Volume 7 (2024) Issue 2 No.4 Pages 49-55
Abstract
Infection is a major contributor to non-relapse mortality in allogeneic hematopoietic stem cell transplantation (allo-HSCT). Detecting infectious diseases in febrile patients during pretransplant conditioning is crucial for subsequent transplant success. Procalcitonin (PCT) is an auxiliary diagnostic marker of severe bacterial infections and has been proposed as a useful predictor of infection in patients undergoing allo-HSCT. Pre-transplant use of anti-thymocyte globulin (ATG) can cause side effects, such as fever and hypotension, which must be distinguished from infectious diseases. Although ATG administration may increase PCT levels, data on PCT levels in febrile patients after ATG administration are limited. Furthermore, no studies have compared PCT levels during allo-HSCT conditioning using ATG or non-ATG regimens. To investigate whether ATG increases PCT levels during febrile episodes in pre-transplant conditioning and whether PCT could be used to discriminate infections during this period, we analyzed 17 ATG and 59 non-ATG patients with fever and who underwent PCT level measurements during pre-transplant conditioning. Our findings revealed that ATG administration was the only significant factor that increased PCT positivity during fever (p = 0.01). In contrast, infectious diseases did not affect PCT positivity in the ATG group (p = 0.24). Furthermore, bloodstream infection was a significant risk factor for PCT positivity in patients who received non-ATG regimens (p < 0.01). Incorporating PCT levels into the diagnostic workup for infectious diseases requires careful consideration, particularly for patients receiving ATG regimens.
Introduction
Infection is a significant factor for non-relapse mortality during allogeneic hematopoietic stem cell transplantation (allo-HSCT)1. During the pre-transplant conditioning stage of allo-HSCT, the risk of infection increases, and the presence of infection during this stage affects subsequent transplant success2. Therefore, the early detection and appropriate treatment of infectious diseases during the conditioning stage are critical for successful transplantation.
In 1992, procalcitonin (PCT) was identified by Nylen as a marker of severe inflammation3. In severe bacterial or fungal infections, pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), are produced, and PCT is secreted into the blood by various organs throughout the body4. PCT is widely used as an auxiliary diagnostic marker for severe bacterial infections. PCT levels are also elevated in patients with hematological or immunosuppressive disorders, both before and after allo-HSCT, and several studies have suggested that PCT can be used to predict infections in this setting5–8. However, there have been some negative reports9, 10, and opinions on this issue are inconsistent.
Antithymocyte globulin (ATG) is commonly used as a conditioning regimen to prevent graft rejection and graft-versus-host disease (GVHD) by removing the donor lymphocytes11. However, it can cause systemic reactions, such as fever and hypotension12. Therefore, it is important to distinguish these symptoms from those of infectious diseases. Although ATG administration in allo-HSCT conditioning regimens is known to increase PCT levels13, 14, the PCT levels in febrile patients following ATG administration have not yet been reported. Furthermore, no study has directly compared the increase in PCT levels during conditioning for allo-HSCT and its risk factors between ATG and non-ATG regimens.
To investigate whether ATG administration could increase PCT levels during febrile episodes in the context of pretransplant conditioning and whether PCT could be used to discriminate infections during this period, we retrospectively analyzed 76 patients who developed febrile episodes during pretransplant conditioning and had their PCT levels measured at our institution.
Patients and Methods
Patients and clinical data
Between January 2012 and September 2014, when ATG was actively used at our institution, 94 patients underwent allo-HSCT at the Kyushu University Hospital. Among them, 76 patients who had no fever or documented infections (DIs) before conditioning (ATG administration for patients on an ATG regimen) and who developed fever followed by PCT measurements during conditioning for allo-HSCT were retrospectively analyzed. For all 76 patients, complete clinical data, such as age, sex, donor stem cell source, primary disease, disease status prior to allo-HSCT, and conditioning regimen, were collected (Table 1). Reduced-intensity conditioning was defined as a regimen with total body irradiation of less than 8 Gy, a melphalan dose of 140 mg/sqm or less, and a busulfan dose of 8 mg/kg or less administered orally or intravenously at equivalent dosages15. This retrospective study was approved by the Institutional Review Board of Kyushu University Hospital (No. 22327-00). The Institutional Review Board approved this study with opt-out consent.
