Volume 7 (2024) Issue 1 No.4 Pages 25-32
Abstract
Autologous stem cell transplantation (ASCT) is the standard treatment for many high-risk solid tumors. Patients undergoing ASCT should be managed in a dedicated hematopoietic stem cell transplantation (HSCT) unit with isolation rooms, high-efficiency particulate air (HEPA) filters, and positive pressure. We report the outcomes of the first 20 pediatric patients who underwent ASCT in isolation rooms with no HEPA filters or positive pressure. Moreover, the isolation rooms were not part of a dedicated HSCT unit.
Data from 20 patients were analyzed. All patients included in the study underwent ASCT after harvest and cryopreservation of the hematopoietic stem cells (HSC). Furthermore, all patients also underwent myeloablative conditioning.
The most common indications for ASCT included high-risk neuroblastoma (HR-NB) (n=9) and refractory/relapsed Hodgkin's lymphoma (HL) (n=6). The median CD-34 positive HSC administered was 4.5 (0.8-21.9) million per kg. The median time to neutrophil and platelet engraftment was 16.5 (10-35) and 19 (10-87) days, respectively. Additionally, only one transplant-related mortality was observed and the mean time to discharge from the hospital was 27.6+8.3 days. The overall survival for all our patients was 75% at a median follow-up of 33.2 months (15 out of 20 patients survived), and the disease-free survival was 60% (median follow-up, 28.4 months). The overall survival for the patients with HL was 85.7% at a median of 45.3 months and for the HR-NB was 66.7% at a median of 34.9 months.
This study provides evidence that ASCT can be safely performed in isolation rooms without HEPA filters and positive pressure if expertise and supportive care are available. In settings with limited resources, such a model could help establish low-cost HSCT units.
Introduction
Bone marrow toxicity, which leads to myeloablation, is often a limiting factor in the treatment of malignancies by escalation of chemotherapy. The ability to harvest and store a patient's hematopoietic stem cells (HSC) and reinfuse them after myeloablative doses of chemotherapy allow better control of many tumors, making autologous stem cell transplantation (ASCT) an effective modality for the treatment of malignancies. Moreover, ASCT is a standard treatment for solid tumors such as high-risk neuroblastomas (HR-NB), stage IV Ewing's sarcoma, and relapsed/refractory lymphomas. Recently, ASCT has been used as a clinical option for stage IV-A retinoblastoma (RB), Wilms tumor, brain tumors, and autoimmune diseases1,2.
As infections are a major concern after highly myeloablative chemotherapy, it is recommended that patients undergoing ASCT be cared for in a dedicated hematopoietic stem cell transplant (HSCT) unit. In general, HSCT units should have a minimum number of single-bed rooms, depending on the volume of the center. Additionally, it is recommended that the single rooms be positively pressured with differential pressure between the rooms and the hallway along with self-closing doors. Rooms should have more than 12 exchanges of air per hour, continuous air pressure monitoring, and high-efficiency particulate filters (HEPA filters)3.
In low-income and middle-income countries, the aforementioned units should be made accessible. Whether acceptable outcomes can be achieved through the best utilization of existing resources is a question that needs to be addressed. In this article, we report the outcomes of the first 20 pediatric patients who underwent ASCT as part of their treatment at a center that initiated the HSCT program. Moreover, ASCT procedures were performed in rooms without HEPA filters. The rooms were not part of the HSCT unit, and adult and pediatric patients of other medical and surgical specialties were admitted to the unit along with pediatric HSCT patients.
Patients and Methods
The ASCT program for the Division of Pediatric Oncology, Department of Pediatrics at the All India Institute of Medical Sciences, New Delhi, commenced in August 2017. We present the outcomes of the first 20 pediatric patients, with ages equal to or less than 14 years, who underwent ASCT treatment. Informed consent was obtained from all patients. The patients were followed up until April 2023. This retrospective study was approved by the Institutional Review Board of the All India Institute of Medical Sciences, New Delhi, India (IEC-396/17.07.2023).
Description of the rooms where the ASCT was performed
The ASCT procedures were performed in one of the 12 rooms located on the top floor of a five-story hospital wing. The main door to the passage was a manually operated glass door, followed by a sensor-operated automatic door. The rooms in which the ASCT was performed were on either side of the passage with self-closing hand-operated wooden doors. Patients from other specialties and those undergoing HSCT were admitted to the rooms (as the rooms were not a part of the dedicated HSCT unit). The rooms were air-conditioned, and before the admission of a patient, they were fumigated with a fogging machine using a solution of stabilized hydrogen peroxide 11%w/v and 0.01%w/v diluted silver nitrate solution. The machine was operated for 40 min, after which the room was closed for another 45 min.
