Volume 7 (2024) Issue 2 No.1 Pages 33-36
Abstract
Melphalan-induced encephalopathy is a rare complication observed in patients undergoing autologous stem cell transplantation (ASCT) and is characterized by symptoms ranging from drowsiness to seizures. Previous reports have described similar cases, including a review of a large cohort of patients in whom melphalan-associated encephalopathy was identified in 2% of the patients undergoing ASCT. We describe the case of a 63-year-old male with Multiple Myeloma and underlying chronic kidney disease (CKD) who underwent ASCT with a reduced dose of melphalan due to renal dysfunction in complete remission following induction therapy and subsequent neurological deterioration, which necessitated an extensive evaluation of several neurological and infective etiologies. In this report, we highlight that melphalan-associated encephalopathy is a distinct entity complicating ASCT in patients with myeloma, especially in those with preexisting renal insufficiency, and consider its management.
Introduction
High dose therapy (melphalan) and autologous stem cell transplantation (HDT-ASCT) is a recommended consolidation therapy for eligible patients with Multiple Myeloma (MM)1. Consolidation with autologous stem cell transplantation (ASCT) is equally safe and efficacious in patients with pre-existing chronic kidney disease (CKD)2,3. Melphalan is known to rarely cause neurotoxicity, with a spectrum of presentation ranging from drowsiness to generalised tonic-clonic seizures (GTCS)4. In this report, we present a case of a patient with Myeloma who developed melphalan associated encephalopathy following ASCT and its management in a tertiary care center in India.
Case Report
A 63-year-old male with type II diabetes, hypertension, and stage 4 CKD presented with anemia and worsening renal insufficiency, with IgG kappa monoclonal protein detected by immunofixation electrophoresis (IFE) and diagnosed with Multiple Myeloma (International staging system, ISS stage III). The patient exhibited no high-risk cytogenetic findings. Following four cycles of Bortezomib, Cyclophosphamide and Dexamethasone (CyBorDex), he attained stringent complete remission (sCR). He was scheduled for ASCT with a reduced dose of melphalan (140 mg/m2, 220 mg) because of persistent renal dysfunction. His baseline renal parameters included a serum creatinine of 3.28 mg/dL (eGFR 19.1 mL/min/1.73 m2) while clinical neurological assessment did not reveal any abnormality. He then underwent stem cell mobilization with granulocyte colony stimulating factor (GCSF) and renal dose-adjusted plerixafor, yielding a total nucleated cell dose and CD34 cell dose of 8.88 × 108/kg and 9.56 × 106/kg, respectively. Stem cells were infused 24 h after melphalan administration, with close clinical monitoring of vital parameters and urine output5.
The post-transplantation period was complicated by febrile neutropenia associated with several episodes of small-volume diarrhea on day+5, for which he was administered intravenous (IV) antibiotics at renal-adjusted doses, as described in Figure 1a. Antibiotic escalation was based on the surveillance of stool/throat swab cultures and hemodynamic stability. On day+7, he developed a rapid decline in mentation, which progressed to severe drowsiness, demonstrable flapping tremors, and oliguria (< 15 mL/kg/hour). He was simultaneously scheduled for hemodialysis (HD) from day+7 in view of oliguria and progressive deterioration in mentation, with a maximal pre-dialysis serum creatinine of 6.63 mg/dL (eGFR 8.5 mL/min/1.73 m2). Sepsis screening, including procalcitonin and serial blood and urine cultures, were negative. No focal neurological deficits were observed. Even after 2 HD sessions, his sensorium worsened markedly, necessitating management in the intensive care unit (ICU) with preemptive mechanical ventilation for airway protection. The patient continued to receive renal-dose antibiotics and was supported on HD. A detailed neurological workup was performed, including metabolic screening (glucose, liver function, and serum electrolytes), magnetic resonance imaging (MRI) of the brain, a comprehensive cerebrospinal fluid (CSF) study including multiplex Polymerase chain reaction for
Discussion
High dose therapy (melphalan) and autologous stem cell transplantation is an effective consolidation strategy for patients with multiple myeloma. Guidelines recommend the use of ASCT as consolidation with appropriate melphalan dose reduction in patients with MM even in the presence of CKD6. Studies analysing the impact of ASCT in patients with MM and pre-existing renal dysfunction observed no difference in overall survival (OS)2,3. However, a subgroup of patients with severe renal impairment (defined as estimated glomerular filtration rate below 45 mL/min/1.73 m2) were noted to have a worse OS2. Melphalan 140 mg/m2 was shown to have comparable survival outcomes in patients transplanted in very good partial response or better and this influenced our choice of melphalan dosing7.