Definition of febrile episodes
Fever was defined as an axillary body temperature of 37.5
Laboratory data measurement and assessment
Blood samples were obtained within 24 h (on the same day or the next calendar day) after the fever reached 37.5
Statistical analysis
Fisher's exact test or the chi-square test was used to compare categorical variables, while the Kruskal-Wallis U test was used to compare continuous variables. Univariate analysis was performed using logistic or exact logistic regression analysis, and parameters with p-values less than 0.10 were re-evaluated using multivariate analysis. Multivariate analysis was performed using logistic regression with Firth's bias reduction. A p-value less than 0.05 was considered statistically. Statistical analyses were performed using EZR16 software and GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA).
Results
PCT positivity upon fever during the conditioning stage in non-ATG versus ATG regimens
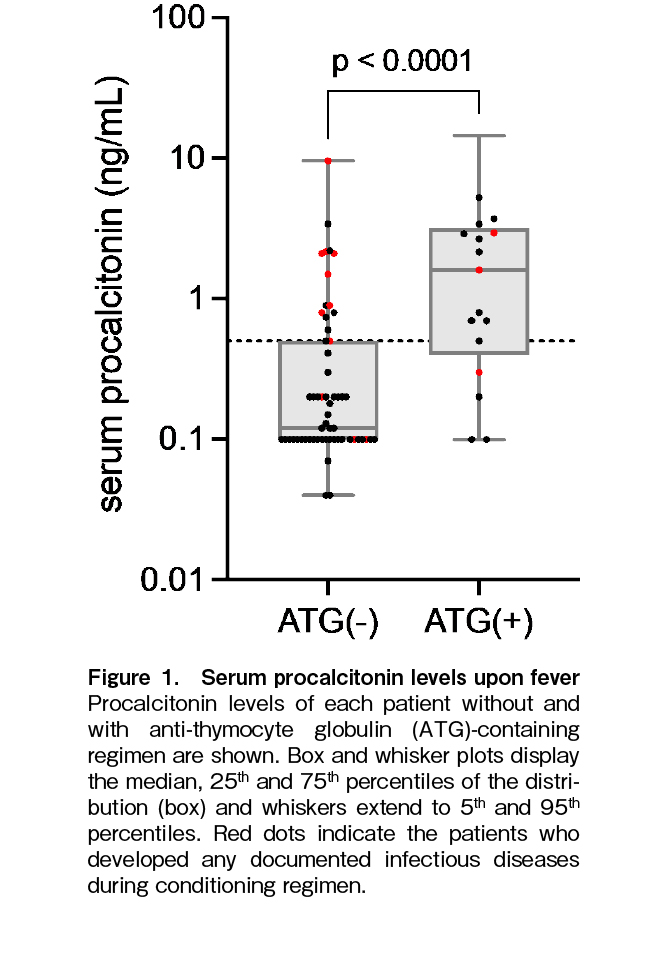
Among the 76 patients who developed fever during the conditioning stage and had their PCT levels measured, 48 were PCT-negative and 28 were PCT-positive (Table 1). Although it is recognized that ATG administration in conditioning regimens for allo-HSCT leads to elevated PCT levels13, 14, there is a lack of reported data on PCT levels in
We further analyzed whether the patients' clinical characteristics had an impact on PCT positivity upon fever onset during the conditioning stage in the non-ATG and ATG groups. Of the 59 patients who received non-ATG regimens, 44 were PCT-negative and 15 were PCT-positive. When clinical characteristics were compared, no significant differences in PCT positivity were observed (Table 2). Of the 17 patients who received an ATG regimen, four were PCT-negative and 13 were PCT-positive. When clinical characteristics were compared, no significant differences in PCT positivity were observed (Table 2).
BSI poses a risk for PCT positivity in patients treated with a non-ATG regimen
PCT is used as a marker for DIs, including BSI and sepsis, in many clinical cases4, and it is important to differentiate infectious diseases when PCT is positive during allo-HSCT conditioning. Therefore, we investigated the effect of infectious disease development on PCT positivity upon fever during conditioning for all-HSCT in the non-ATG and ATG groups.
First, we conducted a comprehensive analysis of DIs and BSIs as indicators of infectious diseases. Among the 44 patients with a negative PCT result in the non-ATG regimen group, infection occurred in three cases, while among the 15 cases with a positive PCT result, eight cases developed infections (p = 0.0003), indicating a significant association between PCT positivity and infection in this group. In contrast, in the ATG regimen group, among the four patients with a negative PCT result, one developed infection, and among the 13 patients with a positive PCT result, two developed infection (p = 1.00), suggesting a mixture of infectious and non-infectious cases in the ATG regimen group (Figure 1).
These data were reevaluated based on the use of ATG and the presence of infectious diseases. As shown in Figure 2, the PCT levels were significantly higher in the non-ATG group with infectious diseases, the ATG group without infectious diseases, and the ATG group with infectious diseases than in the non-ATG group without infectious diseases. However, no significant differences in PCT levels were observed among the three groups.
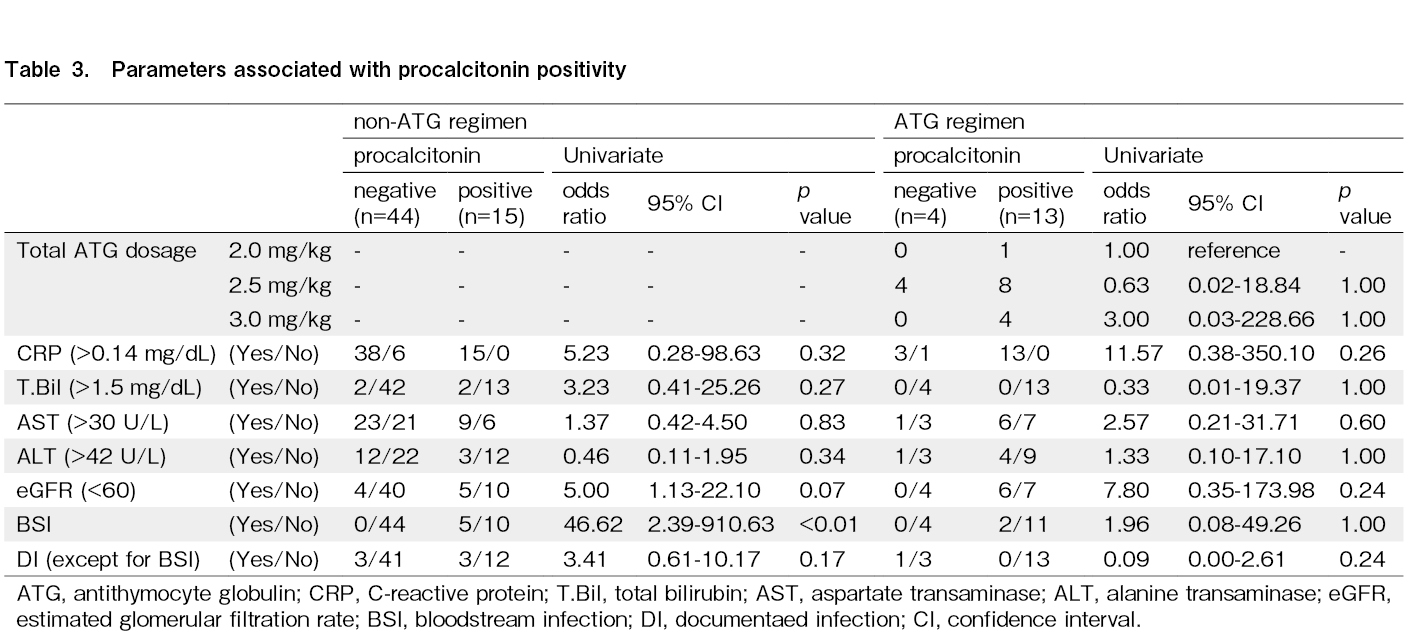
We conducted an analysis to differentiate between infectious diseases, DIs, and BSIs. In addition, the impact of the administered ATG dosage, C-reactive protein (CRP) levels, hepatic function, and renal function on PCT positivity was evaluated. The causative pathogens in the five BSI cases in the non-ATG group comprised two cases of Staphylococcus epidermidis, two cases of Corynebacterium species, and one case of Enterobacter cloacae. Additionally, in the two BSI cases in the ATG group, the pathogens were identified as Corynebacterium jeikeium and Staphylococcus caprae. CRP positivity; elevated total bilirubin, aspartate aminotransferase, or alanine aminotransferase levels; and a reduced estimated glomerular filtration rate had no significant impact on PCT positivity in the non-ATG group. Regarding infectious diseases, while patients with BSI had significant PCT positivity, DIs other than BSI had no effect on PCT positivity in the non-ATG group (Table 3). In the ATG group, the administered ATG dose; CRP positivity; elevated total bilirubin, aspartate aminotransferase, or alanine aminotransferase levels; and reduced estimated glomerular filtration rate had no significant impact on PCT positivity (Table 3).
Discussion
In this study, we revealed that the use of an ATG regimen in pre-transplant conditioning is a risk factor for elevated PCT levels. Furthermore, while elevated PCT levels are indicators of BSI risk in patients receiving non-ATG regimens, they are not predictors of BSI or DI in those receiving ATG regimens.
