Volume 7 (2024) Issue 3 No.1 Pages 64-74
Abstract
This study aimed to investigate the recovery of physical function, muscle mass, and quality of life (QOL) in allogeneic hematopoietic stem cell transplantation (allo-HSCT) patients 1 year after the procedure. A total of 71 patients who underwent allo-HSCT at our institution between February 2010 and June 2020, for whom a physical therapy assessment could be performed before allo-HSCT, at discharge, and 1 year after the procedure, were included. Exercise therapy during hospitalization was provided individually by a physical therapist, and exercise was self-administered after discharge. One year after allo-HSCT, handgrip strength and results of the 6-minute walk test recovered to pre-HSCT levels. However, muscle mass 1 year after allo-HSCT did not reach the pre-HSCT level. All subscales of QOL, 1 year after allo-HSCT, recovered to pre-HSCT levels, but only two of the eight subscales recovered to the national standard of 50. Multivariate analysis revealed factors associated with the recovery of physical function, muscle mass, and QOL, hemoglobin levels and albumin levels, especially among men. In contrast, factors that negatively affected recovery were age, acute graft-versus-host disease, and pre-HSCT intensity conditioning. The results suggest a potential recovery in handgrip strength, endurance, and QOL 1 year after allo-HSCT.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a useful treatment for hematopoietic malignancies, and improved transplant outcomes have increased survival rates1, 2. However, patients' physical function is usually impaired prior to allo-HSCT because of inactivity caused by remission induction and consolidation therapy initiated after diagnosis3. In addition, pre-transplant treatment with high-dose chemotherapy, infections associated with allo-HSCT, and graft-versus-host disease (GvHD) can lead to a decrease in physical activity4, 5. Therefore, rehabilitation is important to prevent a decline in physical function and quality of life (QOL)6, 7. Exercise therapy is beneficial in maintaining physical function, QOL, and lower extremity muscle mass after allo-HSCT4. In addition to physical function and QOL assessment, muscle mass measurement is essential for preventing sarcopenia and frailty8, 9. Recently, it has been suggested that early rehabilitation after diagnosis of a hematological disease is safe and helpful for early recovery of physical function10, 11. Furthermore, most patients independently perform activities of daily living (ADL) but limit outdoor activities when discharged from the hospital. Therefore, a post-discharge approach aimed at early recovery of physical function is essential.
Numerous reports on QOL recovery in allo-HSCT patients have been published, with the recovery time ranging from 6 months to 1 year12, 13. Concerning recovery of physical function, Wiskemann et al. reported an improvement in the 6-min walk test (6 MWT) results at 6-8 weeks after discharge compared with the time of discharge, but not to pre-HSCT levels14. Hayakawa et al. reported that the 6 MWT results at 1 year after allo-HSCT reached pre-HSCT levels, but handgrip strength and knee extension muscle strength did not improve15. Most reports on the recovery of physical function are from the early post-discharge period14, 16, 17, and few studies report the recovery beyond 6 months after allo-HSCT. To the best of our knowledge, no reports exist on the recovery at 1 year after allo-HSCT with respect to changes in muscle mass. This study aimed to investigate factors affecting recovery and the outcomes concerning physical function, muscle mass, and QOL 1 year after allo-HSCT.
Patients and Methods
Inclusion/exclusion criteria
The inclusion criteria comprised patients with hematologic diseases who underwent their first allo-HSCT at our institution and whose physical function, muscle mass, and QOL could be assessed prior to allo-HSCT. Exclusion criteria included patients with bone and joint disorders, severe cardiac dysfunction, severe lung dysfunction, or bone metastasis. Of the 327 patients who underwent allo-HSCT between February 2010 and June 2020, 286 met the inclusion criteria. According to the Japanese ethics guidelines for clinical studies, to obtain a signed informed consent is not required from each patient. Instead, we provided online information regarding this retrospective study, and patients could exclude themselves if they did not want us to use their data. This study was approved by the ethics committee of Imamura General Hospital (NCR21-45) and conducted in accordance with the Declaration of Helsinki and its later amendments.
Exercise intervention
Our institution provides rehabilitation to all patients undergoing allo-HSCT. Physiotherapy assessments are performed routinely before allo-HSCT, at discharge, and during the long-term follow-up (LTFU) outpatient visits.
Exercise therapy during hospitalization began approximately 2 weeks before allo-HSCT and was conducted individually by the physical therapist five times a week for 20 to 40 minutes per session until discharge. The exercise therapy included stretching, strength and balance training, step-ups, and endurance training (walking and cycling). Stretching was a passive exercise conducted for the lower limb and trunk muscles with the assistance of a physical therapist (10 min). Resistance training targeted the hip flexors, hip abductors, knee extensors, and ankle plantar flexors (10-15 min) and was accomplished by manipulating resistance provided by the physical therapist. The target intensity for strength training was
Exercise therapy was self-administered after discharge from the hospital. Before discharge, rehabilitation guidance was provided by the physical therapist, and patients were instructed to continue the exercises they had performed during hospitalization. We distributed exercise therapy leaflets to the patients. In particular, continuing lower extremity strength training and walking exercises was recommended.
Assessment
Physical function, muscle mass, and QOL assessments were performed approximately 2 weeks before allo-HSCT, at discharge, and 1 year after allo-HSCT during LTFU outpatient visits.
Physical function tests
The 6 MWT and handgrip strength were measured as physical function tests. The 6 MWT was based on the American Thoracic Society guidelines20 and performed in a straight corridor with cones at 20 m intervals.
Handgrip strength was measured in a standing position with mild shoulder joint abduction using an adjustable-handle dynamometer (TKK 5101; TAKEI Scientific Instruments Co. Ltd., Niigata, Japan). The average of the left and right handgrip strength measurements was used as the handgrip strength index.
Body composition
Muscle mass and body weight were used to evaluate body composition. Muscle mass was measured by bioelectrical impedance analysis using Physion MD (Physion Ltd., Kyoto, Japan) in a resting supine position, with mild shoulder and hip abduction.
Health-related QOL
Health-related QOL was assessed using the Medical Outcome Study 36-item Short Form Health Survey (SF-36)21, 22. SF-36 consists of eight subscales: physical functioning (PF), role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. Each subscale was calculated on a 0 to 100-point scale. A score of zero indicated the worst health status, and a score of 100 indicated the best health status for the subscale. The calculated score based on the national standard was 50, with higher values indicating a better QOL. In addition, summary scores were calculated for each of the following three components: physical, mental, and role/social.
Clinical factors
Blood tests (albumin [Alb], total protein [TP], and hemoglobin [Hb]), fever (over 38
Statistical analysis
The results for each index are presented as mean or median. Normality was tested using the Kolmogorov-Smirnov method. Changes in physical function, muscle mass, QOL, and clinical factors over time were analyzed using the one-way analysis of variance (Bonferroni's correction for multiple comparisons) or the Friedman test (Scheffe's test). Multiple regression analysis was used to examine the factors associated with physical function, muscle mass, and QOL recovery 1 year after allo-HSCT. The independent variables were the rate of change in clinical factors (pre-HSCT to 1-year post-HSCT), change in physical function (pre-HSCT to 1-year post-HSCT), steroid dosage, adherence to exercise therapy during hospitalization, pre-HSCT intensity conditioning, age, and GvHD. These were used to perform single regression analysis, and factors with P < 0.15 were used for multiple regression analysis. The change in each indicator from pre-HSCT to 1 year after HSCT was expressed as a percentage. Similarly, we analyzed factors affecting physical function and QOL 1 year after allo-HSCT. Adherence to exercise therapy was calculated as the percentage of days in which exercise therapy was performed relative to the scheduled days. Statistical analysis was performed using EZR version 1.5524 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), and a P-value of < 0.05 was considered statistically significant.
Results
Study population
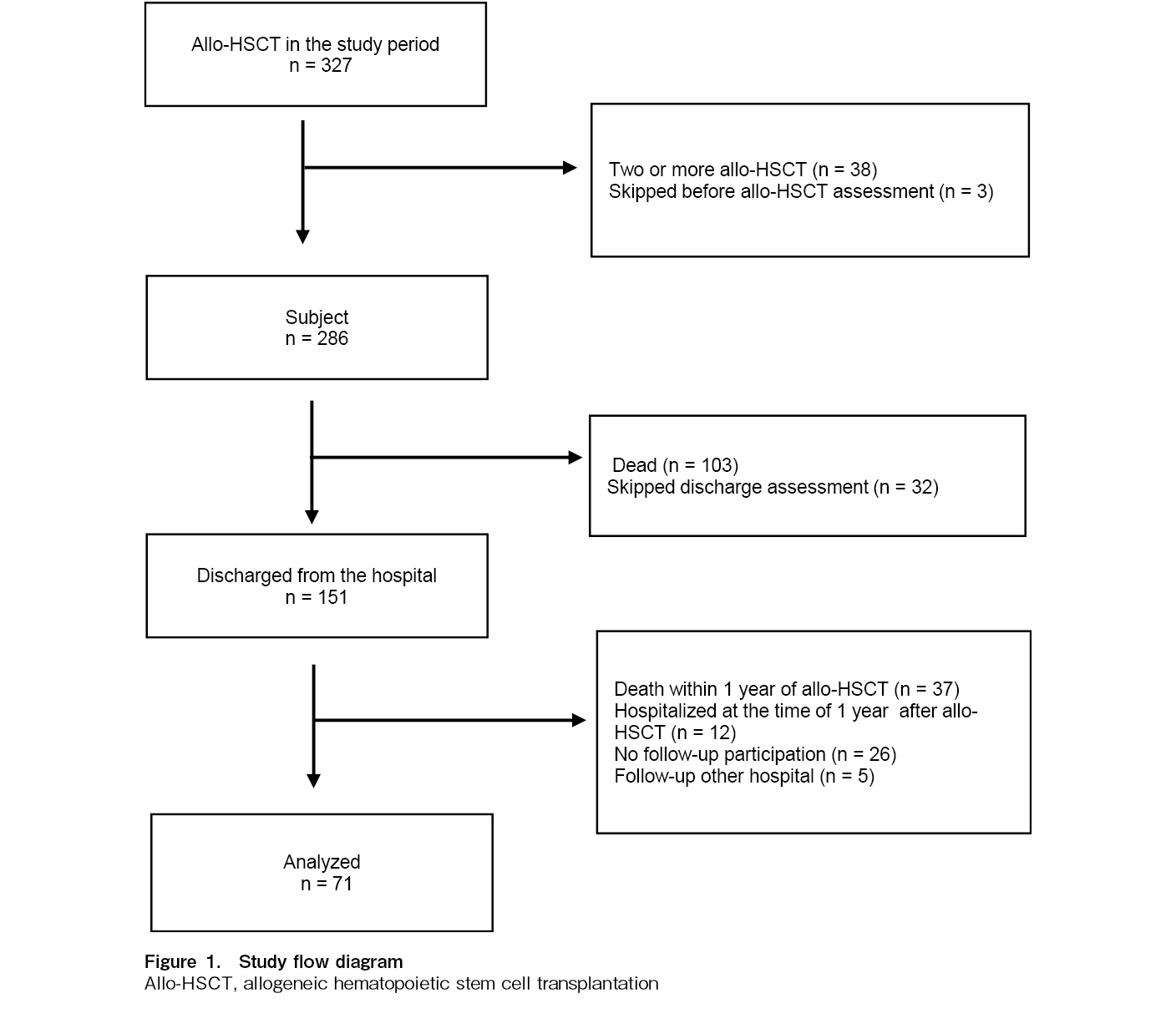
A flow diagram of the study population is shown in Figure 1. Of the 286 patients, 103 died during hospitalization and 32 could not be evaluated at discharge owing to physical problems. Of the 151 patients who were evaluated at discharge, 71 patients were included in the final analysis: 37 patients who died within 1 year of allo-HSCT, 12 patients who were in inpatient care 1 year after allo-HSCT, 26 patients who did not participate in LTFU, and 5 patients who were followed at other hospitals were excluded.
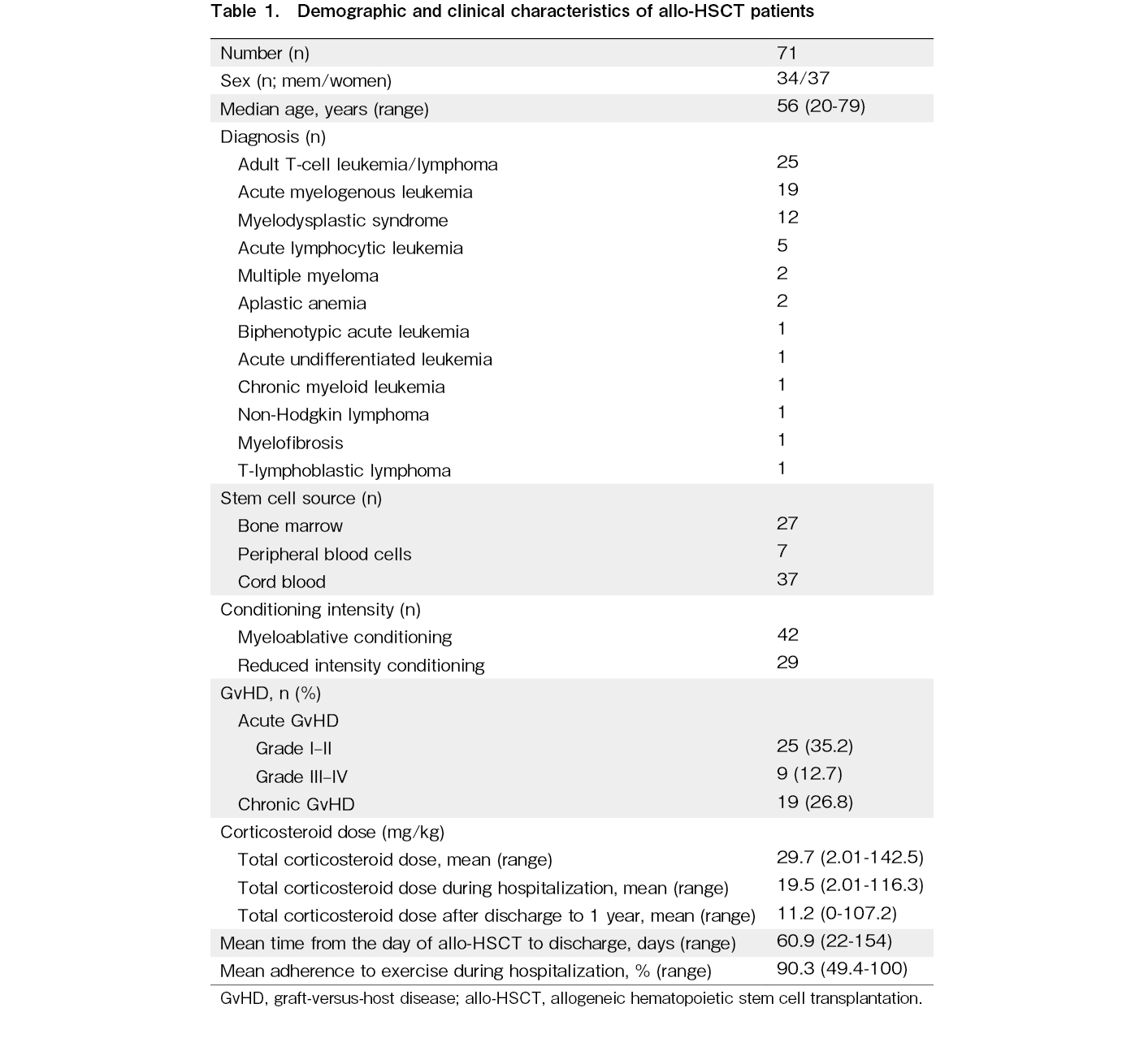
Patient characteristics are shown in Table 1. An almost equal distribution of men and women was observed, with adult T-cell leukemia/lymphoma (ATL) being the most common disease (our hospital is in an endemic area for the HTLV-1 virus). Most of the factors that reduced conditioning intensity were due to age. Acute GvHD was observed in 34 patients (severe GvHD in nine patients) during hospitalization. Chronic GvHD was observed in 19 patients but was limited to mild skin issues, mucous membrane disorders, dry eyes, and joint symptoms (joint contractures and pain), and the median total corticosteroid dose after discharge was 1.3 mg/kg. Of the 71 patients, 4 (with ATL) relapsed within 1 year of allo-HSCT, and two received additional treatment.
Physical function and body composition change before and after allo-HSCT
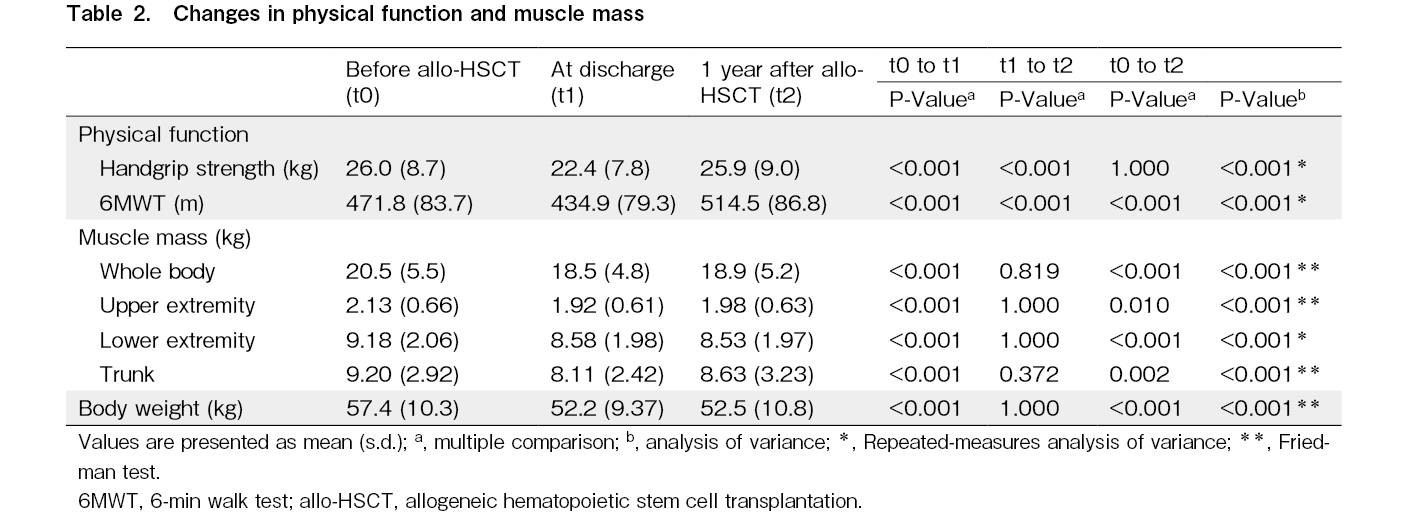
Handgrip strength decreased significantly at the time of discharge assessment (P < 0.001) but improved considerably 1 year after allo-HSCT (P < 0.001), reaching pre-HSCT levels (Table 2). Likewise, the 6 MWT results decreased significantly at discharge (P < 0.001) but markedly improved 1 year after allo-HSCT (P < 0.001), recovering to pre-HSCT levels.
Regarding changes in muscle mass, the whole-body muscle mass significantly decreased at discharge (P < 0.001) and showed a slight improvement 1 year after allo-HSCT; however, pre-HSCT levels were not achieved. Similar outcomes were noted in body weight and the upper extremity, lower extremity, and trunk muscle mass.
Changes in the QOL before and after allo-HSCT
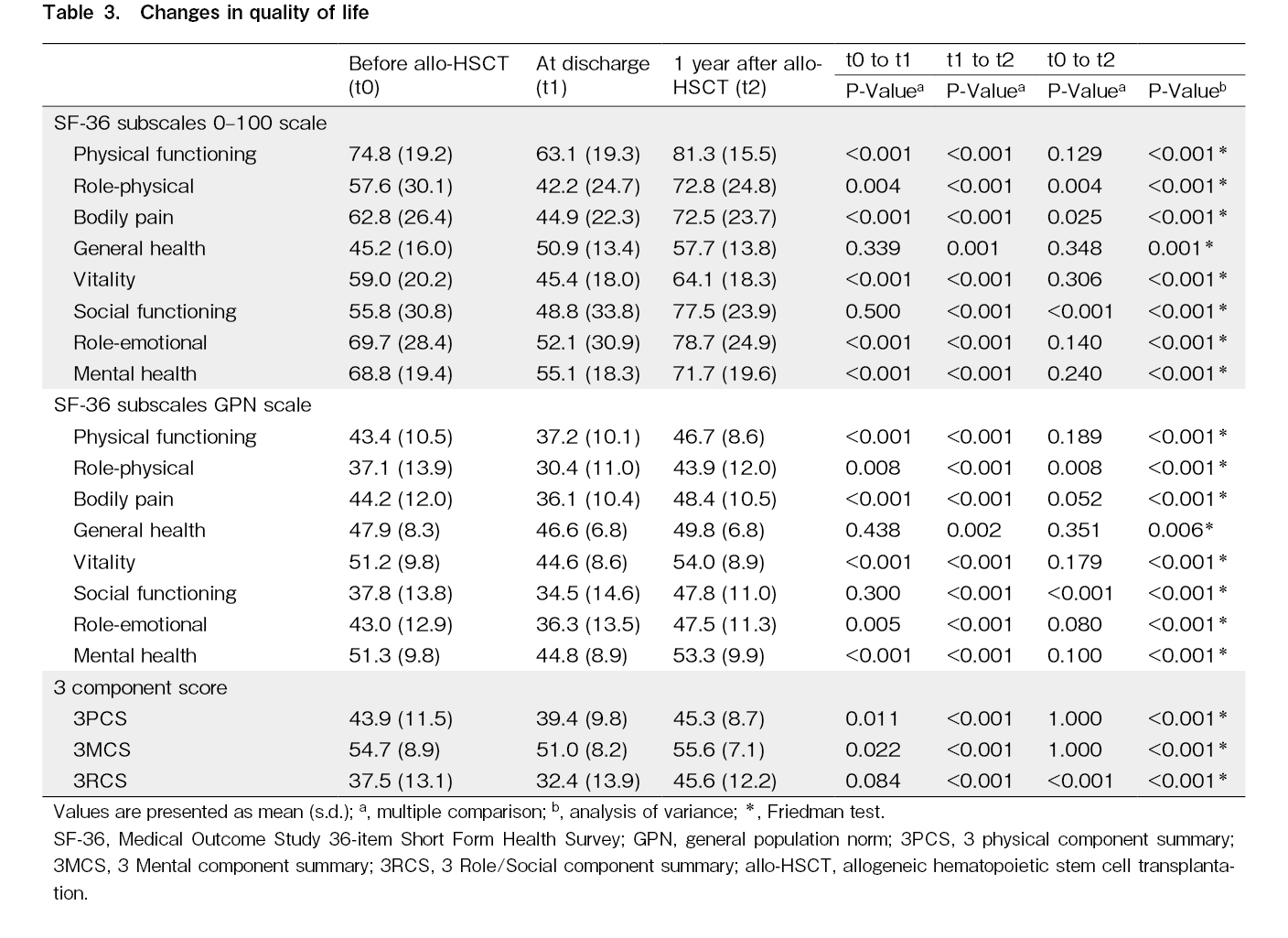
Seven of the eight subscales, excluding general health, decreased significantly at discharge (Table 3). However, all eight subscales improved substantially from discharge to 1 year after allo-HSCT, reaching pre-HSCT levels. Of the eight subscales, only vitality and mental health exceeded the national standard of 50 at 1 year after allo-HSCT.
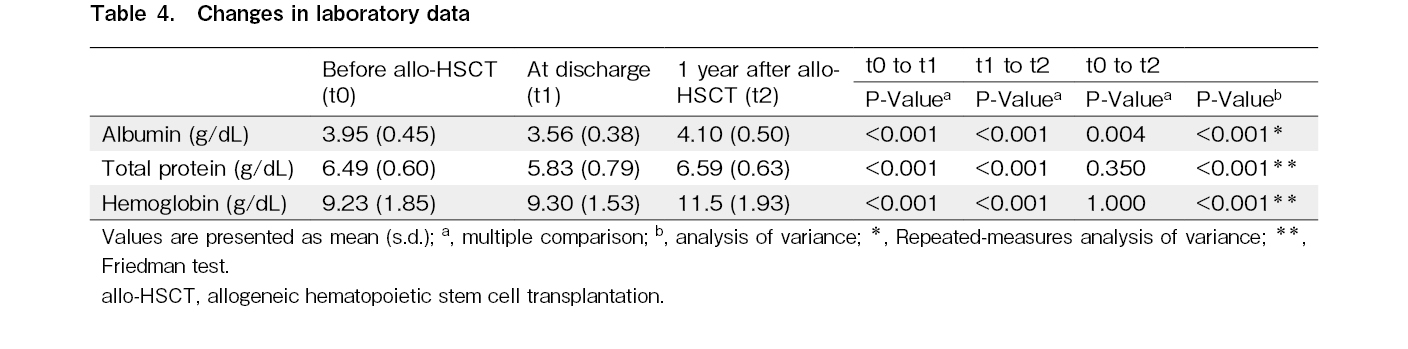
Changes in laboratory data before and after allo-HSCT
Alb, TP, and Hb levels decreased significantly after allo-HSCT (P < 0.001) but showed significant recovery from discharge to 1 year after allo-HSCT (Table 4). In addition, Alb values at 1-year post-HSCT showed better results than pre-transplant levels.
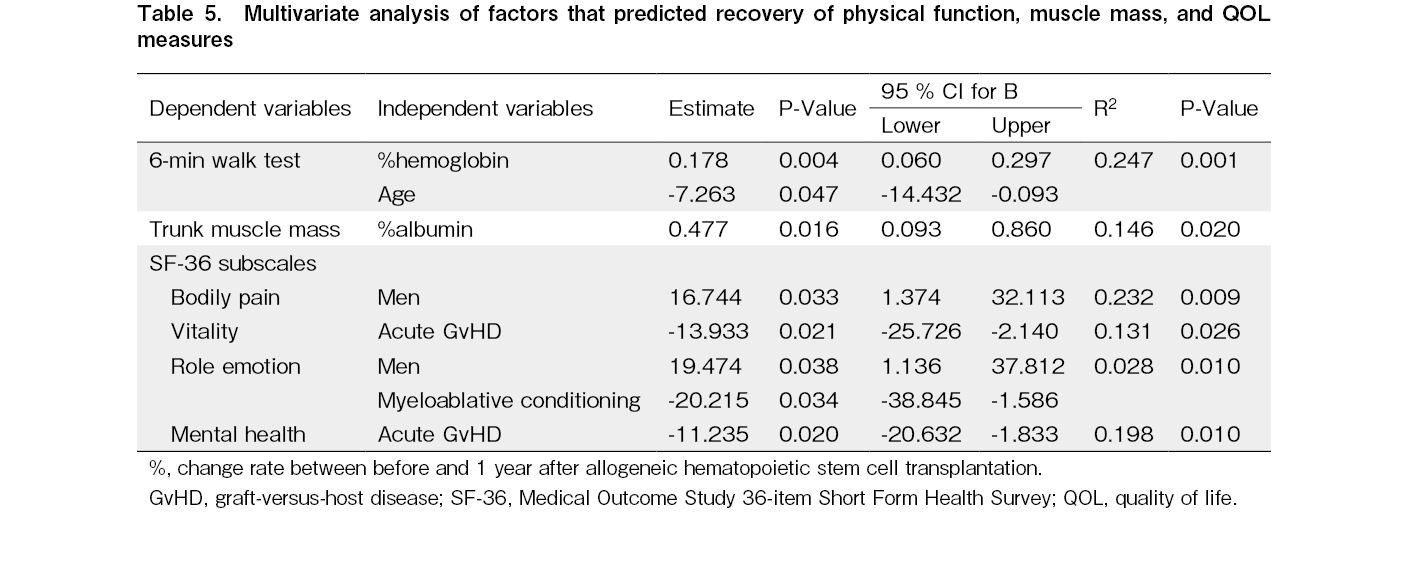
Factors associated with recovery to pre-HSCT levels
An improvement in Hb levels was positively associated with improvement in the 6 MWT results (R2 = 0.247, P < 0.001). Also, an improvement in Alb levels was positively associated with improvement in trunk muscle mass (R2 = 0.146, P = 0.020) (Table 5).
Men were positively associated with QOL (bodily pain [R2 = 0.232, P = 0.009] and role emotion [R2 = 0.028, P = 0.010]). Acute GvHD was negatively associated with recovery of QOL (vitality [R2 = 0.131, P = 0.026] and mental health [R2 = 0.198, P = 0.010]) at 1-year post-HSCT. Conditioning intensity (myeloablative conditioning; MAC) was negatively associated with recovery of QOL (role emotion [R2 = 0.028, P = 0.034]) at 1-year post-HSCT.
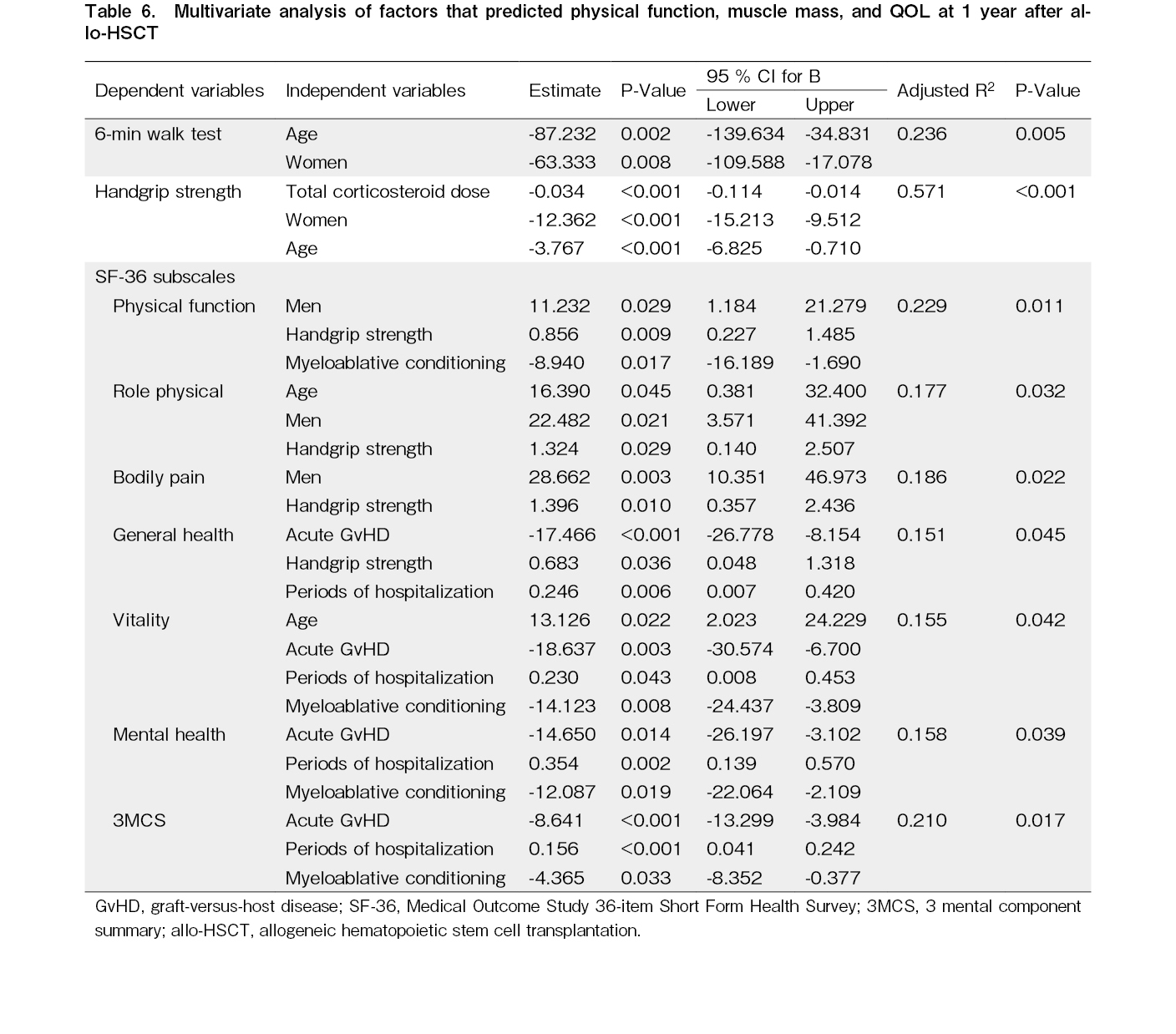
Factors associated with physical function and QOL at 1 year after allo-HSCT
Handgrip strength and the 6 MWT results at 1 year after allo-HSCT were negatively related to older age and women (Table 6). QOL was positively correlated with handgrip strength, age (older), sex (men), and periods of hospitalization, and negatively correlated with acute GvHD and conditioning intensity (MAC).
Discussion
We investigated the recovery of physical function, muscle mass, and QOL in patients undergoing initial allo-HSCT. One year after allo-HSCT, physical function and QOL scores reached pre-HSCT levels.
Handgrip strength and 6 MWT results decreased significantly at discharge but improved considerably 1 year after allo-HSCT. In this study, exercise therapy was initiated before allo-HSCT and continued until discharge, with an implementation rate of 90.3%, similar to other reports (74-90%)14, 25, 26. Continuation of exercise therapy contributed to maintaining patients' activities and ADL abilities, enabling discharge at the ambulatory level. At discharge, patients were instructed to perform the exercises at home. In addition, we advised them to continue stretching, strength training, and walking. Patients were also guided on how to maintain and improve their activities during the daytime. Most patients led active daily lives in the first few months after discharge from the hospital, although some had decreased activity due to fatigue. By maintaining moderate activity after discharge from the hospital, handgrip strength and 6 MWT results seemed to recover to pre-HSCT levels 1 year after allo-HSCT.
Multivariate analysis showed that the recovery of the 6 MWT results was associated with improved Hb levels, which are indicative factors for the recovery of physical function to pre-HSCT levels at 1-year post-allo-HSCT. As factors associated with physical function at 1 year of allo-HSCT, age (older) and sex (women) were negatively associated with the 6 MWT, and corticosteroid administration and age were negatively associated with handgrip strength. A low Hb status is a hypoxic risk to tissues throughout the body because of decreased oxygen-carrying capacity concerning physical function and Hb levels27. Therefore, a low Hb level is an independent risk factor for decreased physical function (especially decreased endurance)28, 29. In patients undergoing allo-HSCT, there is a negative association between pre-HSCT Hb levels and the 6 MWT results3. In this study, Hb improved compared with the pre-HSCT level 1 year after allo-HSCT. Increased activity and improved Hb level after discharge from the hospital may have contributed to the recovery of the 6 MWT.
On the other hand, handgrip strength 1 year after allo-HSCT was negatively associated with total corticosteroid dosage. In allo-HSCT, corticosteroids are administered as a treatment for acute and chronic GvHD. Corticosteroids for acute GvHD are usually prescribed in high doses, while for chronic GvHD, they are administered for long periods after discharge from the hospital. In patients who undergo allo-HSCT, corticosteroid administration is associated with decreased physical function during hospitalization30. In the present study, chronic GvHD was observed in 26.8% of the cases. Symptoms of chronic GvHD include mild skin issues, mucous membrane disorders, and dry eyes, many of which can be treated with topical medication. Therefore, the median corticosteroid dose after discharge from the hospital was very low, 1.3 mg/kg. The median corticosteroid dosage in the present study was 18.5 mg/kg, a relatively low dosage compared with the median dosage of 25.96 mg/kg in a previous study of patients with chronic GvHD15. In this study, corticosteroid dosage was not associated with recovery of handgrip strength 1 year after allo-HSCT but rather was identified as a factor that negatively affected it. Thus, corticosteroids administered after transplantation may affect handgrip strength after allo-HSCT.
Changes in body composition, muscle mass, and body weight at 1-year post-HSCT improved slightly but did not reach pre-HSCT levels. Loss of appetite in long-term post-HSCT patients is reported to persist after discharge from the hospital13. In the present study, taste disturbance and loss of appetite seen during hospitalization continued after discharge in many cases, and improvement in food intake was slow. An increased Alb level was associated with trunk muscle mass recovery to pre-HSCT level at 1-year allo-HSCT. Serum Alb is a nutritional status that correlates with muscle mass31. In the hematological data, TP and Alb reached pre-HSCT levels 1 year after allo-HSCT. Improvements in TP and Alb were associated with recovery of physical function. However, muscle mass and body weight recovery required more time. It is necessary for physicians and nurses to interview patients about their health and post-transplant progress (GvHD and other complications) as well as their dietary intake during routine visits, and to provide nutritional guidance as needed.
Improvement in the QOL after allo-HSCT is reported to take approximately 1 year12, 13. In this study, all SF-36 subscales 1 year after allo-HSCT reached the pre-HSCT levels. QOL in long-term post-HSCT patients has been reported to be affected by chronic GvHD32, 33. In the present study, most patients with chronic GvHD had relatively mild symptoms, including skin issues, dry eyes, and dry mouth. Chronic GvHD was observed in 26.8% of the patients and was relatively mild in most cases, and thus, had little impact on the QOL. At 1-year post-HSCT, six of the eight subscale scores of the SF-36 were below the national standard of 50. Gifford et al. reported the QOL equivalent to the national standard 2 years after allo-HSCT34. Therefore, QOL scores at 1-year post-HSCT recovered to pre-HSCT levels but not to the national standard. As indicated in previous study34, recovery to the national standard level may take more than 1 year after allo-HSCT.
The results of the multivariate analysis showed that sex (men) was a positive factor associated with the pre-HSCT levels of QOL at 1 year after allo-HSCT, and acute GvHD was a negative factor. In addition, sex (men), periods of hospitalization and handgrip strength were positive factors in QOL at 1-year post-HSCT, while acute GvHD and conditioning regimens (MAC) were negative factors. With regard to sex differences in QOL after allo-HSCT, women have been reported to experience a greater decline in QOL than men35. Men may also recover QOL earlier than women. Physical function is associated with QOL in allo-HSCT recipients3, and there is a positive correlation between handgrip strength and the subscale SF-36 (PF)35. In long-term post-HSCT patients, those with higher physical function have a better QOL36. In terms of QOL and GvHD, although chronic GvHD is associated with lower QOL33, acute GvHD was also considered an influential factor at the relatively early time point of the first year of HSCT. We expected QOL recovery to be delayed in cases with longer hospital stays, but the opposite was true. The factors for this are unknown.
The American College of Sports Medicine promotes exercise therapy to restore physical function in cancer survivors37. Therefore, for the early recovery of physical function, muscle mass, and QOL in allo-HSCT recipients, it is important to implement exercise therapy during hospitalization, continue exercise at home after discharge, and conduct regular assessments in the outpatient setting.
However, this study has some limitations. First, this was a single-center, retrospective study involving only 71 cases. Second, exercise therapy after discharge was self-administered, and quantitative activity levels could not be assessed because no progress records were kept. However, at the 1-year post-HSCT assessment, we confirmed that the patients had gradually expanded their ADL by interviewing them about their activity level at home, any possible complications, and dietary intake. Third, many patients dropped out at the 1-year post-HSCT assessment. Factors contributing to non-participation included the fact that LTFU participation was voluntary, the length of time required for the LTFU, and the cost of the treatment, which was not covered by insurance. Of the 32 patients attending our hospital who could not be evaluated 1 year after allo-HSCT, there were eight cases of relapse, but the performance status was maintained. Furthermore, among these patients, only a small number had severely limited muscle strength or endurance; however, these results may be biased and require further investigation. Future large-scale studies that include post-discharge activity assessments are needed to clarify the time necessary to recover physical function, muscle mass, and QOL after allo-HSCT.
Muscle strength, endurance, and QOL of allo-HSCT recipients reached pre-HSCT levels 1 year after the treatment. However, muscle mass and body weight did not. Factors associated with the recovery of physical function and QOL included improved Hb levels and Alb levels, particularly in men. Physical function may be restored earlier by maintaining sufficient activity after discharge from the hospital.
Acknowledgments
The authors thank the study participants, physical therapists at the Rehabilitation Department, and physicians at the Hematology and Rehabilitation Medicine Department at the Imamura General Hospital.
Author Contributions
T.T., K.D., A.U., and N. Nakano designed the study, reviewed and analyzed the data, and wrote the paper. The clinical data collection was performed by T.T., N. Nakashima, and T.O., who critically reviewed the previous versions of the manuscript. All authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
The authors thank the study participants, physical therapists at the Rehabilitation Department, and physicians at the Hematology and Rehabilitation Medicine Department at the Imamura General Hospital.
Ethical approval
This study was approved by the ethics committee of Imamura General Hospital (NCR21-45) and conducted in accordance with the Declaration of Helsinki and its later amendments. Per the Japanese ethics guidelines for clinical studies, written informed consent was not required from each patient.
References
1.Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, et al. Indications for Autologous and Allogeneic Hematopoietic Cell Transplantation: Guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2015; 21: 1863-9.
2.Zhang M, Xiao H, Shi J, Tan Y, Zhao Y, Yu J, et al. Improved survival for young acute leukemia patients following a new donor hierarchy for allogeneic hematopoietic stem cell transplantation: A phase III randomized controlled study. Am J Hematol. 2021; 96: 1429-40.
3.Morishita S, Kaida K, Ikegame K, Yoshihara S, Taniguchi K, Okada M, et al. Impaired physiological function and health-related QOL in patients before hematopoietic stem-cell transplantation. Support Care Cancer. 2012; 20: 821-9.
4.Takekiyo T, Dozono K, Mitsuishi T, Murayama Y, Maeda A, Nakano N, et al. Effect of exercise therapy on muscle mass and physical functioning in patients undergoing allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2015; 23: 985-92.
5.Ishikawa A, Otaka Y, Kamisako M, Suzuki T, Miyata C, Tsuji T, et al. Factors affecting lower limb muscle strength and cardiopulmonary fitness after allogeneic hematopoietic stem cell transplantation. Support Care Cancer. 2019; 27: 1793-800.
6.Morishita S, Tsubaki A, Hotta K, Fu JB, Fuji S. The benefit of exercise in patients who undergo allogeneic hematopoietic stem cell transplantation. J Int Soc Phys Rehabil Med. 2019; 2: 54-61.
7.Mohananey D, Sarau A, Kumar R, Lewandowski D, Abreu-Sosa SM, Nathan S, et al. Role of Physical Activity and Cardiac Rehabilitation in Patients Undergoing Hematopoietic Stem Cell Transplantation. JACC Cardio Oncol. 2021; 3: 17-34.
8.DeFilipp Z, Troschel FM, Qualls DA, Li S, Kuklinski MW, Kempner ME, et al. Evolution of Body Composition Following Autologous and Allogeneic Hematopoietic Cell Transplantation: Incidence of Sarcopenia and Association with Clinical Outcomes. Biol Blood Marrow Transplant. 2018; 24: 1741-7.
9.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010; 42: 1409-26.
10.Rupnik E, Skerget M, Sever M, Zupan IP, Ogrinec M, Ursic B, et al. Feasibility and safety of exercise training and nutritional support prior to haematopoietic stem cell transplantation in patients with haematologic malignancies. BMC Cancer. 2020; 20: 1142.
11.Mina DS, Dolan LB, Lipton JH, Au D, Pérez EC, Franzese A, et al. Exercise before, during, and after Hospitalization for Allogeneic Hematological Stem Cell Transplant: A Feasibility Randomized Controlled Trial. J Clin Med. 2020; 9: 1854.
12.Wettergren L, Sprangers M, Björkholm M, Langius-Eklöf A. Quality of life before and one year following stem cell transplantation using an individualized and a standardized instrument. Psychooncology. 2008; 17: 338-46.
13.Grulke N, Albani C, Bailer H. Quality of life in patients before and after haematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 2012; 47: 473-82.
14.Wiskemann J, Dreger P, Schwerdtfeger R, Bondong A, Huber G, Kleindienst N, et al. Effects of a partly self-administered exercise program before, during, and after allogeneic stem cell transplantation. Blood. 2011; 117: 2604-13.
15.Hayakawa J, Miyamura D, Kimura SI, Gomyo A, Tamaki M, Akahoshi Y, et al. Negative impact of chronic graft-versus-host disease and glucocorticoid on the recovery of physical function after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019; 54: 994-1003.
16.Hacker ED, Collins E, Park C, Peters T, Patel P, Rondelli D. Strength Training to Enhance Early Recovery after Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2017; 23: 659-69.
17.Knols RH, de Bruin ED, Uebelhart D, Aufdemkampe G, Schanz U, Stenner-Liewen F, et al. Effects of an outpatient physical exercise program on hematopoietic stem-cell transplantation recipients: a randomized clinical trial. Bone Marrow Transplant. 2011; 46: 1245-55.
18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982; 14: 377-81.
19.Karvonen MJ, Kentala E, Mustala O. The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn. 1957; 35: 307-15.
20.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002; 166: 111-7.
21.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, et al. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992; 305: 160-4.
22.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. J Clin Epidemiol. 1998; 51: 1037-44.
23.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15: 825-8.
24.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplantation. 2013; 48: 452-8.
25.Jarden M, Baadsgaard MT, Hovgaard DJ, Boesen E, Adamsen L. A randomized trial on the effect of a multimodal intervention on physical capacity, functional performance and quality of life in adult patients undergoing allogeneic SCT. Bone Marrow Transplant. 2009; 43: 725-37.
26.Morishita S, Kaida K, Setogawa K, Kajihara K, Ishii S, Ikegame K, et al. Safety and feasibility of physical therapy in cytopenic patients during allogeneic haematopoietic stem cell transplantation. Eur J Cancer Care. 2013; 22: 289-99.
27.Dy SM, Lorenz KA, Naeim A, Sanati H, Walling A, Asch SM. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Oncol. 2008; 26: 3886-95.
28.Aung KC, Feng L, Yaq KB, Sitoh YY, Leong IY, Ng TP. Serum albumin and hemoglobin are associated with physical function in community-living older persons in Singapore. J Nutr Health Aging. 2011; 15: 877-82.
29.Penninx BW, Guralnik JM, Onder G, Ferrucci L, Wallace RB, Pahor M. Anemia and decline in physical performance among older persons. Am J Med. 2003; 115: 104-10.
30.Morishita S, Kaida K, Yamauchi S, Sota K, Ishii S, Ikegame K, et al. Relationship between corticosteroid dose and declines in physical function among allogeneic hematopoietic stem cell transplantation patients. Support Care Cancer. 2013; 21: 2161-9.
31.Mithal A, Bonjour JP, Boonen S, Burckhardt P, Degens H, El Hajj Fuleihan G, et al. Impact of nutrition on muscle mass, strength, and performance in older adults. Osteoporos Int. 2013; 24: 1555-66.
32.Pallua S, Giesinger J, Oberguggenberger A, Kemmler G, Nachbaur D, Clausen J, et al. Impact of GvHD on quality of life in long-term survivors of haematopoietic transplantation. Bone Marrow Transplant. 2010; 45: 1534-9.
33.Wong FL, Francisco L, Togawa K, Bosworth A, Gonzales M, Hanby C, et al. Long-term recovery after hematopoietic cell transplantation: predictors of quality-of-life concerns. Blood. 2010; 115: 2508-19.
34.Gifford G, Sim J, Horne A, Ma D. Health status, late effects and long-term survivorship of allogeneic bone marrow transplantation: a retrospective study. Intern Med J. 2014; 44: 139-47.
35.Morishita S, Kaida K, Yamauchi S, Wakasugi T, Yoshihara S, Taniguchi K, et al. Gender differences in health-related quality of life, physical function and psychological status among patients in the early phase following allogeneic haematopoietic stem cell transplantation. Psychooncology. 2013; 22: 1159-66.
36.Inoue J, Kai M, Doi H, Okamura A, Yakushiji K, Makimura D, et al. Association between physical function and health-related quality of life in survivors of hematological malignancies undergoing hematopoietic stem cell transplantation. Trends in Transplantation. 2021; 14: 1-5.
37.Schmitz KH, Courneya KS, Matthews C, Demark-Wahnefried W, Galvão DA, Pinto BM, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010; 42: 1409-26.
Search
News