Volume 6 (2023) Issue 4 No.1 Pages 104-113
Abstract
Background: Chronic graft-versus-host disease (cGVHD) is a serious complication after allogeneic stem cell transplantation. Poor prognosis has been shown in patients with cGVHD after the failure of primary steroid-based treatments. A previous report demonstrated the efficacy and safety of ibrutinib in these patients, leading to the approval of ibrutinib for cGVHD in Japan. Here, we report the extended follow-up of patients in this study.
Objectives: To evaluate the safety and efficacy of ibrutinib in Japanese patients with steroid-dependent or refractory cGVHD.
Study Design: An open-label, single-arm, multicenter study of ibrutinib in Japanese patients with steroid-dependent or refractory cGVHD (NCT No.: NCT03474679; Clinical Registry No.: CR108443).
Results: At the time of the final data cutoff, 7/19 (36.8%) patients completed the study treatment, and 12/19 (63.2%) patients discontinued ibrutinib. After a median follow-up of 31.11 months (range:1.9 to 38.6 months), the best overall response rate was 84.2% (16/19 patients; 95% CI:60.4%, 96.6%) in all treated populations, with a median time to response of 2.81 (range:1.0 to 27.6) months. Of 15 responders with
Conclusions: The final results support previous conclusions, demonstrating a clinically meaningful response and acceptable safety profile of ibrutinib in Japanese patients with steroid-dependent or refractory cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) is a major life-threatening complication and a leading cause of late non-relapse mortality after allogeneic hematopoietic stem cell transplantation (allo-HSCT), which leads to poor health-related quality of life (HRQoL)1–3. Overall, cGVHD occurs in 30-70% of patients after allo-HSCT, depending on several factors, including donor and graft sources4–6.
Corticosteroid-based treatment is the standard first-line therapy for cGVHD; however, a significant proportion of patients develop steroid-dependent or steroid-refractory disease and require second-line treatment within 2 years7–9. For many years, there have been few effective treatment options for patients with cGVHD, with poor prognosis after failure to corticosteroids8.
Ibrutinib is a first-in-class, potent inhibitor of Bruton's tyrosine kinase (BTK) and also an inhibitor of interleukin-2-inducible T-cell kinase (ITK), which was approved by the US Food and Drug Administration (FDA) for the treatment of adult patients with cGVHD after the failure of one or more lines of systemic therapy in August 2017, and the indication was expanded for pediatric patients in August 2022 (
In Japan, an open-label, single-arm, multicenter study of ibrutinib that involved 19 Japanese patients (
Materials and Methods
The detailed methodology for this study has been published previously11. In brief, eligible patients had steroid-dependent or refractory cGVHD defined according to the modified NIH criteria (2014)12 with no more than three previous systemic treatments for cGVHD. Patients received oral ibrutinib (420 mg) once daily until they experienced unacceptable toxicity or met other criteria for ibrutinib treatment discontinuation. The dose was reduced to 280 mg/day with the concomitant use of voriconazole.
The study was continued after the primary analysis (at week 37 of the last enrolled patient) until the planned end of the study, resulting in an additional 22 months of follow-up (median follow-up was 31.11 months at final analysis). The efficacy and safety in the additional follow-up period after the primary analysis were assessed according to previously described criteria11.
The primary efficacy endpoint was the best ORR as defined by the NIH Consensus Development Project Criteria (2014)13. Major secondary efficacy endpoints were a sustained response (CR or PR) of
Safety was assessed until 30 days after the final dose of ibrutinib. All enrolled patients who received at least one dose of ibrutinib were included in the safety analysis set. The AEs recorded by the investigators in the case report form (CRF) were coded using the Medical Dictionary for Regulatory Activities (MedDRA) version 24.1.
Institutional review board/Independent ethics committee approval was obtained from each participating institution. This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines. All patients provided written informed consent.
Results
The study was continued from January 21, 2020 (the primary cut-off date), to November 29, 2021 (the final cut-off date). The median follow-up was extended from 11.9 (range:1.9 to 16.7) months at primary analysis11 to 31.1 (range:1.9 to 38.6) months at the final analysis, and the median duration of ibrutinib exposure, from 9.6 (range:0.6 to 16.7) months11 to 16.3 (range:0.6 to 36.2) months. At the time of the final data cutoff, 7/19 (36.8%) patients completed the study treatment, and 12/19 (63.2%) patients discontinued ibrutinib. The reasons for ibrutinib discontinuation were AEs and physician decisions (3/19 [15.8%] patients each), cGVHD progression and withdrawal by the patient (2/19 [10.5%] patients each), relapse of the underlying malignancy, and others (1/19 [5.3%] patients each). A total of 11/19 (57.9%) patients completed study participation and 8/19 (42.1%) patients terminated the study prematurely. The reasons for terminating study participation were death (7/19 [36.8%]) and withdrawal by patient (1/19 [5.3%]) (Supplementary Table 1).