Broska et al. reported that PCT levels increased 1 day after ATG administration in patients undergoing allo-HSCT conditioning, and this increase persisted for 3 days, despite the absence of any obvious infection13. However, only one of the 26 patients, who received ATG treatment in their study, had fever, with a 3% incidence, which is significantly lower than the reported incidence of fever above 38
With the widespread use of posttransplant cyclophosphamide (PTCy) regimens in recent years18, the use of ATG regimens in our hospital has decreased. Given the recent progress in supportive therapies, such as antibiotics and antifungal agents, the incidence of infections may vary depending on the use of different supportive therapies. Therefore, we limited this study to cases transplanted before the introduction of the PTCy regimen in our institution and found that the PCT positivity rate was 76.5%, which was significantly higher than that in the non-ATG conditioning group during fever (25.4%).
PCT is produced in a variety of organs, including the lungs, kidneys, liver, adipose tissue, and muscles, in response to inflammatory cytokines such as TNF-α and interleukin-6 (IL-6), which are typically released following infections19. While PCT levels significantly increase after ATG treatment in the context of pre-transplant conditioning13, the mechanism underlying the lack of PCT increase in some patients remains unclear. Kuse reported that PCT levels increased to 12.1 ± 6 ng/mL within 24 h of administering anti-thymocyte OKT3 to liver transplant patients, despite the absence of infection20. They suggested that the TNF-α released from necrotic tumors caused by OKT3 contributed to the increase in PCT levels. However, no correlation was found between the presence or absence of tumors (remission or non-remission) and the rate of PCT positivity following ATG administration in our study. Furthermore, Pihusch et al. reported an increase in PCT levels in patients who received ATG for GVHD prevention after HSCT, and suggested a relationship between the increase in CRP and IL-6 levels21. However, because these patients had previously received immunosuppressive therapy, a direct comparison with our data is difficult. Although TNF-α and IL-6 levels were not measured in our study, no significant correlation was found between the PCT positivity rate and the increase in CRP levels in the ATG group. As ATG is a polyclonal antibody22, differences in immunogenicity between different lots may affect the extent of induction of inflammatory cytokines. ATG also contributes to the elimination of T-cells via mechanisms such as antibody-dependent cellular cytotoxicity, complement-dependent cellular cytotoxicity, and apoptosis22. Thus, differences in antigenicity, including genetic polymorphisms, or in the activity of residual immune cells, such as natural killer cells, may be related to the differences in PCT levels after ATG administration. Accumulating data on lot-to-lot comparisons, genetic polymorphism analyses, and residual immune cell activity analyses are necessary to clarify the mechanisms underlying the increase in PCT levels with the ATG regimen.
Notably, when fever occurs during ATG administration and PCT yields a positive result, false-positive results may occur. Nonetheless, 15% of the cases with positive PCT results in the ATG group were attributed to BSIs in our study. Furthermore, DIs were observed in some patients, both with and without ATG use, despite no increase in PCT levels. Given the importance of promptly diagnosing and treating infections in the context of transplant therapy, physicians must exercise caution when relying heavily on PCT findings. While false positives for PCT were more prevalent in the ATG group, it is imperative to thoroughly screen for infections in patients with positive PCT outcomes. In the future, other biomarkers in addition to PCT may have potential value in the detection of infections and ATG-induced fever.
The findings of this study indicate that elevated PCT levels are not an indicator of infection in febrile recipients receiving an ATG-containing pre-conditioning regimen. However, it is worth noting that this study was retrospective and only investigated data related to fever onset. To elucidate the significance of ATG-induced PCT elevation and the associated risks of infection, a large-scale prospective study on PCT and IL-6 and TNF-α levels, as well as other relevant factors, from pre-treatment to post-ATG administration over a sufficient duration is warranted.
Acknowledgments
The authors thank the medical and nursing staff of Kyushu University Hospital.
Author Contributions
T.S. coordinated the project, performed the allo-SCT, designed and analyzed the data, and wrote the manuscript; M.M., T.T., Y.K., F.J., T.Y., Y.M., G.Y., S.M., T.M., and K.K. performed allo-SCT, organized patient information and reviewed the manuscript; and K.A. designed the study, reviewed the manuscript, and edited the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Styczyński J, Tridello G, Koster L, Iacobelli S, van Biezen A, van der Werf S, et al. Death after hematopoietic stem cell transplantation: changes over calendar year time, infections and associated factors. Bone Marrow Transplant. 2020; 55: 126-36.
2.Gea-Banacloche J. Risks and Epidemiology of Infections After Hematopoietic Stem Cell Transplantation. In: Ljungman P, Snydman D, Boeckh M, guest editors. Transplant Infections, 4th ed., Springer Nature. 2016; 81-99.
3.Nylen ES, O'Neill W, Jordan MH, Snider RH, Moore CF, Lewis M, et al. Serum procalcitonin as an index of inhalation injury in burns. Horm Metab Res. 1992; 24: 439-43.
4.Vijayan AL, Vanimaya, Ravindran S, Saikant R, Lakshmi S, Kartik R, et al. Procalcitonin: a promising diagnostic marker for sepsis and antibiotic therapy. J Intensive Care. 2017; 5: 51.
5.Pihusch M, Pihusch R, Fraunberger P, Pihusch V, Andreesen R, Kolb H-J, et al. Evaluation of C-reactive protein, interleukin-6, and procalcitonin levels in allogeneic hematopoietic stem cell recipients. Eur J Haematol. 2006; 76: 93-101.
6.Koya J, Nannya Y, Ichikawa M, Kurokawa M. The clinical role of procalcitonin in hematopoietic SCT. Bone Marrow Transplant. 2012; 47: 1326-31.
7.Sato M, Nakasone H, Terasako-Saito K, Sakamoto K, Yamazaki R, Tanaka Y, et al. Prediction of infectious complications by the combination of plasma procalcitonin level and localized infection before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2014; 49: 553-60.
8.Ortega M, Rovira M, Filella X, Almela M, Puig de la Bellacasa J, Carreras E, et al. Prospective evaluation of procalcitonin in adults with febrile neutropenia after haematopoietic stem cell transplantation. Br J Haematol. 2004; 126: 372-6.
9.Mori Y, Miyawaki K, Kato K, Takenaka K, Iwasaki H, Harada N, et al. Diagnostic value of serum procalcitonin and C-reactive protein for infections after allogeneic hematopoietic stem cell transplantation versus nontransplant setting. Intern Med. 2011; 50: 2149-55.
10.Lyu Y-X, Yu X-C, Zhu M-Y. Comparison of the diagnostic value of procalcitonin and C-reactive protein after hematopoietic stem cell transplantation: a systematic review and meta-analysis. Transpl Infect Dis. 2013; 15: 290-9.
11.Bonifazi F, Rubio M-T, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transplant. 2020; 55: 1093-102.
12.Marsh JC, Gordon-Smith EC. The role of antilymphocyte globulin in the treatment of chronic acquired bone marrow failure. Blood Rev. 1988; 2: 141-8.
13.Brodska H, Drabek T, Malickova K, Kazda A, Vitek A, Zima T, et al. Marked increase of procalcitonin after the administration of anti-thymocyte globulin in patients before hematopoietic stem cell transplantation does not indicate sepsis: a prospective study. Crit Care. 2009; 13: R37.
14.Garg A, Patel K, Shah K, Raj A, Shah S. Surge in Procalcitonin Levels Post ATG During Stem Cell Transplantation for Aplastic Anemia: A Diagnostic Dilemma with Sepsis?. Ann Hematol Oncol. 2021; 8: 1377.
15.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009; 15: 367-9.
16.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
17.Kobayashi R, Kaneda M, Watanabe N, Iguchi A, Cho Y, Yoshida M, et al. [Adverse effects of anti-thymocyte globulin/anti-lymphocyte globulin therapy]. Rinsho Ketsueki. 1999; 40: 531-5.
18.Rimando J, McCurdy SR, Luznik L. How I prevent GVHD in high-risk patients: posttransplant cyclophosphamide and beyond. Blood. 2023; 141: 49-59.
19.Christ-Crain M, Müller B. Procalcitonin in bacterial infections–hype, hope, more or less?. Swiss Med Wkly. 2005; 135: 451-60.
20.Kuse ER, Jaeger K. Procalcitonin increase after anti-CD3 monoclonal antibody therapy does not indicate infectious disease. Transpl Int. 2001; 14: 55.
21.Pihusch M, Pihusch R, Fraunberger P, Pihusch V, Andreesen R, Kolb H-J, et al. Evaluation of C-reactive protein, interleukin-6, and procalcitonin levels in allogeneic hematopoietic stem cell recipients. Eur J Haematol. 2006; 76: 93-101.
22.Ippoliti G, Lucioni M, Leonardi G, Paulli M. Immunomodulation with rabbit anti-thymocyte globulin in solid organ transplantation. World J Transplant. 2015; 5: 261-6.
Search
News