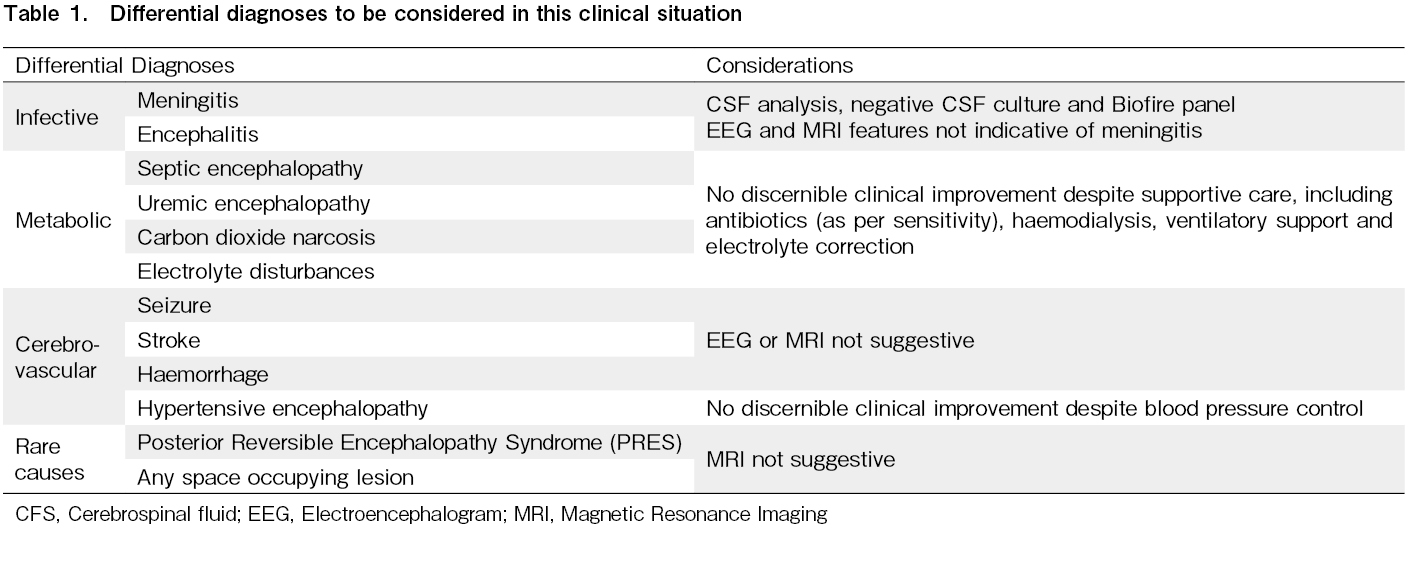
While post-dialysis neurological recovery in uremic encephalopathy can be slow, the rapid worsening of symptoms despite being on HD and receiving optimum antibiotics forced us to consider the possibility of an alternate diagnosis8. A reasonable attempt was made to check for plausible infective, vascular, and metabolic (including alternate drugs) causes of the altered mental status, as described in Table 1. However, they were non-contributory. Sepsis-associated encephalopathy is an important cause of an altered mental state. The lack of sepsis-defining events and the lack of improvement in clinical condition despite optimal antimicrobial use led us to consider drug (melphalan) induced encephalopathy as a possible diagnosis.
The occurrence of encephalopathy following melphalan conditioning was first described in 1999 by Schuh et al. They reported two patients with myeloma and AL amyloidosis, both with pre-existing renal insufficiency, who had a similar clinical course. One of the two patients displayed recovery by day+18 post-transplant, while the other succumbed to respiratory complications by day+259. Alayón-Laguer et al. reported a similar case of a 60-year lady with MM and CKD who developed slow mentation progressing rapidly over a few days. They also reported a similar sudden improvement in the neurological status with no sequelae of note10.
The largest consolidated review of this entity was reported by the MD Anderson Cancer Center, in which 2% of patients undergoing ASCT after melphalan conditioning (n=451) developed melphalan-associated encephalopathy at a median of 13 days (range 4-22) after ASCT4. Patients developed symptoms ranging from drowsiness and confusion to loss of consciousness, while one patient had GTCS. The study patients had no identifiable cause on evaluation, and complete resolution of neurological symptoms was observed in all patients prior to hospital discharge, as observed in our patient. Notably, only two of the nine patients (22%) had baseline renal insufficiency in this study. Whether pre-existing renal insufficiency portends the development of melphalan encephalopathy remains unclear owing to its rare occurrence and conflicting data. However, what is certain is that our patient had a comparably distinct clinical course, a suboptimal response to therapeutic interventions and infection control measures as well as an extensive battery of investigations that yielded no conclusive evidence of a definitive diagnosis.
These situations can be challenging during the post-transplantation period. However, recognition of the clinical entity (melphalan-induced encephalopathy) can help optimize management with reasonable expectations of recovery. Collaborative multicenter observational studies are required to establish the epidemiology and provide evidence-based management strategies.
Conclusion
Melphalan-induced encephalopathy is a distinct condition that complicates ASCT in patients with myeloma, especially in those with pre-existing renal insufficiency. Management involves multidisciplinary team efforts, optimal critical care strategies, and adequate laboratory support to assist in excluding other diagnoses. Patients typically experience a complete clinical and neurological recovery.
Author Contributions
Concept and design: AN, RN; Literature search: VAG, SS, AN; Clinical Management: AN, VAG, DP, JK, SB, RN; Laboratory studies: DKM; Data acquisition, data analysis: VAG, SS, AN; Manuscript preparation: VAG, SS, AN; Manuscript editing: AN, RN; Manuscript review: RN, DP, DC, DKM, JK, SB, MC; IA Guarantors: AN, RN
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Data Sharing Statement
Data and materials are available to the corresponding author upon reasonable request.
Ethics Approval
Institutional Review Board (Ethics Committee) waiver No. EC/WV/TMC/20/23; Case report with no patient personal identifiers.
Consent for Publication
Yes, consent was obtained from all patients.
References
1.Mikhael J, Ismaila N, Cheung MC, Costello C, Dhodapkar MV, Kumar S, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019; 37: 1228-63.
2.Antlanger M, Dust T, Reiter T, Böhm A, Lamm WW, Gornicec M, et al. Impact of renal impairment on outcomes after autologous stem cell transplantation in multiple myeloma: a multi-center, retrospective cohort study. BMC Cancer. 2018; 18: 1008.
3.Lazana I, Floro L, Christmas T, Shah S, Bramham K, Cuthill K, et al. Autologous stem cell transplantation for multiple myeloma patients with chronic kidney disease: a safe and effective option. Bone Marrow Transplant. 2022; 57: 959-65.
4.Najera JE, Sudhakar T, Bashir Q, Shah N, Champlin RE, Qazilbash MH, et al. Neurotoxicity after high-dose melphalan. J Clin Oncol. 2012; 30 (Suppl 15): 6546.
5.Shuman K, Palmer S, Anders B, Moore D, Ptachcinski J, Grgic T, et al. Correlation of Engraftment and Time from Melphalan Administration to Stem Cell Infusion. Transplant Cell Ther. 2023; 29: 36.e1-5.
6.Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E, et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J Clin Oncol. 2016; 34: 1544-57.
7.Auner HW, Iacobelli S, Sbianchi G, Knol-Bout C, Blaise D, Russell NH, et al. Melphalan 140 mg/m2 or 200 mg/m2 for autologous transplantation in myeloma: results from the Collaboration to Collect Autologous Transplant Outcomes in Lymphoma and Myeloma (CALM) study. A report by the EBMT Chronic Malignancies Working Party. Haematologica. 2018; 103: 514-21.
8.Wijdicks EFM. Identifying encephalopathies from acute metabolic derangements. J Intern Med. 2022; 292: 846-57.
9.Schuh A, Dandridge J, Haydon P, Littlewood TJ, et al. Encephalopathy complicating high-dose melphalan. Bone Marrow Transplant. 1999; 24: 1141-3.
10.Alayón-Laguer D, Alsina M, Ochoa-Bayona JL, Ayala E. Melphalan Culprit or Confounder in Acute Encephalopathy during Autologous Hematopoietic Stem Cell Transplantation?. Case Rep Transplant. 2012; 2012: 942795.
Search
News