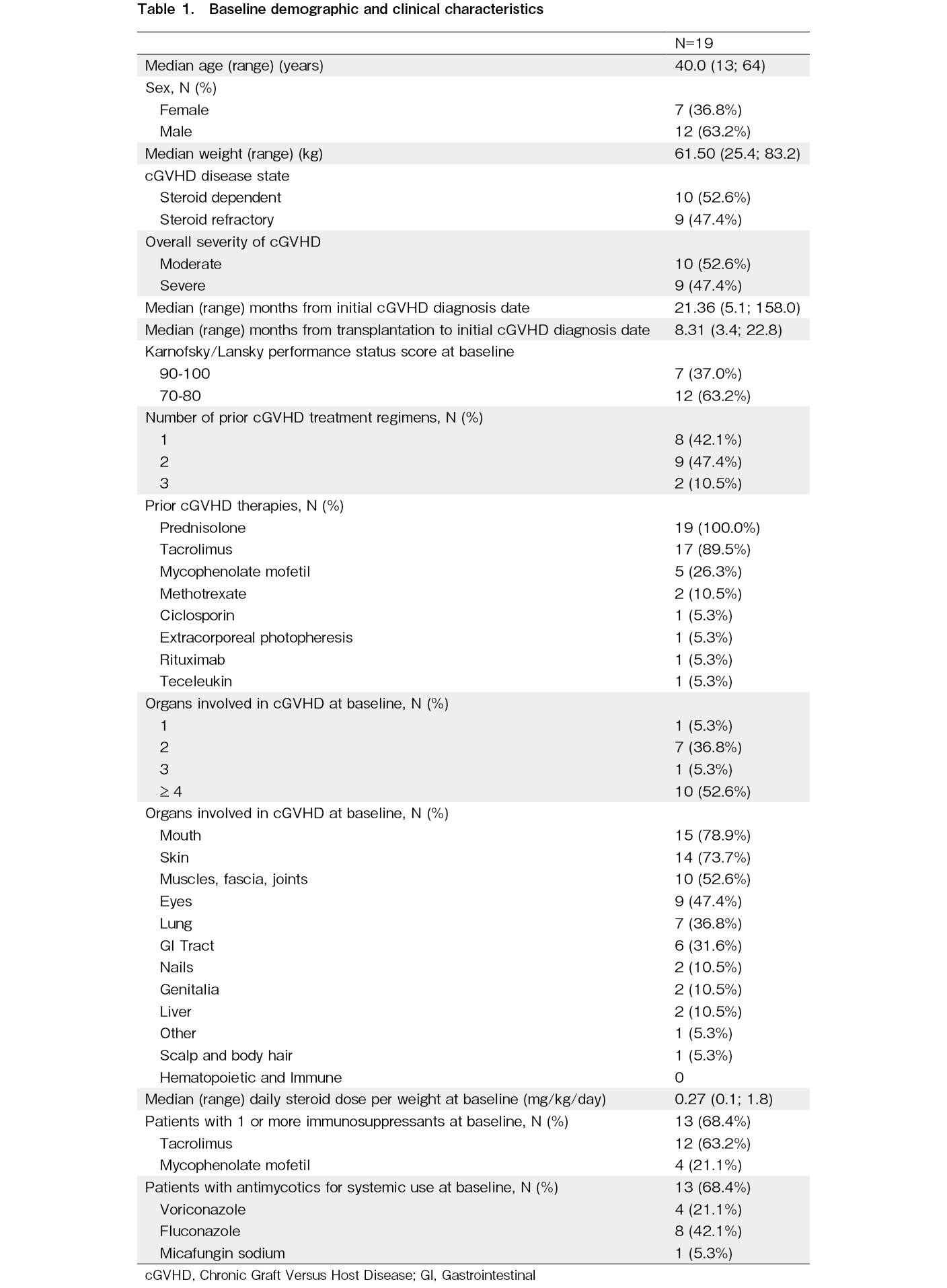
At baseline, approximately half of the patients (10/19 [52.6%]) were steroid-dependent and nine (47.4%) were steroid-refractory. The majority of patients (94.7%) had two or more organs involved at baseline, with the most commonly involved being the mouth (15/19 [78.9%]), skin (14/19 [73.7%]), muscles, fascia, and joints (10/19 [52.6%]), and eyes (9/19 [47.4%]). One patient was reconsidered to have mouth involvement at baseline by the investigator after primary analysis. All patients had a history of at least one transplant and an underlying malignancy leading to transplantation (Table 1).
Efficacy Findings
Best overall response rate
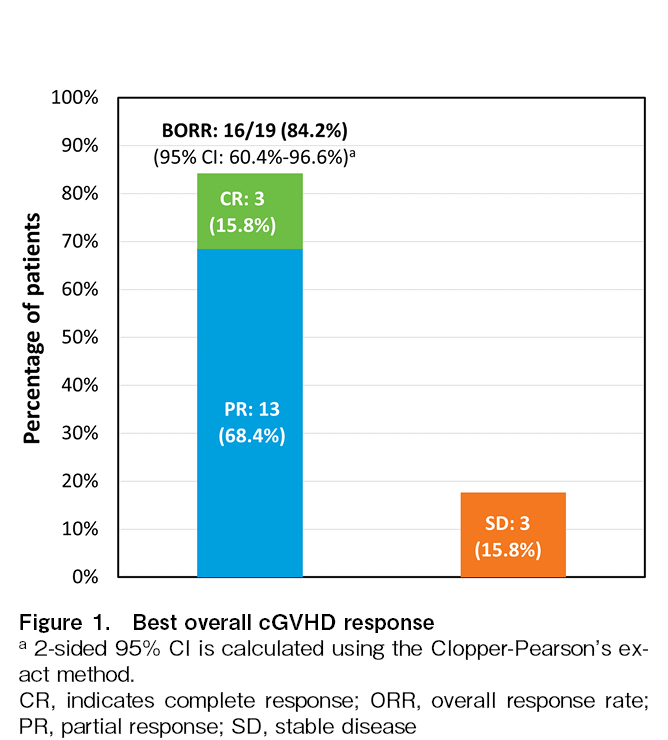
The prespecified criteria for positive primary efficacy results (lower bound of the 95% CI exceeding 25% for the best ORR) of the study were already met in the primary analysis. With this additional 22 months of follow-up, the best ORR increased from 73.7% (14/19 patients, 95% CI:48.8%, 90.9%) in the primary analysis to 84.2% (16/19 patients; 95% CI:60.4%, 96.6%) in the final analysis in all treated analysis sets, including three patients who achieved CR and 13 patients who achieved PR (Figure 1).
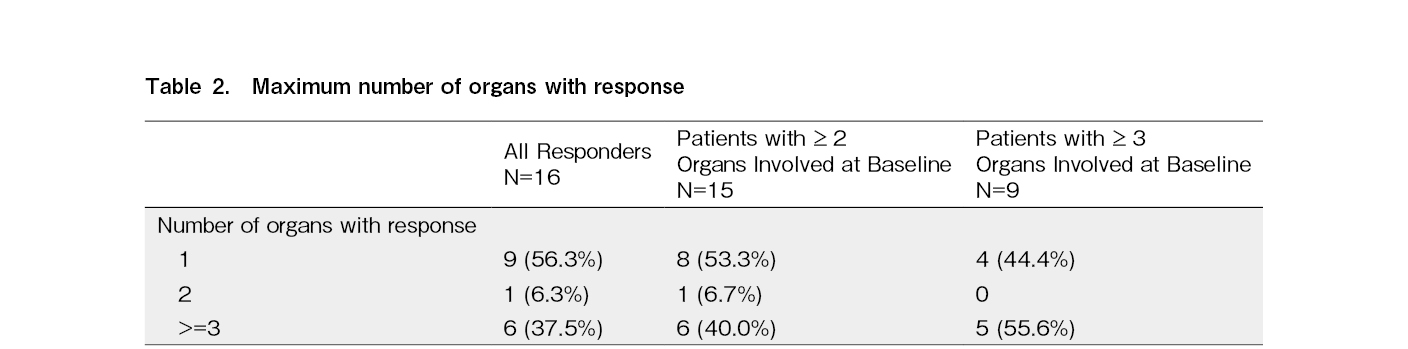
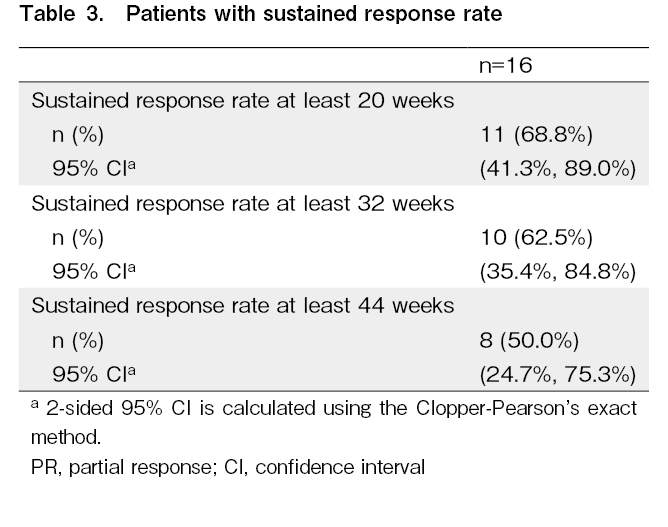
Approximately 50% of responders with
Despite the extended duration of ibrutinib exposure, the median DOR could not be estimated based on Kaplan-Meier estimates (range:1.0, 34.5+). The Kaplan-Meier estimate for the DOR at 30 months was 56.2% (95% CI:26.9%, 77.6%). The median time to response was 2.81 (range:1.0 to 27.6) months for 16 responders, and that to CR was 5.59 (range:1.0 to 19.5) months for three patients with CR, respectively. Responses were observed across the organs involved in cGVHD (Supplementary Table 2).
The organ response rates (PR/CR) for the skin, eyes, mouth, and esophagus increased from 35.7%, 11.1%, 35.7%, and 40.0% in the primary analysis to 57.1%, 22.2%, 40.0%, and 60.0%, respectively, in the final analysis. Among patients with sclerotic features (n=6), 3/6 (50.0%) patients reached a skin response with improved skin features score (a score for sclerotic features), and 4/6 (66.7%) patients had
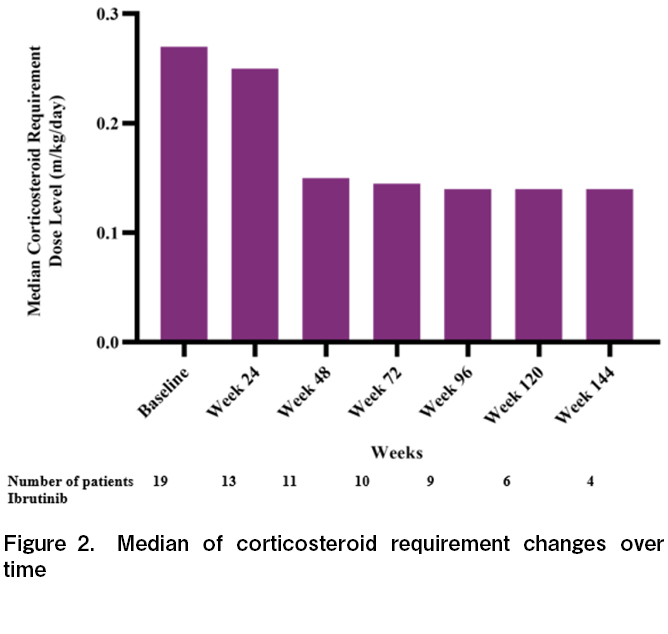
The corticosteroid trend in this updated analysis was consistent with that in the primary analysis. At ibrutinib initiation, the median daily corticosteroid dose requirement decreased over time in all treatment analysis sets. The median daily corticosteroid dose requirement for all treated analysis sets was 0.27 mg/kg/day at baseline, 0.15 mg/kg/day at week 48, 0.14 mg/kg/day at week 96, and 0.14 mg/kg/day at week 144 (Figure 2). A total of 4/19 (21.1%) patients stopped steroids for at least 28 days and 12/19 (63.2%) patients used a < 0.15 mg/kg/day average daily dose of steroid for at least one week.
Exploratory Findings
With the extended duration of ibrutinib exposure, the median FFS (range:1.9, 37.3+) and OS (range:1.9,
Improvement rate in the lee cGVHD symptom scale
After the primary analysis, two additional patients achieved an improvement in the total summary score on the Lee cGVHD Symptom Scale. The improvement rate in the Lee cGVHD Symptom Scale total summary score was 52.6% (10/19 patients; 95% CI:28.9%, 75.6%) for all patients (including both responders and non-responders). At the 6- and 12-month landmarks, the improvement rates in the Lee cGVHD Symptom scores were 26.3% (5/19 patients; 95% CI:9.1%, 51.2%) and 42.1% (8/19 patients; 95% CI:20.3%, 66.5%), respectively.
Safety Findings
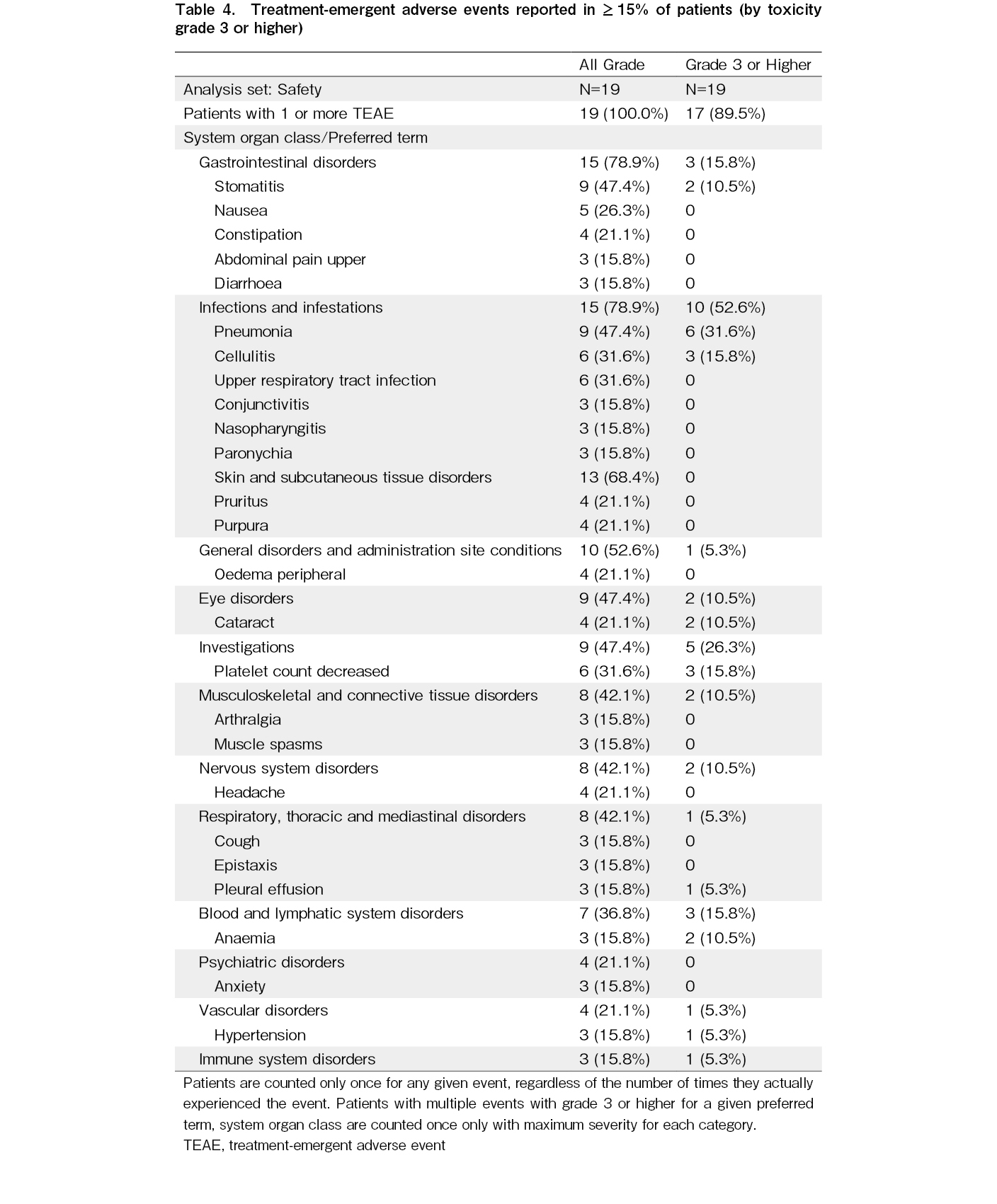
The safety findings of this updated analysis were consistent with those of the primary analysis. All 19 treated patients had at least one reported TEAE. The most common TEAEs (
Grade 3 or higher TEAEs were reported in 17/19 (89.5%) patients. The most common (
Treatment-emergent SAEs were reported in 12/19 (63.2%) patients during the study. The most common (
The incidence of TEAEs was highest in the first six months and decreased in subsequent periods during ibrutinib treatment. There were no TEAEs with a higher incidence over the course of treatment. Infections (defined as TEAEs in the SOC of infections and infestations) including pneumonia had a decreasing trend over time; 14/19 (73.7%) patients in the first 6 months, 9/16 (56.3%) patients from 6 to
The incidences of Grade 3 or higher infections in patients with and without antimycotic use at baseline were 46.2% (6/13 patients) and 66.7% (4/6 patients), respectively. One patient who received micafungin sodium at baseline developed fungal pneumonia (Supplementary Table 4).
Despite the extended duration of ibrutinib exposure, no new TEAEs leading to death, treatment discontinuation, or dose reduction were reported. Three of the 19 (15.8%) patients died because of TEAEs: one had multiple organ dysfunction syndrome (ibrutinib-related), one had fungal pneumonia (ibrutinib-related), and one had subarachnoid hemorrhage (not ibrutinib-related), as previously reported. Among the other four patients who died during the study, one patient had cGVHD progression, one patient had progression of an underlying malignancy, and two patients had other safety events that occurred > 30 days after the last dose of ibrutinib.
TEAEs leading to treatment discontinuation were reported in 3/19 (15.8%) patients (one each with Grade 3 stomatitis, fatal multiple organ dysfunction syndrome, and fatal subarachnoid hemorrhage, respectively). TEAEs leading to dose reduction were reported in 2/19 (10.5%) patients. During the study, TEAEs leading to dose reduction were gastrointestinal hemorrhage (Grade 3) and stomatitis (Grade 3). All nonfatal TEAEs that led to treatment discontinuation or dose reduction were resolved.
Three of the 19 (15.8%) patients reported major hemorrhagic events in the primary analysis, and no new patients reported major hemorrhage after the primary analysis. One patient had Grade 3 gastrointestinal hemorrhage and Grade 4 immune thrombocytopenia, one patient had Grade 5 subarachnoid hemorrhage, and a third had Grade 3 traumatic hemorrhage.
In other safety observations, 1/19 (5.3%) patients reported cardiac arrhythmia events (serious Grade 2 atrial flutter) after the primary analysis. Infections were reported in 15/19 (78.9%) patients. The most common infection was pneumonia (9/19 [47.4%] patients), cellulitis, and upper respiratory tract infection (6/19 [31.6%] patients each). The most common Grade 3 or higher infections were pneumonia (6/19 [31.6%] patients) and cellulitis (3/19 [15.8%] patients) (Table 4). No new safety signs were observed despite the extended duration of ibrutinib exposure.
Pharmacodynamic evaluation
ITK binding occupancy was measured; however, the signal-to-noise ratio was inadequate for quantitative analysis to return precise occupancy.
Discussion
Treatment with ibrutinib at 420 mg/day showed high overall response rates, clinically meaningful sustained responses, and durable clinical activity based on the NIH cGVHD response criteria (2014)13. Efficacy results for this updated analysis were generally consistent with those in the primary analysis11 and comparable with the final results of a phase 1b/2 study of ibrutinib in patients with cGVHD after the failure of prior therapy (the PCYC-1129 study)14.
With extended treatment, the best ORR increased from 73.7% in the primary analysis11 to 84.2% in the final analysis (additional one PR and CR each). The rate of sustained response for
Similarly, the median corticosteroid dose continued to decrease over time, to doses associated with minimal toxicity, an important goal in the treatment of patients with cGVHD. This result suggests that ibrutinib has a steroid-sparing effect that could lead to a reduction in the side effects associated with the use of corticosteroids in cGVHD16. This is an essential clinical benefit of ibrutinib given the numerous serious side effects of corticosteroids, including infection, avascular necrosis, hypertension, poor glycemic control, mood swings, osteoporosis, and weight gain17, 18. Although extended ibrutinib treatment led to increased ORR and discontinuation of steroids in several patients, the median corticosteroid dose levels remained similar after week 48. This may be due to the careful consideration of further steroid tapering in other patients, which did not result in a decrease in the median value.
The clinical efficacy of ibrutinib in cGVHD is further supported by the continued overall improvement in the Lee cGVHD Symptom Scale score in 52.6% of patients, demonstrating the patient-perceived benefit of ibrutinib on reducing symptom burden3, 19–21. The FFS and OS at 30 months were 62.7% and 62.0%, respectively.
Furthermore, the efficacy results in this study were generally similar to those from a phase 3 study of ruxolitinib and a phase 2 study of belumosudil, with best ORR of 76.4% and 76%, respectively22, 23. The improvement rate in the Lee cGVHD Symptom Scale score was 24.2% at 24 weeks for ruxolitinib and 61% at any time point for belumosudil. The FFS (18 months-FFS:68.4% in this study, 60.7% in the ruxolitinib study, and 44% in the belumosudil study) may have been affected by a higher number of prior lines of therapy in the belumosudil study than in the other two studies. Given the limited sample size of this study, the ORR of each organ could not be compared with those of other studies.
The safety findings of this updated analysis were consistent with those of the primary analysis. The safety profile of ibrutinib 420 mg/day was generally consistent with the known safety profile of ibrutinib. Given the disease characteristics of moderate and severe cGVHD and the toxicities of corticosteroids, the TEAEs observed in this study can also be considered consistent with the expected safety profile for cGVHD patients with continuous steroid use17, 24, 25. No new safety signals were observed since the primary analysis. No increase in the incidence of TEAEs leading to death, treatment discontinuation, or dose reduction was observed after the primary analysis, suggesting that ibrutinib can be used safely in the long term in patients with cGVHD. One patient reported an additional episode of Grade 3 gastrointestinal hemorrhage and one episode of Grade 4 immune thrombocytopenia during long-term follow-up. Additional Grade 3 gastrointestinal hemorrhage resolved after ibrutinib interruption; however, ibrutinib treatment was discontinued after the event was resolved by the investigator, given the risk of recurrent gastrointestinal hemorrhage. Grade 4 immune thrombocytopenia occurred during ibrutinib interruption and was considered unrelated to ibrutinib by the investigator. Infections were reported in 15/19 patients, with 10 patients having Grade 3 or higher infections, including serious Grade 4 pneumonia reported after the primary analysis. However, no increased incidence of infections was observed with long-term treatment. Cardiac arrhythmia (Grade 2 atrial flutter) was reported in one (5.3%) patient, but no ibrutinib discontinuation or reduction was required, and the event did not recur during the study. Overall, the TEAEs reported in this study were generally manageable and consistent with the known safety profile of ibrutinib. No new safety signs were observed in patients with steroid-dependent or refractory cGVHD after extended ibrutinib exposure.
Conclusions
After an additional follow-up of 22 months, the final results of Study 54179060GVH3001 were generally consistent with those of the primary analysis, showing clinically meaningful results for the best ORR and sustained response. The overall safety profile of ibrutinib at 420 mg/day was acceptable for patients aged 12 years and older. No new safety signs were observed in the primary results. The final results support previous conclusions demonstrating a positive benefit-risk profile of ibrutinib in 12 years and older Japanese patients with steroid-dependent or refractory cGVHD.
The major limitations of this analysis were its open-label design, lack of a comparator group, and small sample size. However, it is noteworthy that this study reaffirmed a clinically meaningful response in adolescent and adult patients with cGVHD treated with ibrutinib.
Acknowledgments
The authors appreciate the study participants and appreciate the investigators and study coordinators for their contributions to this study. The writing support for the manuscript was provided by Swapnil Lanjewar from Syneos Health.
Author Contributions
M.T., N.D., S.S., T.O., M.O., T.K., M.S., T.I., Y.U., N.Y., and E.F. were involved in the conduct of the research. N.Y. and E.F. were additionally involved in the design, conceptualization, management, and coordination of the research. T.H. performed the statistical analysis. All authors reviewed the manuscript for important intellectual content, approved the final manuscript, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Acknowledgments
The authors appreciate the study participants and appreciate the investigators and study coordinators for their contributions to this study. The writing support for the manuscript was provided by Swapnil Lanjewar from Syneos Health.
Financial Support
The study was funded by Janssen Pharmaceutical
Acknowledgments
The authors appreciate the study participants and appreciate the investigators and study coordinators for their contributions to this study. The writing support for the manuscript was provided by Swapnil Lanjewar from Syneos Health.
Ethics approval
Institutional review board/Independent ethics committee approval was obtained from each participating institution. This study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines.
Acknowledgments
The authors appreciate the study participants and appreciate the investigators and study coordinators for their contributions to this study. The writing support for the manuscript was provided by Swapnil Lanjewar from Syneos Health.
Consent for publication
Informed consent was obtained from each participant or their legally acceptable representative.
Conflicts of Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: M.T. received payment or honoraria from Janssen Pharmaceutical, Sanofi, Takeda Pharmaceutical Co., Nippon Shinyaku Co., Beckman Coulter Inc., Novo Nordisk Pharma., Daiichi Sankyo Co., Chugai Pharmaceutical Co., Asahi Kasei Pharma Corp., and Sumitomo Dainippon Pharma Co. for lectures, presentations, speakers' bureaus, manuscript writing, or educational events. M.S. received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Kyowa Kirin Co. Ltd., Chugai, Pfizer, Astellas, Nippon-Shinyaku, Ono, MSD, Bristol-Myers-Squibb, Asahi Kasei, Novartis, Eisai, Otsuka, Sumitomo-Dainippon, Sanofi, Takeda, Celgene, Mochida, Shire, Mundipharma, abbvie, CSL Behring, Sym-Bio, Janssen, AstraZeneca, DAIICHI SANKYO, and GlaxoSmithKline. N.D. received payment or honoraria for lectures, presentations, speakers' bureaus, manuscript writing, or educational events from Novartis Pharma and Janssen Pharma. Y.U. participated in a Data Safety Monitoring Board or Advisory Board with Sanofi and Otsuka Pharmaceutical. M.O. received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Otsuka Pharmaceutical Co. Ltd. N.Y. is an employee of Janssen Pharmaceutical K.K. and holds stock ownership with Johnson & Johnson. T.H. and E.F. are employees of Janssen Pharmaceutical K.K.
S.S., T.O., T.I., and T.K. declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
Acknowledgments
The authors appreciate the study participants and appreciate the investigators and study coordinators for their contributions to this study. The writing support for the manuscript was provided by Swapnil Lanjewar from Syneos Health.
Data Sharing Statement
The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at
References
1.Lee JW, Deeg HJ. Prevention of chronic GVHD. Best Pract Res Clin Haematol. 2008; 21: 259-70.
2.Arai S, Arora M, Wang T, Spellman SR, He W, Couriel DR, et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: a report from the Center for International Blood and Marrow Transplant Research. Biol Blood Marrow Transplant. 2015; 21: 266-74.
3.Pidala J, Kurland B, Chai X, Majhail N, Weisdorf DJ, Pavletic S, et al. Patient-reported quality of life is associated with severity of chronic graft-versus-host disease as measured by NIH criteria: report on baseline data from the Chronic GVHD Consortium. Blood. 2011; 117: 4651-7.
4.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008; 1: 134-41.
5.Kanda J, Nakasone H, Atsuta Y, Toubai T, Yokoyama H, Fukuda T, et al. Risk factors and organ involvement of chronic GVHD in Japan. Bone Marrow Transplant. 2014; 49: 228-35.
6.Ohwada C, Sakaida E, Igarashi A, Kobayashi T, Doki N, Mori T, et al. A prospective, longitudinal observation of the incidence, treatment, and survival of late acute and chronic graft-versus-host disease by National Institutes of Health Criteria in a Japanese Cohort. Biol Blood Marrow Transplant. 2020; 26: 162-70.
7.Baird K, Pavletic SZ. Chronic graft versus host disease. Curr Opin Hematol. 2006; 13: 426-35.
8.Wolff D, Fatobene G, Rocha V, Kröger N, Flowers ME. Steroid-refractory chronic graft-versus-host disease: treatment options and patient management. Bone Marrow Transplant. 2021; 56: 2079-87.
9.Flowers ME, Apperley JF, van Besien K, Elmaagacli A, Grigg A, Reddy V, et al. A multicenter prospective phase 2 randomized study of extracorporeal photopheresis for treatment of chronic graft-versus-host disease. Blood. 2008; 112: 2667-74.
10.Zeiser R, Lee SJ. Three US Food and Drug Administration-approved therapies for chronic GVHD. Blood. 2022; 139: 1642-5.
11.Doki N, Toyosaki M, Shiratori S, Osumi T, Okada M, Kawakita T, et al. An open-label, single-arm, multicenter study of ibrutinib in Japanese patients with steroid-dependent/refractory chronic graft-versus-host disease. Transplant Cell Ther. 2021; 27: 867.e1-9.
12.Martin PJ, Lee SJ, Przepiorka D, Horowitz MM, Koreth J, Vogelsang GB, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: VI. The 2014 Clinical Trial Design Working Group report. Biol Blood Marrow Transplant. 2015; 21: 1343-59.
13.Lee SJ, Wolff D, Kitko C, Koreth J, Inamoto Y, Jagasia M, et al. Measuring therapeutic response in chronic graft-versus-host disease. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IV. The 2014 Response Criteria Working Group report. Biol Blood Marrow Transplant. 2015; 21: 984-99.
14.Waller EK, Miklos D, Cutler C, Arora M, Jagasia MH, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy: 1-year update of a phase 1b/2 study. Biol Blood Marrow Transplant. 2019; 25: 2002-7.
15.Palmer J, Williams K, Inamoto Y, Chai X, Martin PJ, Santo Tomas LH, et al. Pulmonary symptoms measured by the national institutes of health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014; 20: 337-44.
16.Wolff D, Schleuning M, von Harsdorf S, Bacher U, Gerbitz A, Stadler M, et al. Consensus conference on clinical practice in chronic GVHD: second-line treatment of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2011; 17: 1-17.
17.Dignan FL, Amrolia P, Clark A, Cornish J, Jackson G, Mahendra P, et al. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol. 2012; 158: 46-61.
18.Miklos D, Cutler CS, Arora M, Waller EK, Jagasia M, Pusic I, et al. Ibrutinib for chronic graft-versus-host disease after failure of prior therapy. Blood. 2017; 130: 2243-50.
19.Mitchell SA, Leidy NK, Mooney KH, Dudley WN, Beck SL, LaStayo PC, et al. Determinants of functional performance in long-term survivors of allogeneic hematopoietic stem cell transplantation with chronic graft-versus-host disease (cGVHD). Bone Marrow Transplant. 2010; 45: 762-9.
20.Lee SK, Cook EF, Soiffer R, Antin JH. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002; 8: 444-52.
21.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003; 9: 215-33.
22.Zeiser R, Polverelli N, Ram R, Hashmi SK, Chakraverty R, Middeke JM, et al. Ruxolitinib for Glucocorticoid-Refractory Chronic Graft-versus-Host Disease. N Engl J Med. 2021; 385: 228-38.
23.Cutler C, Lee SJ, Arai S, Rotta M, Zoghi B, Lazaryan A, et al. Belumosudil for chronic graft-versus-host disease after 2 or more prior lines of therapy: the ROCKstar Study. