the clinical significance of oral MMF interruption in GVHD prophylaxis.
Acknowledgments
The authors thank all of the physicians and staff at our hospital.
Author Contributions
K.F. collected the data and analyzed the data. T.K. designed the research, collected the data, analyzed the data, performed the statistical analysis, and wrote the manuscript. Y.N. contributed to the critical review of the manuscript. All the other authors contributed to data collection. All authors approved the final version.
Conflicts of Interest
The author declares no conflict of interest. Disclosure form provided by the author are available on the website.
Satoshi Takahashi is one of the Editors of Blood Cell Therapy. He was not involved in the editorial evaluation or decision to accept this article for publication.
Acknowledgments
The authors thank all of the physicians and staff at our hospital.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank all of the physicians and staff at our hospital.
Statements
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. The Institutional Review Board of the Institute of Medical Science, the University of Tokyo approved this retrospective study (2023-33-0810), and the use of an opt-out consent mechanism in this retrospective study.
References
1.Ram R, Yeshurun M, Vidal L, Shpilberg O, Gafter-Gvili A. Mycophenolate mofetil vs. methotrexate for the prevention of graft-versus-host-disease–systematic review and meta-analysis. Leuk Res. 2014; 38: 352-60.
2.Kharfan-Dabaja M, Mhaskar R, Reljic T, Pidala J, Perkins JB, Djulbegovic B, et al. Mycophenolate mofetil versus methotrexate for prevention of graft-versus-host disease in people receiving allogeneic hematopoietic stem cell transplantation. Cochrane Database Syst Rev. 2014; 7: CD010280.
3.Bolwell B, Sobecks R, Pohlman B, Andresen S, Rybicki L, Kuczkowski E, et al. A prospective randomized trial comparing cyclosporine and short course methotrexate with cyclosporine and mycophenolate mofetil for GVHD prophylaxis in myeloablative allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004; 34: 621-5.
4.Iida M, Fukuda T, Ikegame K, Yoshihara S, Ogawa H, Taniguchi S, et al. Use of mycophenolate mofetil in patients received allogeneic hematopoietic stem cell transplantation in Japan. Int J Hematol. 2011; 93: 523-31.
5.Iida M, Fukuda T, Uchida N, Murata M, Aotsuka N, Minagawa K, et al. Mycophenolate mofetil use after unrelated hematopoietic stem cell transplantation for prophylaxis and treatment of graft-vs.-host disease in adult patients in Japan. Clin Transplant. 2014; 28: 980-9.
6.Isobe M, Konuma T, Masuko M, Uchida N, Miyakoshi S, Sugio Y, et al. Single cord blood transplantation for acute myeloid leukemia patients aged 60 years or older: a retrospective study in Japan. Ann Hematol. 2021; 100: 1849-61.
7.Terakura S, Kuwatsuka Y, Yamasaki S, Wake A, Kanda J, Inamoto Y, et al. GvHD prophylaxis after single-unit reduced intensity conditioning cord blood transplantation in adults with acute leukemia. Bone Marrow Transplant. 2017; 52: 1261-7.
8.Konuma T, Kanda J, Inamoto Y, Hayashi H, Kobayashi S, Uchida N, et al. Improvement of early mortality in single-unit cord blood transplantation for Japanese adults from 1998 to 2017. Am J Hematol. 2020; 95: 343-53.
9.Atkinson K, Downs K. Omission of day 11 methotrexate does not appear to influence the incidence of moderate to severe acute graft-versus-host disease, chronic graft-versus-host disease, relapse rate or survival after HLA-identical sibling bone marrow transplantation. Bone Marrow Transplant. 1995; 16: 755-8.
10.Kumar S, Wolf RC, Chen MG, Gastineau DA, Gertz MA, Inwards DJ, et al. Omission of day +11 methotrexate after allogeneic bone marrow transplantation is associated with increased risk of severe acute graft-versus-host disease. Bone Marrow Transplant. 2002; 30: 161-5.
11.Bensinger W, Stem Cell Trialists' Collaborative Group. Individual patient data meta-analysis of allogeneic peripheral blood stem cell transplant vs bone marrow transplant in the management of hematological malignancies: indirect assessment of the effect of day 11 methotrexate administration. Bone Marrow Transplant. 2006; 38: 539-46.
12.Honda A, Kakihana K, Aoki J, Kobayashi T, Doki N, Sakamaki H, et al. Omission of day-11 MTX, in combination with tacrolimus, is not associated with increased risk of acute graft-versus-host disease after allo-BMT. Bone Marrow Transplant. 2013; 48: 307-9.
13.Hamilton BK, Rybicki L, Haddad H, Abounader D, Yurch M, Majhail NS, et al. Does day 11 omission of methotrexate due to toxicity influence the outcome in myeloablative hematopoietic cell transplant? Results from a single-center retrospective cohort study. Blood Cancer J. 2015; 5: e344.
14.Lin A, Brown S, Maloy M, Ruiz JD, Devlin S, DeRespiris L, et al. Impact of omitting post-transplant minidose-methotrexate doses in allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2022; 63: 1686-93.
15.Kharfan-Dabaja MA, Reljic T, Kumar A, Yassine F, Keller K, Fernandez A, et al. Omission of day +11 methotrexate dose and allogeneic hematopoietic cell transplantation outcomes: results of a systematic review/meta-analysis. Bone Marrow Transplant. 2022; 57: 65-71.
16.Konuma T, Kato S, Isobe M, Mizusawa M, Oiwa-Monna M, Takahashi S, et al. Reduced-Toxicity Myeloablative Conditioning Consisting of Fludarabine/Busulfan/Low-Dose Total Body Irradiation/Granulocyte Colony-Stimulating Factor-Combined Cytarabine in Single Cord Blood Transplantation for Elderly Patients with Nonremission Myeloid Malignancies. Biol Blood Marrow Transplant. 2019; 25: 764-70.
17.Konuma T, Monna-Oiwa M, Isobe M, Okabe M, Takahashi S, Tojo A. Radiation-free myeloablative conditioning consisting of fludarabine added to full-dose busulfan and cyclophosphamide in single-unit cord blood transplantation for adults. Eur J Haematol. 2021; 107: 374-6.
18.Konuma T, Kato S, Oiwa-Monna M, Tanoue S, Ogawa M, Isobe M, et al. Cryopreserved CD34+ Cell Dose, but Not Total Nucleated Cell Dose, Influences Hematopoietic Recovery and Extensive Chronic Graft-versus-Host Disease after Single-Unit Cord Blood Transplantation in Adult Patients. Biol Blood Marrow Transplant. 2017; 23: 1142-50.
19.Mizusawa M, Konuma T, Kato S, Isobe M, Shibata H, Suzuki M, et al. Clinical outcomes of persistent colonization with multidrug-resistant Gram-negative rods in adult patients undergoing single cord blood transplantation. Int J Hematol. 2020; 111: 858-68.
20.Konuma T, Oiwa-Monna M, Mizusawa M, Isobe M, Kato S, Nagamura-Inoue T, et al. Red blood cell transfusion burden by day 30 predicts mortality in adults after single-unit cord blood transplantation. Bone Marrow Transplant. 2019; 54: 1836-46.
21.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15: 825-8.
22.Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S, et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol. 1991; 28: 250-9.
23.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106: 2912-9.
24.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014; 123: 3664-71.
25.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR' for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
26.Nash RA, Johnston L, Parker P, McCune JS, Storer B, Slattery JT, et al. A phase I/II study of mycophenolate mofetil in combination with cyclosporine for prophylaxis of acute graft-versus-host disease after myeloablative conditioning and allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2005; 11: 495-505.
27.Giaccone L, McCune JS, Maris MB, Gooley TA, Sandmaier BM, Slattery JT, et al. Pharmacodynamics of mycophenolate mofetil after nonmyeloablative conditioning and unrelated donor hematopoietic cell transplantation. Blood. 2005; 106: 4381-8.
28.Gupta V, Daly A, Lipton JH, Hasegawa W, Chun K, Kamel-Reid S, et al. Nonmyeloablative stem cell transplantation for myelodysplastic syndrome or acute myeloid leukemia in patients 60 years or older. Biol Blood Marrow Transplant. 2005; 11: 764-72.
29.Baron F, Maris MB, Storer BE, Sandmaier BM, Stuart MJ, McSweeney PA, et al. HLA-matched unrelated donor hematopoietic cell transplantation after nonmyeloablative conditioning for patients with chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005; 11: 272-9.
30.Baron F, Maris MB, Storer BE, Sandmaier BM, Panse JP, Chauncey TR, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005; 19: 822-8.
31.Minagawa K, Yamamori M, Katayama Y, Matsui T. Mycophenolate mofetil: fully utilizing its benefits for GvHD prophylaxis. Int J Hematol. 2012; 96: 10-25.
32.Zhang D, Chow DS. Clinical Pharmacokinetics of Mycophenolic Acid in Hematopoietic Stem Cell Transplantation Recipients. Eur J Drug Metab Pharmacokinet. 2017; 42: 183-9.
33.Mattsson J, Westin S, Edlund S, Remberger M. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transplant. 2006; 38: 629-33.
34.van Lier YF, Vos J, Blom B, Hazenberg MD. Allogeneic hematopoietic cell transplantation, the microbiome, and graft-versus-host disease. Gut Microbes. 2023; 15: 2178805.
35.Bhatia M, Militano O, Jin Z, Figurski M, Shaw L, Moore V, et al. An age-dependent pharmacokinetic study of intravenous and oral mycophenolate mofetil in combination with tacrolimus for GVHD prophylaxis in pediatric allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2010; 16: 333-43.
36.Kurata K, Yakushijin K, Okamura A, Yamamori M, Ichikawa H, Sakai R, et al. Pharmacokinetics of intravenous mycophenolate mofetil in allogeneic hematopoietic stem cell-transplanted Japanese patients. Cancer Chemother Pharmacol. 2018; 81: 839-46.
37.Sano H, Hilinski JA, Qayed M, Applegate K, Newton JG, Watkins B, et al. Early blood stream infection following allogeneic hematopoietic stem cell transplantation is a risk factor for acute grade III-IV GVHD in children and adolescents. Pediatr Blood Cancer. 2018; 65: e26821.
38.Zhou X, O'Dwyer DN, Xia M, Miller HK, Chan PR, Trulik K, et al. First-Onset Herpesviral Infection and Lung Injury in Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med. 2019; 200: 63-74.
39.Uchida N, Wake A, Nakano N, Ishiwata K, Takagi S, Tsuji M, et al. Mycophenolate and tacrolimus for graft-versus-host disease prophylaxis for elderly after cord blood transplantation: a matched pair comparison with tacrolimus alone. Transplantation. 2011; 92: 366-71.
40.Kim DH, Sohn SK, Jeon SB, Baek JH, Kim JG, Lee NY, et al. Prognostic significance of platelet recovery pattern after allogeneic HLA-identical sibling transplantation and its association with severe acute GVHD. Bone Marrow Transplant. 2006; 37: 101-8.
41.Ramírez P, Brunstein CG, Miller B, Defor T, Weisdorf D. Delayed platelet recovery after allogeneic transplantation: a predictor of increased treatment-related mortality and poorer survival. Bone Marrow Transplant. 2011; 46: 981-6.
Search
News