Only one attendant (in most cases, the mother) was allowed to be with the child during admission for ASCT treatment. Patients were kept in isolation from admission until discharge.
Stem cell collection and harvest
Peripheral blood HSCs were used in all patients. The patients underwent a femoral line insertion 1 day before harvest. Stem cell mobilization was performed using granulocyte colony-stimulating factor (G-CSF) alone, or G-CSF and plerixafor. Harvesting was performed via a Spectra-Optia apheresis system (Terumo BCT, Lakewood, Colorado, USA) using a continuous mononuclear cell collection program.
Stem cell cryopreservation and transfusion
After collection, the HSCs were cryopreserved at our stem cell facility using established protocols. The harvested stem cells were mixed with a freshly prepared cold cryoprotectant mixture (normal saline, dimethylsulfoxide, albumin, and heparin) in a ratio of 1:1, with a constant swirling of the cryobag. An aliquot of the harvested cryopreserved HSC was sent for sterility testing. The cryobag was properly labeled, sealed, and stored at -80℃ for later use. At the time of infusion, the HSC cryobags were retrieved from the -80℃ freezer and thawed at 37℃ using a water bath at the patient's bedside. An aliquot of the thawed HSC was tested for stem cell viability using the trypan blue dye assay.
Central venous access
Central venous access for patients during autologous HSCT involved Hickman line insertion or the use of a peripherally inserted central catheter line.
Medical and Nursing care
The patients had a dedicated superspecialty trainee doctor assigned to their care. Each patient was examined by consultants twice daily. The nurse-to-patient ratio varied from 1:3 to 1:4. All nurses were experienced in the care of patients undergoing HSCT; however, they also managed other patients. Only healthcare personnel were allowed to enter the rooms after donning a cap and face mask, changing footwear, and thoroughly washing their hands with water or using an antiseptic handrub. The patients were provided cooked food from a common hospital kitchen. Patients were allowed to drink reverse osmosis-filtered water and commercially available mineral water.
Conditioning regimens
The conditioning regimens used for the lymphomas included carmustine, etoposide, cytarabine, and melphalan (BEAM). In most cases, the regimen consisted of carmustine 300 mg/m2 for 1 day, etoposide 200 mg/m2 for 4 days, Ara-C 200 mg/m2 for 4 days, and melphalan 140 mg/m2 for 1 day4. We also used gemcitabine-based conditioning in Hodgkin lymphoma (HL) if the refractory disease was sensitive to gemcitabine-containing salvage chemotherapy. Furthermore, in one patient, we used carmustine 300 mg/m2 for 1 day, gemcitabine 1,000 mg/m2 for 2 days, and melphalan 140 mg/m2 for 1 day5. In another study, busulfan was administered at 80 mg/m2 for 4 days, gemcitabine at 2,000 mg/m2 for 2 days, and melphalan at 120 mg/m2 for 2 days6.
The conditioning regimen used in the HR-NB group was a combination of busulfan 4 mg/kg/day for 4 days and melphalan 70 mg/m2 for 2 days7 or a combination of carboplatin (doses based on Calvert's formula) for 4 days, etoposide 338 mg/m2 for 4 days, and melphalan 70 mg/m2 for 3 days8.
Patients with stage IV-A RB who underwent ASCT received either carboplatin 350 mg/m2 for 5 days, etoposide 350 mg/m2 for 5 days, and cyclophosphamide 1,600 mg/m2 for 4 days9 or carboplatin 500 mg/m2 for 3 days, thiotepa 300 mg/m2 for 3 days, and etoposide 250 mg/m2 for 3 days10.
Supportive care
All patients received fluconazole and acyclovir for antifungal and antiviral prophylaxis, respectively. All patients received ursodeoxycholic acid as prophylaxis against veno-occlusive disease (VOD). The treatments were terminated on Day +90. Co-triamoxazole was stopped before conditioning and restarted on day +30 after engraftment had been achieved.
Blood component and growth factor support
Packed red blood cells (PRBC) were transfused when the hemoglobin level was less than 7 g/dL, and platelets were transfused when they were less than 20,000/cumm. All patients received G-CSF from day +1 until the neutrophils were > 1,000/cumm. All the PRBC and platelets were transfused after irradiation.
Treatment of infections
Any episode of fever after conditioning defined as a single temperature of more than 38.3℃ (101℉) or more than 38.0℃ (100.4℉) lasting for more than 1 hour was treated with broad-spectrum antibiotics. The initial antibiotics of choice were piperacillin-tazobactam and amikacin. Teicoplanin was added upfront in the presence of mucositis or 48 hours later if the fever persisted. If no response was observed, meropenem was added. Systemic antifungals were initiated if the fever persisted for 5 days or more despite antibiotic treatment or if suspicion of an invasive fungal infection was present based on radiological/computed tomography scan findings. If fungal infection was suspected, serum galactomannan levels were evaluated. Cytomegalovirus (CMV) infection was suspected if fever or cytopenia persisted even after the addition of antifungals, and CMV-polymerase chain reaction (PCR) was requested from the blood samples.
Recording of data and statistical analysis
The data of all patients were prospectively recorded after ASCT. For this retrospective study, the data were retrieved and analyzed. Non-normally distributed continuous data were reported as median (range), and normally distributed data were presented as mean ± standard deviation. Qualitative data were expressed as numbers and percentages. Kaplan-Meier survival curves were prepared for individual diseases, as well as for the entire cohort.
Results
Twenty pediatric patients underwent ASCT between August 2017 and the present. The indications for ASCT included HR-NB (n=9), refractory/relapsed HL (n=6), relapsed or refractory non-HL (NHL) (n=3), and stage IV-A RB (n=2). Sixteen patients were males and four were females. The mean age of the patients at the time of transplant was 84.9
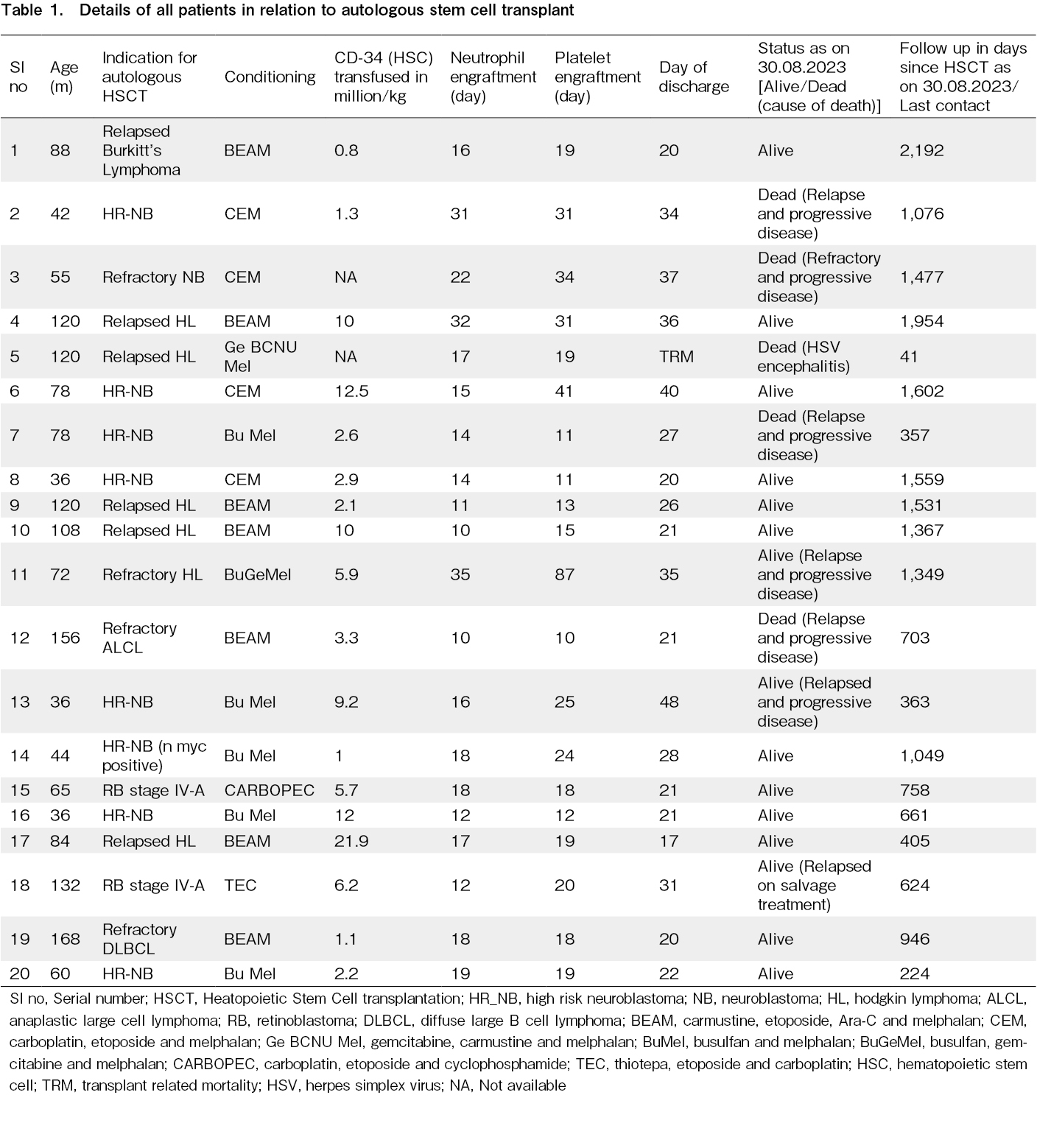
Myeloablative conditioning chemotherapy was used in all cases. Carboplatin, etoposide, and melphalan (n=4) and busulfan and melphalan (BuMel) (n=5) were the conditioning chemotherapies administered to patients with HR-NB. In patients with HL, BEAM (n=5) or gemcitabine-based conditioning (n=2) was used. For NHL, the conditioning chemotherapy was BEAM, and for RB (n=2), the chemotherapy included thiotepa, etoposidem, and carboplatin in one patient and carboplatin, etoposide, and cyclophosphamide in the other. (Table 1)
The stem cell dose administered was available for 18 patients. The median CD-34 positive HSCs administered were 4.5 (0.82-1.9) million stem cells per kg of recipient body weight. The median time to neutrophil engraftment was 16.5(10-35) days and the median time to platelet engraftment was 19 (10-87) days. One transplant-related mortality (TRM) was observed and the mean time to discharge from the hospital for the remaining patients (n=19) from the day of ASCT was 27.6
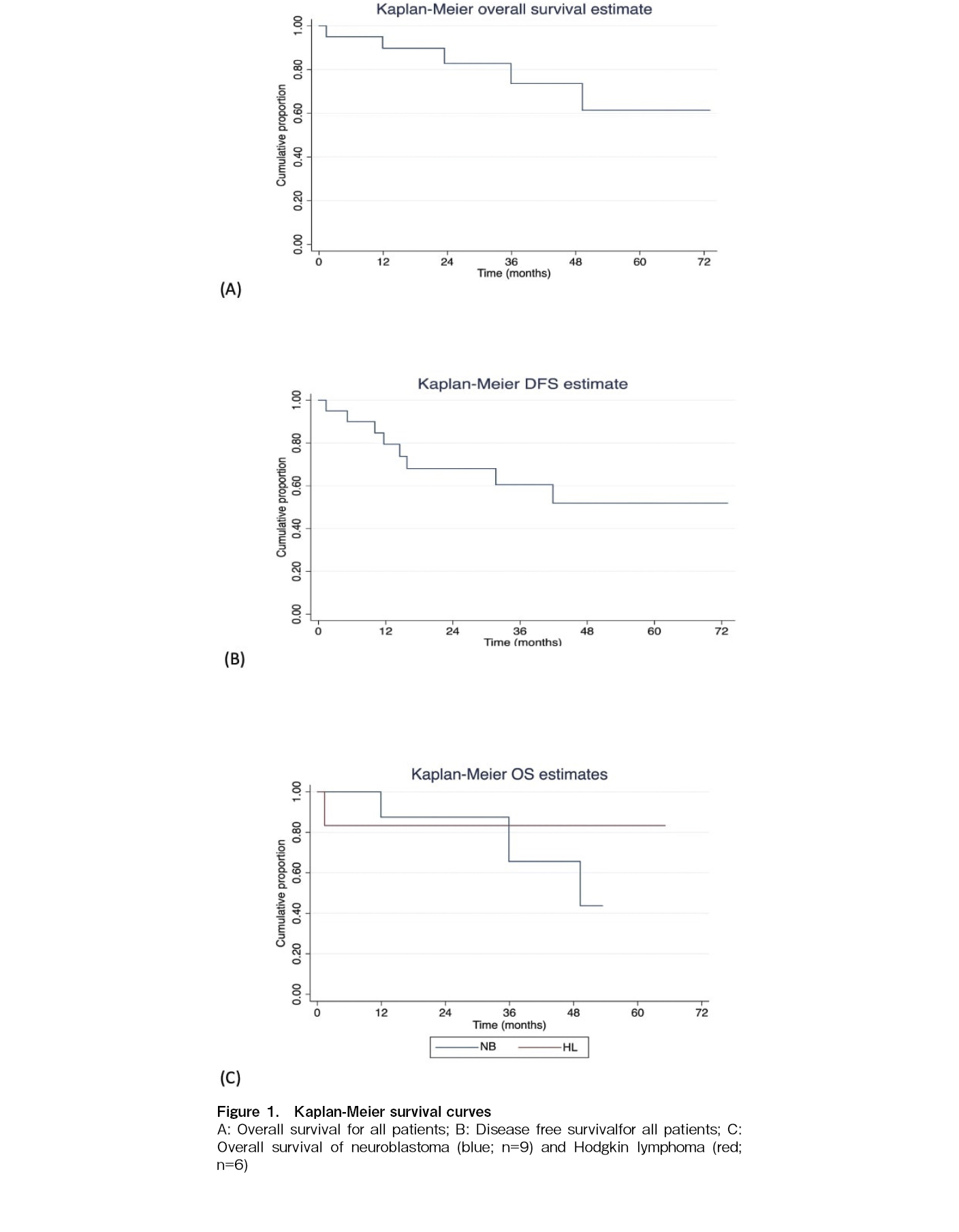
The overall survival (OS) for all our patients was 75% at a median follow-up of 33.2 months (15 out of 20 patients survived), and the disease-free survival (DFS) was 60% (at a median follow-up of 28.4 months). The overall survival for the HL patients was 85.7% at a median of 45.3 months and the HR-NB was 66.7% at a median of 34.9 months. One TRM was observed in a patient with relapsed HL due to herpes simplex virus (HSV) encephalitis on day +41 post-HSCT. The magnetic resonance imaging (MRI) findings of the patient's brain were suggestive of HSV encephalitis, and HSV PCR of the cerebrospinal fluid (CSF) demonstrated positive results. Additionally, four deaths (three for HR-NB and one for NHL) were observed, all of which were attributed to relapse and progressive disease in all cases. (Table 1) The Kaplan-Meier curves for OS and DFS for all patients and OS for HR-NB and relapsed/refractory HL are displayed in Figure 1.
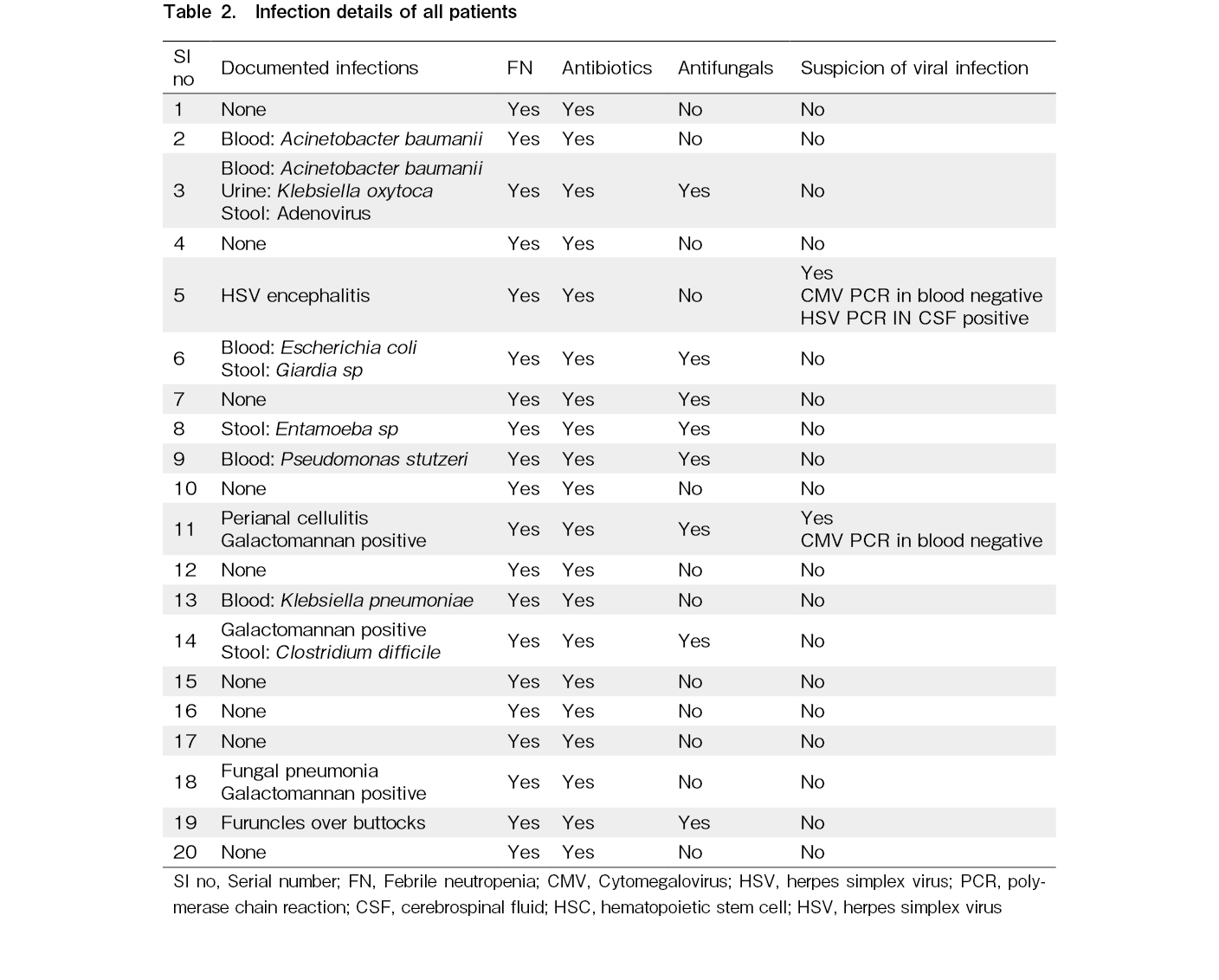
During admission for ASCT, all the patients experienced febrile neutropenia (FN) and required antibiotics. Need for systemic antifungal treatment was required in 40% of cases. Blood cultures were positive in five cases. Moreover, two cases exhibited positivity for serum galactomannan, of which one also demonstrated evidence of IFI on imaging (Table 2).
Additionally, CMV reactivation was suspected in two cases but was ruled out by quantitative PCR. None of the patients had VOD.
Discussion
Good short- and long-term outcomes were observed in the first 20 pediatric ASCT cases performed at our center. These ASCTs were performed in normal isolation rooms in a common ward; the rooms did not have HEPA filters or positive pressure.
The use of HEPA-filtered rooms is recommended for ASCT recipients to reduce the chances of nosocomial infections as a result of prolonged neutropenia11. On the other hand, improved supportive care and timely engraftment due to the use of peripheral blood HSCs have made ASCT a safe procedure with low mortality12. The need for HEPA filtration and positive pressure rooms, especially for ASCT, has been debated. Ruiz-Argüelles et al in 2022 published a review in which they recommended that ASCT be performed in an outpatient setting. They considered the need for HEPA-filtered rooms with laminar flow to be optional13. A similar experience has been published by Kumar et al., who demonstrated acceptable infection rates and survival in 40 patients who underwent allogeneic HSCT in non-HEPA-filtered rooms without positive pressure14. Tsai et al. demonstrated that the 100-day mortality in adult patients with multiple myeloma did not differ if the room used was without a HEPA filter15.
In our experience, we identified an OS of 75% at a median follow-up of 33.2 months. In patients with HR-NB, a disease with a poor prognosis, the OS was 66%. Although our study was limited to 20 patients, these were the first ASCTs performed in our unit.
Sharma et al. demonstrated a 55% OS in a retrospective analysis of 36 patients, (half of them with HR-NB) who underwent ASCT at their center16. In another study from India, which also included adult patients with multiple myeloma, Yadav et al. demonstrated a 55.6% OS at a median follow-up of 114 days (range: 21-617 days). The median duration of neutrophil engraftment in their study was 12 days (range, 9-30)17. Relapsed/refractory HL had a 5-year OS of about 68%18 and in HR NB, an EFS of 50% has been demonstrated by Landenstein et al. in patients undergoing ASCT with BuMel conditioning19. In our experience, relapsed/refractory HL and HR-NB were the common conditions for which autologous HSCT was performed and the OS observed in our patients was acceptable.
Rates of FN in ASCT up to 93% have been documented, with microbiologically or clinically documented infections in 50%20. A FN rate of 89% has been documented among 113 ASCT recipients, in adult patients not receiving any antibiotic prophylaxis21. Some studies have demonstrated an improvement in FN rates with the use of prophylactic antibiotics, however, we did not use prophylactic antibiotics during the ASCT22,23. All patients had FN and required antibiotics, but all of them recovered completely. Blood culture results were positive in five instances. Our FN and blood culture positivity rates were acceptable in comparison with those reported in other studies. In our study, one case of TRM was observed due to HSV encephalitis.
The neutrophil and platelet engraftment time in patients is comparable to the time documented by others24. Jain et al reported the outcomes of 35 patients with HR-NB who underwent ASCT in regular multi-bedded ward beds without HEPA filtration and cryopreservation of the HSC. They reported a median time to neutrophil engraftment of 11 days, antibiotic usage of 86%, and an OS at 3 years of 41%25.
Our study has some limitations because of the retrospective nature and the inclusion of only 20 patients. However, the study provides evidence that ASCT can be safely performed in non-HEPA filtered and non-positive pressure isolation rooms if expertise and supportive care are available. In resource-limited settings, such a model could help establish low-cost HSCT units.
Acknowledgments
We acknowledge the contribution and support of the CanKids KidsCan, a national society for change for childhood cancer in India, in providing access, holistic care and financial support to our patients.
Author Contributions
AKG designed the study, analyzed the data, and wrote the manuscript; AKG, JPM, RS, PN, SM and PC were involved in the clinical care of the patients and reviewed the manuscript; All the authors approve the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
We acknowledge the contribution and support of the CanKids KidsCan, a national society for change for childhood cancer in India, in providing access, holistic care and financial support to our patients.
Funding
None to declare
Acknowledgments
We acknowledge the contribution and support of the CanKids KidsCan, a national society for change for childhood cancer in India, in providing access, holistic care and financial support to our patients.
Ethical Approval
This retrospective study was approved by the institutional review board of All India Institute of Medical Sciences, New Delhi, India (IEC-396/17.07.2023).
Acknowledgments
We acknowledge the contribution and support of the CanKids KidsCan, a national society for change for childhood cancer in India, in providing access, holistic care and financial support to our patients.
Informed Consent
Informed consent was obtained from all participants or their parents.
References
1.Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022; 57: 1217-39.
2.Kreissman SG, Rackoff W, Lee M, Breitfeld PP. High dose cyclophosphamide with carboplatin: a tolerable regimen suitable for dose intensification in children with solid tumors. J Pediatr Hematol Oncol. 1997; 19: 309-12.
3.Rasheed W, Niederwieser DW, Aljurf M. The HSCT Unit. In: Carreras E, Dufour C, Mohty Mo, et al. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. 7th ed., Springer. 2019; 27-34.
4.Mills W, Chopra R, McMillan A, Pearce R, Linch DC, Goldstone AH. BEAM chemotherapy and autologous bone marrow transplantation for patients with relapsed or refractory non-Hodgkin's lymphoma. J Clin Oncol. 1995; 13: 588-95.
5.Rapoport AP, Guo C, Badros A, Hakimian R, Akpek G, Kiggundu E, et al. Autologous stem cell transplantation followed by consolidation chemotherapy for relapsed or refractory Hodgkin's lymphoma. Bone Marrow Transplantation. 2004; 34: 883-90.
6.Nieto Y, Thall PF, Ma J, Valdez BC, Ahmed S, et al. Phase II Trial of High-Dose Gemcitabine/Busulfan/Melphalan with Autologous Stem Cell Transplantation for Primary Refractory or Poor-Risk Relapsed Hodgkin Lymphoma. Biol Blood Marrow Transplant. 2018; 24: 1602-09.
7.Ladenstein RL, Poetschger U, Luksch R, Brock P, Castel V, Yaniv I, et al. Busulfan-melphalan as a myeloablative therapy (MAT) for high-risk neuroblastoma: Results from the HR-NBL1/SIOPEN trial.Journal of Clinical Oncology. 2011; 29 (Suppl): 2.
8.Stram DO, Matthay KK, O'Leary M, Reynolds CP, Haase GM, Atkinson JB, et al. Consolidation chemotherapy and autologous bone marrow transplantation vs. continued chemotherapy for metastatic neuroblastoma: A report of two concurrent Children's Cancer Group studies. J Clin Oncol. 1996; 14: 2417-26.
9.Namouni F, Doz F, Tanguy ML, Quintana E, Michon J, Pacquement H, et al. High-dose chemotherapy with carboplatin, etoposide and cyclophosphamide followed by a haematopoietic stem cell rescue in patients with high-risk retinoblastoma: a SFOP and SFGM study. Eur J Cancer. 1997; 33: 2368-75.
10.Dunkel IJ, Aledo A, Kernan NA, Kushner B, Bayer L, Gollamudi SV, et al. Successful treatment of metastatic retinoblastoma. Cancer. 2000; 89: 2117-21.
11.Dykewicz CA, Centers for Disease Control and Prevention (U.S.), Infectious Diseases Society of America, American Society of Blood and Marrow Transplantation. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001; 33: 139-44.
12.Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L, et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995; 85: 588-96.
13.Ruiz-Argüelles GJ, Seber A, Ruiz-Delgado GJ. Conducting hematopoietic stem cell transplantation in low and middle income countries. Hematology. 2022; 27: 809-12.
14.Kumar R, Naithani R, Mishra P, Mahapatra M, Seth T, Dolai TK, et al. Allogeneic hematopoietic SCT performed in non-HEPA filter rooms: initial experience from a single center in India. Bone Marrow Transplant. 2009; 43: 115-9.
15.Tsai CK, Yeh CM, Hong YC, Chen PM, Liu JH, et al. The influence of high-efficiency particulate air filtration on mortality among multiple myeloma patients receiving autologous stem cell transplantation. Sci Rep. 2021; 11: 11789.
16.Sharma A, Rastogi N, Kapoor R, Chatterjee G. Outcomes of pediatric autologous hematopoietic stem cell transplant: a ten years experience. Pediatric Hematology Oncology Journal. 2020; 5: S50-1.
17.Yadav SP, Kalra M, Sachdeva A, Dinand V, Parashar N, Kohli S, et al. The Experience of Hematopoietic Stem Cell Transplantation From An Emerging Centre in North India. Blood. 2009; 114: 4317.
18.Lieskovsky YE, Donaldson SS, Torres MA, Wong RM, Amylon MD, Link MP, et al. High-dose therapy and autologous hematopoietic stem-cell transplantation for recurrent or refractory pediatric Hodgkin's disease: results and prognostic indices. J Clin Oncol. 2004; 22: 4532-40.
19.Ladenstein R, Pötschger U, Pearson ADJ, Brock P, Luksch R, Castel V, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017; 18: 500-14.
20.Celebi H, Akan H, Akçağlayan E, Ustün C, Arat M. Febrile neutropenia in allogeneic and autologous peripheral blood stem cell transplantation and conventional chemotherapy for malignancies. Bone Marrow Transplant. 2000; 26: 211-4.
21.Signorelli J, Zimmer A, Liewer S, Shostrom VK, Freifeld A. Incidence of Febrile Neutropenia in Autologous Hematopoietic Stem Cell Transplant (HSCT) Recipients on levofloxacin prophylaxis. Transpl Infect Dis. 2020; 22: e13225.
22.Kimura S, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, et al. Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. J Infect. 2014; 69: 13-25.
23.Vehreschild JJ, Moritz G, Vehreschild MJ, Arenz D, Mahne M, Bredenfeld H, et al. Efficacy and safety of moxifloxacin as antibacterial prophylaxis for patients receiving autologous haematopoietic stem cell transplantation: a randomised trial. Int J Antimicrob Agents. 2012; 39: 130-4.
24.Hassan MN, Fauzi HM, Husin A, Mustaffa R, Hassan R, Ibrahim MI, et al. Autologous Peripheral Blood Stem Cell Transplantation Among Lymphoproliferative Disease Patients: Factors Influencing Engraftment. Oman Med J. 2019; 34: 34-43.
25.Jain R, Hans R, Totadri S, Trehan A, Sharma RR, Menon P, et al. Autologous stem cell transplant for high-risk neuroblastoma: Achieving cure with low-cost adaptations. Pediatr Blood Cancer. 2020; 67: e28273.
Search
News