Volume 7 (2024) Issue 1 No.1 Pages 1-9
Abstract
Introduction: Temcell is a mesenchymal stem cell (MSC) product approved for steroid-refractory acute graft-versus-host disease (SR-aGVHD) in Japan. However, reports regarding Temcell's efficacy in pediatric patients have been scarce, and the appropriate use of MSC therapy against pediatric SR-aGVHD also remains to be determined.
Patients and Methods: We retrospectively assessed a cohort of pediatric patients treated with Temcell for SR-aGVHD following allogeneic hematopoietic transplantation. MSCs were infused intravenously at a dose of 2 × 106 cells/kg according to the manufacturer's instructions.
Results: Twelve patients received eighteen cycles of MSC therapy (median age, 10.3 [1.7-17.8] years), with four receiving additional cycles (one cycle: n = 3, three cycles: n = 1). The severity of aGVHD before MSC therapy was grade I-II in three patients and grade III-IV in nine patients (gut stage 3-4, n= 7; liver stage 3-4; n =2). The median number of immunosuppressive therapy regimens received prior to MSC administration was two (range: 1-5). The first MSC cycle displayed the best overall response rate of 83%, including six patients with a complete response (CR) and with a 49% reduction in the mean daily dose of prednisone after eight weeks. The median time to first response was 3.5 days (range: 2-15 days). Two of the four patients who were re-administered MSCs for recurrent or persistent GVHD achieved a CR. The three-year overall survival rate was 69.4%, while the three-year failure free survival (FFS) rate was 22.2%, with a median FFS of 4.9 months. There were no observable side effects of MSC therapy.
Conclusions: MSC therapy appears to be an effective and safe treatment for pediatric SR-aGVHD, with a steroid-sparing effect and satisfactory efficacy upon re-administration. Further studies are needed to determine its appropriate combination with additional treatments and the optimal use of re-administration of MSCs.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is a well-established therapeutic approach for pediatric patients with malignant and nonmalignant hematopoietic diseases, primary immunodeficiencies, and metabolic inborn errors. Acute graft-versus-host disease (aGVHD) is a common complication associated with allogeneic HSCT, and systemic corticosteroids are currently the standard first-line treatment for aGVHD. However, the response rates are unsatisfactory; ranging from 40% to 60%1–3. Outcome predictions for patients with steroid-refractory aGVHD (SR-aGVHD) remain inadequate, and no clear consensus has yet been reached on the second- and additional next-line treatments for SR-aGVHD. Ruxolitinib is the only drug that has been proven to be significantly more effective than the control in a randomized trial, and has become the first US Food and Drug Administration-approved treatment for SR-aGVHD in patients over 12 years of age4. However, the durable overall response rate to ruxolitinib is still only approximately 40%, necessitating the implementation of complementary strategies to more effectively treat SR-aGVHD.
Mesenchymal stem cell (MSC) therapy is a treatment option for SR-aGVHD. A large amount of clinical data has been published on the use of MSCs in the treatment of GVHD. Data from various clinical trials is considerably heterogeneous, owing to variations in MSC dosage, sources, and patient characteristics, with reported overall response rates (ORR) for aGVHD ranging from 40.6% to 100%5. A phase 3 randomized trial comparing the commercially available MSC product remestemcel-L (Ryoncil, Mesoblast, Ltd, Melbourne, Australia) with placebo, in addition to second-line therapy to treat SR-aGVHD, failed to attain the primary endpoint of a more durable complete response; however, post hoc analysis revealed that pediatric patients in the remestemcel-L arm had a considerably higher ORR than those in the placebo arm (64% vs. 23%; P = 0.05)6. Temcell (JCR Pharmaceuticals Co. Ltd, Ashiya, Japan) is an MSC product equivalent to remestemcel-L and has been approved for the treatment of SR-aGVHD in Japan7,8. Recently, Murata et al. reported that Temcell had an ORR of approximately 60% in adult and pediatric patients with acute GVHD9. This retrospective study found no significant differences in the efficacy of Temcell treatment across age groups. However, there have been scarce reports on the efficacy of remestemcel-L/Temcell in pediatric patients, and the appropriate use of MSC therapy for SR-aGVHD remains unknown. Furthermore, only a limited number of studies have examined the efficacy and safety of MSC re-administration.
Here, we present our findings based on observations from pediatric patients who received Temcell to treat SR-aGVHD following allogeneic HSCT, including those who received re-administration of MSCs.
Patients and Methods
We retrospectively studied pediatric patients who received Temcell for SR-aGVHD after allogeneic HSCT at the Saitama Children's Medical Center between April 2016 and October 2021. Data were collected through a retrospective chart review. The data cut-off date was May 1, 2022. The study protocol was approved by the Institutional Review Board of Saitama Children's Medical Center (No.2021-02-033). The aGVHD was diagnosed and graded using standard criteria10. The analysis also included cases of aGVHD that recurred or developed more than 100 days after transplantation11. When aGVHD was diagnosed, corticosteroids and an optimal dose of calcineurin inhibitors were initially administered. The SR-aGVHD was defined as aGVHD progression within 3-5 days from initialization of first-line therapy with 2 mg/kg/day of prednisolone (PSL) equivalent, or failure to improve within 5-7 days after beginning of the treatment12. The MSCs were infused intravenously at a dose of 2 × 106 cells/kg as directed by the manufacturer. A four-week cycle of MSC therapy consisted of eight biweekly infusions, with the option of four additional weekly infusions. Patients who demonstrated a partial response to MSCs but poor GVHD control were administered MSCs again. Response to MSC treatment was defined as follows: Complete response (CR); Resolution of all symptoms of GVHD. Partial response (PR); Improvement in at least one stage of acute GVHD severity in one organ without progression in others. Stable disease (SD); Absence of improvement. Progressive disease (PD); Progression of one or more organs without improvement in others. The ORR represents the sum of the CR and PR rates. Clinical responses were assessed at 28, 56, and 100 days following the first MSC infusion. The definitions for a myeloablative conditioning (MAC) regimen included: (i) total body irradiation of ≥5 Gy as a single fraction or ≥8 Gy if fractionated, (ii) >8 mg/kg of oral busulfan or >6.4 mg/kg of intravenous busulfan, (iii) >140 mg/
The cumulative incidence method was used to calculate the probability of achieving CR or PR, together with the associated 95% confidence intervals (CI). The cumulative incidence of the response was examined using the competing risk method, with death or disease progression in the absence of a response considered as the competing risk. The Kaplan-Meier method was used to calculate the overall survival (OS) and failure-free survival (FFS) rates, as well as their associated 95% CI. Survival time was measured from the first MSC infusion to the date of death or last follow-up. The FFS was defined as the time from the start of MSCs therapy to the relapse or progression of hematologic disease, non-relapse-related death, or addition of new systemic therapy for aGVHD.
Results
We examined 12 pediatric patients who received MSC therapy at our facility, and patient characteristics are summarized in Table 1 and the
The median onset of aGVHD after transplantation was 21.5 days (range, 10-52 days), while that of SR-aGVHD was 27 days (range, 11-57 days) (Table 2). Four patients developed persistent or recurrent SR-aGVHD after more than 100 days. Before MSC therapy, one patient presented with grade I aGVHD (overlapping chronic skin GVHD), two with grade II, four with grade III, and five with grade IV, while nine patients exhibited two or more organ abnormalities. Skin, gut, and liver involvement was observed in 92%, 58%, and 25% of the patients, respectively. The median time from the diagnosis of SR-GVHD to the start of MSC administration was 37 days (range, 6-259 days). The median time to begin the first cycle after transplantation was 64 days (range, 34-276 days). MSC therapy was administered after a median of two prior ineffective immunosuppressive therapies (range: 1-5 days). The number of MSCs per infusion was 2 × 106 cells/kg in all patients. The most common number of MSC doses for initial treatment was 12, the maximum number of doses per cycle, for nine cases, eight doses for two cases, and only one case was terminated early due to death from a cause other than GVHD. Eight patients received one cycle of MSC treatment, and four received additional cycles (one cycle, n = 3; three cycles, n = 1).
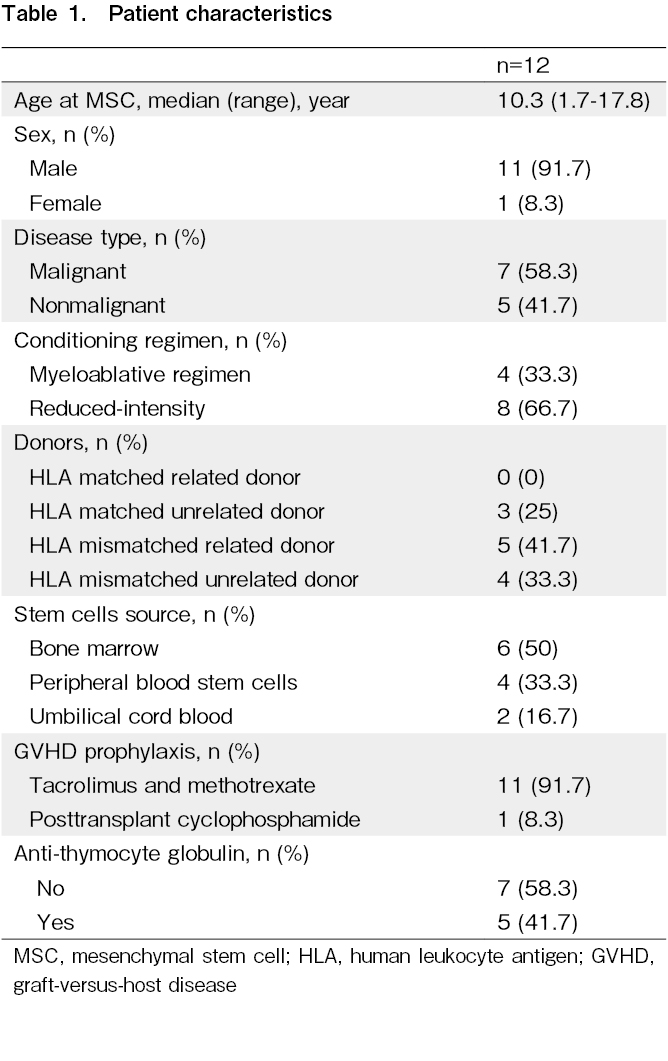
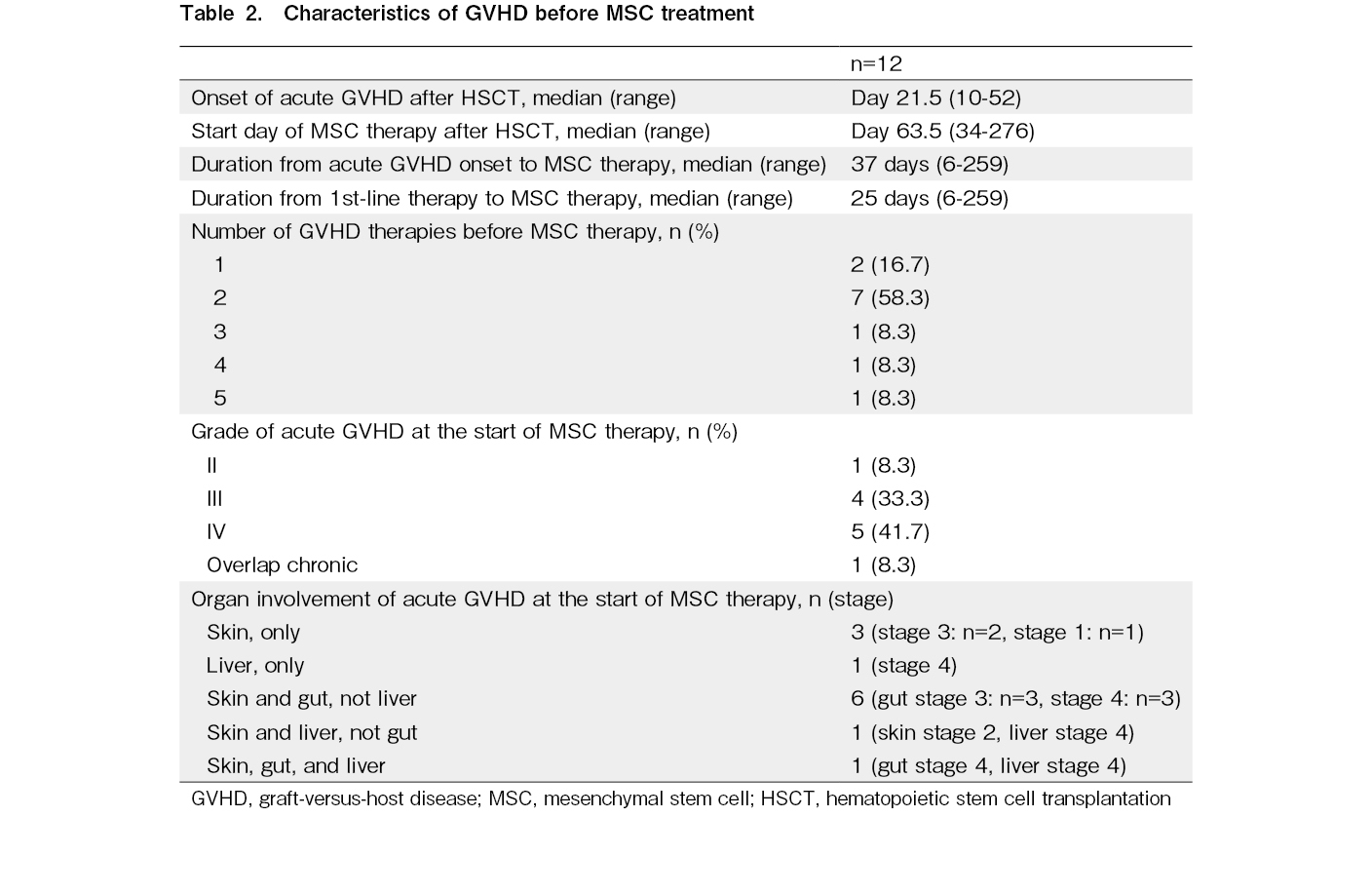
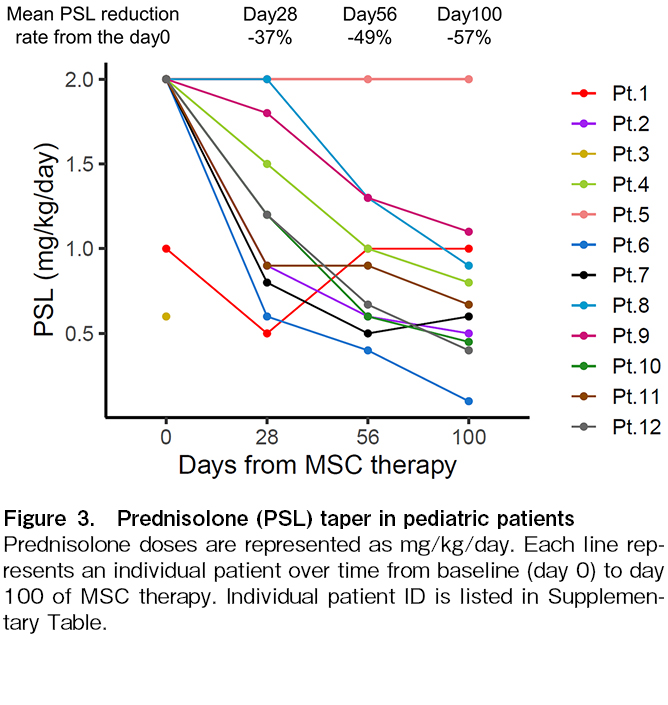
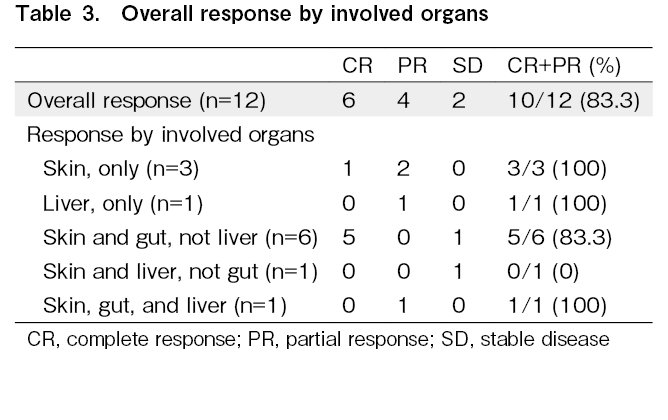
The ORR for aGVHD on day 28 after the first MSC was 75% (25% for CR, and 50% for PR) (Figure 1). The durable ORR on days 56 and 100 were 75% (50% for CR, and 25% for PR) and 67% (42% for CR, and 25% for PR), respectively. One patient died of TMA and intra-abdominal hemorrhage within 30 days of starting MSC therapy and was not evaluated for response on days 56 and 100. The 28-day ORR and CR rates for the nine patients with grade III-IV aGVHD were 67% and 22%, respectively, and the corresponding 56-day ORR and CR rates were 67% and 56%. The cumulative incidence of overall response was 83% (95% CI, 40.9%-95.3%). Many patients demonstrated a therapeutic effect soon after treatment began, with the median onset of the therapeutic effect being 3.5 days (range, 2-15) (Figure 2). The steroid-sparing effect was investigated as a treatment-response metric. The average daily dose of steroids gradually declined after the start of MSC therapy, with a 49% reduction (range, 0-80) by day 56 (Figure 3). In terms of the best overall response in GVHD-affected organs, CR was achieved in 33% (n = 1) of patients with skin GVHD alone, 83% (n = 5) of patients with skin and intestinal but not liver GVHD, while patients with liver GVHD (n = 3) did not show evidence of CR (Table 3).
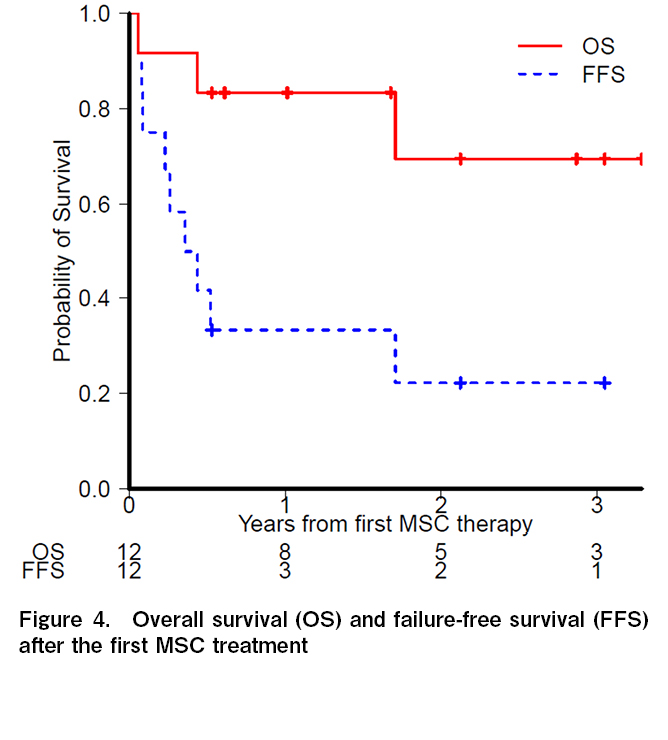
The median follow-up period for the surviving patients was 755 days (range, 193-2,177 days). The three-year OS rate was 69.4% (95% CI, 29.7%-89.6%) (Figure 4). The leading causes of death were primary disease (n = 2) and TMA (n = 1). No deaths were associated with aGVHD progression. During the 18 cycles of MSC treatment, there were no considerable adverse events that could be attributed to the MSCs. No infusion reactions or ectopic tissue formation were observed. TMA (n = 3), viral hemorrhagic cystitis (n = 2), sepsis (n = 1), and chronic inflammatory demyelinating polyneuropathy (n = 1) were observed within 100 days of MSC administration; however, there was no clear association with the MSC treatment. The 3-year FFS rate was 22.2% (95% CI, 4.1%-49.2%), with a median FFS of 4.9 months. Of the patients who achieved CR or PR following MSC treatment, six patients (Pt. 2, 4, 6, 9, 10, and 11 in
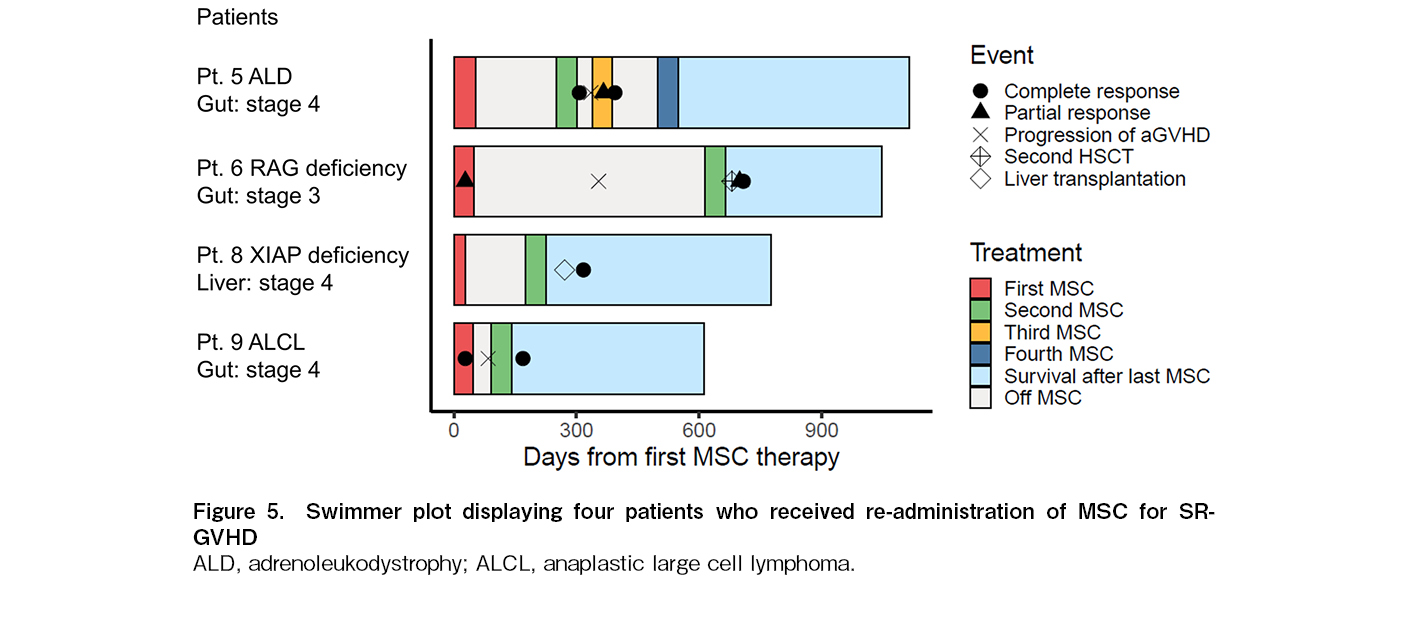
The MSCs were re-administered to four patients (Pt. 5, 6, 8, and 9) with relapsed or persistent GVHD (Figure 5); one patient (Pt. 5) received four cycles, and the remaining three patients received two cycles. Three patients underwent HSCT for non-malignant diseases. Two patients (Pt. 5 and 9) responded after the second administration of MSCs. Although Pt. 5 did not respond to the first round of MSC treatment for steroid-resistant stage 4 gut GVHD, CR was achieved by re-administration of MSCs (197 days after the first MSC dose administration) with ATG for persistent GVHD, after the failure of PSL and MMF. Subsequently, the provided third and fourth MSC treatments (37 and 111 days after the last dose, respectively) with ATG for gut GVHD relapse, resulted in CR and PSL reduction in each cycle. Pt.9, who presented with stage 4 gut GVHD that was refractory to PSL, MMF, ruxolitinib, and ATG, achieved CR after the first administration of MSCs. The patient subsequently experienced a relapse of stage 4 gut GVHD with a poor response to PSL treatment. Therefore, he was administered a second MSC treatment, 43 days after the end of the first MSC regimen and achieved CR. Despite no clinical improvement in GVHD grade after MSC administration for Pt. 6 and 8, Pt. 6 underwent a second HSCT with the addition of MSCs (565 days after the first MSCs) for persistent stage 4 gut GVHD that was resistant to steroids, methotrexate, ruxolitinib, and vedolizumab. Pt. 8 had refractory stage 4 liver GVHD and was treated with MSCs again (147 days after the first MSC regimen), which allowed for a reduction in the steroid dosage and underwent liver transplantation. All patients are currently alive, and there are no apparent side effects from the re-administration of MSCs.
Discussion
The findings presented by our retrospective study herein, showcase encouraging outcomes in a group of pediatric patients treated with Temcell for SR-GVHD. Previous research on remestemcel-L treatment for SR-aGVHD in pediatric patients has yielded promising results14–16. Despite the small number of patients included in this study, the best ORR for SR-aGVHD is 83%, confirming the existing evidence in current literature regarding the efficacy of MSCs against pediatric SR-aGVHD. Our observations are also comparable to previously reported response rates for other GVHD therapies, including ruxolitinib4,17. In addition to a relatively high ORR, MSC therapy may have the advantage of a lower non-relapse mortality associated with infectious complications18. Indeed, the infectious complication rate in the present work was 25% (3/12 cases), which was lower than that reported in other second-line GVHD treatments18. While the ORR at day 28 for ruxolitinib and MSCs was reported to be comparable (62% vs. 60%)4, MSCs are likely to be easier to use in patients with cytopenia and infection, as ruxolitinib has been associated with adverse events such as myelosuppression and viral infections.
There are several possible reasons for the comparatively higher efficacy of MSCs in pediatric patients than that in adult patients. MSC therapy has been shown to shift the Th1/Treg ratio toward a more anti-inflammatory direction in pediatric patients than in adults, which may explain why pediatric patients have a higher ORR, relative to adult patients19,20. Furthermore, adult patients frequently switch to other immunosuppressive agents before MSCs, usually after two to six lines of failure, whereas in children, MSCs are frequently administered shortly after steroids21. The benefit of using MSCs early is that patients do not require extensive pretreatment and do not develop irreversible tissue damage or life-threatening infections5. Temcell has been used more frequently in recent years for SR-aGVHD, either in conjunction to steroids, or in the early stages after adding methotrexate until Temcell could be arranged, which may explain why the observed ORR is so high in our study. Further research is needed to validate the early use of Temcell in the treatment of SR-type aGVHD in pediatric patients.
Previous studies have found that cutaneous GVHD has a higher OR than gut or hepatic GVHD after MSC treatment22–24. Ball et al. reported that pediatric patients with skin GVHD responded well and rapidly to MSC infusion, however, the complete normalization of gastrointestinal symptoms and liver function required a longer recovery time14. MSC therapy has been shown to have a positive clinical effect on gut aGVHD. In a multicenter phase I/II clinical trial using Temcell to treat SR-aGVHD, 10 out of 14 patients had bowel involvement, and all but one achieved CR after MSC treatment7. Other clinical trials have returned similar promising results for the treatment of gastrointestinal GVHD using MSCs8,25,26. The effect of MSCs on gut GVHD may be attributed not only to their immunomodulatory effect, but also to their ability of repairing damaged intestinal epithelium27,28. In our work, Temcell showed promising efficacy in gut GVHD, underscoring its preferential usage to treat intestinal SR-GVHD in particular. In contrast, our results showed that none of the three patients with liver GVHD achieved CR, implying that the treatment response was insufficient for severe liver GVHD. This is consistent with previous observations that have identified liver GVHD as a significant predictor of a poor response to MSC therapy24,25,29–31. However, because liver GVHD is not always diagnosed based on histopathology but rather on clinical features, such as elevated bilirubin levels, it has been suggested that the effect of treatment on GVHD may not be accurately evaluated when other causes of GVHD are present6. Further research is warranted to determine how accurately liver GVHD may predict a poor response to MSC therapy.
The re-administration of MSCs for recurrent aGVHD is reportedly safe and efficient. Muroi et al. observed that when Temcell was re-administered to only 1 out of 25 patients with SR-GVHD, CR was preserved, and the patient survived8. However, to date, no other reports have further described the re-administration of Temcell. It has been proposed that one or two MSC infusions are insufficient to maintain CR26,32, and that additional MSC infusions are required to maintain treatment efficacy6,31,33. Although the optimal number of infusions has not yet been determined, a maximum of 12 infusions per cycle is recommended to preserve CR if a therapeutic effect is observed. Furthermore, in patients who responded to the first MSC cycle but then experienced GVHD relapse after the completion of MSC therapy, Temcell could be re-administered to achieve another remission and extend the CR period. Re-administration of Temcell was safe in severe disease states and no severe complications were observed. More data from clinical studies and follow-up of Temcell re-administered patients, is required to confirm the long-term efficacy and safety of this drug. In contrast, multiple MSC injections did not improve efficacy in patients who did not initially achieve CR34,35. As the initial response to MSCs was observed relatively early in our study, other GVHD treatments should be considered if an early response to MSC treatment is not achieved. Several novel strategies for GVHD have been investigated, including modification of alloreactive T cells, targeting of cytokines, promotion of tissue regeneration, and reconstitution of the microbiome36. Further research on these strategies in pediatric patients with SR-aGVHD who are refractory to MSCs would be beneficial.
Our current work had several limitations. First, this was a retrospective study with a small sample size from a clinically diverse patient population, which may have influenced the accuracy of our findings. Second, MSCs were combined with other immunosuppressive therapies in many cases, and the therapeutic efficacy of MSCs alone was not thoroughly evaluated. Finally, several patients had a relatively short median follow-up period, and assessment of Temcell's long-term efficacy requires further investigation.
In conclusion, MSC therapy may be an effective and safe treatment for pediatric SR-aGVHD, with a steroid-sparing effect and a satisfactory degree of success upon re-administration. The 4.9 months of FFS for MSC treatment in our study was comparable to the 5.0 months achieved by ruxolitinib4, which was unsatisfactory. More in-depth investigation is therefore required, to determine the optimal combination of additional treatments and the most efficient method for re-administering MSCs.
Acknowledgments
The authors appreciate the cooperation of the patients and their families.
Author Contributions
H.K., Y.A. and K.K. designed the study. H.K. collected the data and performed the analyses. H.K., Y.A., Y.M., T.I., M.W., T.I., R.K., M.H., Y.M., K.F., M.M.,
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
The authors appreciate the cooperation of the patients and their families.
Patient Consent Statement
Informed consent was obtained by all participants in this study.
References
1.Van Lint MT, Uderzo C, Locasciulli A, Majolino I, Scimé R, Locatelli F, et al. Early Treatment of Acute Graft-Versus-Host Disease With High- or Low-Dose 6-Methylprednisolone: A Multicenter Randomized Trial From the Italian Group for Bone Marrow Transplantation. Blood. 1998; 92: 2288-93.
2.Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, et al. First- and Second-Line Systemic Treatment of Acute Graft-versus-Host Disease: Recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2012; 18: 1150-63.
3.Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020; 7: e157-67.
4.Zeiser R, von Bubnoff N, Butler J, Mohty M, Niederwieser D, Or R, et al. Ruxolitinib for Glucocorticoid-Refractory Acute Graft-versus-Host Disease. N Engl J Med. 2020; 382: 1800-10.
5.Li Y, Hao J, Hu Z, Yang YG, Zhou Q, Sun L, et al. Current status of clinical trials assessing mesenchymal stem cell therapy for graft versus host disease: a systematic review. Stem Cell Res Ther. 2022; 13: 1-22.
6.Kebriaei P, Hayes J, Daly A, Uberti J, Marks DI, Soiffer R, et al. A Phase 3 Randomized Study of Remestemcel-L versus Placebo Added to Second-Line Therapy in Patients with Steroid-Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020; 26: 835-44.
7.Muroi K, Miyamura K, Ohashi K, Murata M, Eto T, Kobayashi N, et al. Unrelated allogeneic bone marrow-derived mesenchymal stem cells for steroid-refractory acute graft-versus-host disease: A phase I/II study. Int J Hematol. 2013; 98: 206-13.
8.Muroi K, Miyamura K, Okada M, Yamashita T, Murata M, Ishikawa T, et al. Bone marrow-derived mesenchymal stem cells (JR-031) for steroid-refractory grade III or IV acute graft-versus-host disease: a phase II/III study. Int J Hematol. 2016; 103: 243-50.
9.Murata M, Terakura S, Wake A, Miyao K, Ikegame K, Uchida N, et al. Off-the-shelf bone marrow-derived mesenchymal stem cell treatment for acute graft-versus-host disease: real-world evidence. Bone Marrow Transplant. 2021; 56: 2355-66.
10.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995; 15: 825-8.
11.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health Consensus Development Project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Bio Blood Marrow Transplant. 2005; 11: 945-56.
12.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT−NIH−CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018; 53: 1401-15.
13.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-Intensity Conditioning Regimen Workshop: Defining the Dose Spectrum. Report of a Workshop Convened by the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2009; 15: 367-9.
14.Ball LM, Bernardo ME, Roelofs H, van Tol MJD, Contoli B, Zwaginga JJ, et al. Multiple infusions of mesenchymal stromal cells induce sustained remission in children with steroid-refractory, grade III-IV acute graft-versus-host disease. Br J Haematol. 2013; 163: 501-9.
15.Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, et al. Allogeneic human mesenchymal stem cell therapy (Remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014; 20: 229-35.
16.Kurtzberg J, Abdel-Azim H, Carpenter P, Chaudhury S, Horn B, Mahadeo K, et al. A Phase 3, Single-Arm, Prospective Study of Remestemcel-L, Ex Vivo Culture-Expanded Adult Human Mesenchymal Stromal Cells for the Treatment of Pediatric Patients Who Failed to Respond to Steroid Treatment for Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2020; 26: 845-54.
17.Murata M, Ikegame K, Morishita Y, Ogawa H, Kaida K, Nakamae H, et al. Low-dose thymoglobulin as second-line treatment for steroid-resistant acute GvHD: An analysis of the JSHCT. Bone Marrow Transplant. 2017; 52: 252-7.
18.Murata M, Teshima T. Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease Using Commercial Mesenchymal Stem Cell Products. Front Immunol. 2021; 12: 1-8.
19.Dander E, Lucchini G, Vinci P, Introna M, Masciocchi F, Perseghin P, et al. Mesenchymal stromal cells for the treatment of graft-versus-host disease: Understanding the in vivo biological effect through patient immune monitoring. Leukemia. 2012; 26: 1681-84.
20.Keto J, Kaartinen T, Salmenniemi U, Castrén J, Partanen J, Hänninen A, et al. Immunomonitoring of MSC-Treated GvHD Patients Reveals Only Moderate Potential for Response Prediction but Indicates Treatment Safety. Mol Ther Methods Clin Dev. 2018; 9: 109-18.
21.Introna M, Lucchini G, Dander E, Galimberti S, Rovelli A, Balduzzi A, et al. Treatment of graft versus host disease with mesenchymal stromal cells: A phase I study on 40 adult and pediatric patients. Biol Blood Marrow Transplant. 2014; 20: 375-81.
22.Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, et al. Allogeneic human mesenchymal stem cell therapy (Remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014; 20: 229-35.
23.Sánchez-Guijo F, Caballero-Velázquez T, López-Villar O, Redondo A, Parody R, Martínez C, et al. Sequential Third-Party Mesenchymal Stromal Cell Therapy for Refractory Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2014; 20: 1580-85.
24.Resnick IB, Barkats C, Shapira MY, Stepensky P, Bloom AI, Shimoni A, et al. Treatment of severe steroid resistant acute GVHD with mesenchymal stromal cells (MSC). Am J Blood Res. 2013; 3: 225-38.
25.Prasad VK, Lucas KG, Kleiner GI, Talano JAM, Jacobsohn D, Broadwater G, et al. Efficacy and Safety of Ex Vivo Cultured Adult Human Mesenchymal Stem Cells (ProchymalTM) in Pediatric Patients with Severe Refractory Acute Graft-Versus-Host Disease in a Compassionate Use Study. Biol Blood Marrow Transplant. 2011; 17: 534-41.
26.Kebriaei P, Isola L, Bahceci E, Holland K, Rowley S, McGuirk J, et al. Adult Human Mesenchymal Stem Cells Added to Corticosteroid Therapy for the Treatment of Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2009; 15: 804-11.
27.Ringdén O, Uzunel M, Rasmusson I, Remberger M, Sundberg B, Lönnies H, et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation. 2006; 81: 1390-97.
28.Burnham AJ, Daley-Bauer LP, Horwitz EM. Mesenchymal stromal cells in hematopoietic cell transplantation. Blood Adv. 2020; 4: 5877-87.
29.Galleu A, Milojkovic D, Deplano S, Szydlo R, Loaiza S, Wynn R, et al. Mesenchymal stromal cells for acute graft-versus-host disease: response at 1 week predicts probability of survival. Br J Haematol. 2019; 185: 89-92.
30.Hashmi S, Ahmed M, Murad MH, Litzow MR, Adams RH, Ball LM, et al. Survival after mesenchymal stromal cell therapy in steroid-refractory acute graft-versus-host disease: systematic review and meta-analysis. Lancet Haematol. 2016; 3: e45-52.
31.Kurtzberg J, Prockop S, Teira P, Bittencourt H, Lewis V, Chan KW, et al. Allogeneic human mesenchymal stem cell therapy (Remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014; 20: 229-35.
32.Servais S, Baron F, Lechanteur C, Seidel L, Selleslag D, Maertens J, et al. Infusion of bone marrow derived multipotent mesenchymal stromal cells for the treatment of steroid-refractory acute graft-versus-host disease: A multicenter prospective study. Oncotarget. 2018; 9: 20590-604.
33.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008; 371: 1579-86.
34.Pérez-Simon JA, López-Villar O, Andreu EJ, Rifón J, Muntion S, Campelo MD, et al. Mesenchymal stem cells expanded in vitro with human serum for the treatment of acute and chronic graft-versus-host disease: Results of a phase I/II clinical trial. Haematologica. 2011; 96: 1072-76.
35.Godoy JAP, Paiva RMA, Souza AM, Kondo AT, Kutner JM, Okamoto OK. Clinical Translation of Mesenchymal Stromal Cell Therapy for Graft Versus Host Disease. Front Cell Dev Biol. 2019; 7: 1-14.
36.Toubai T, Magenau J. Immunopathology and biology-based treatment of steroid-refractory graft-versus-host disease. Blood. 2020; 136: 429-40.
Search
News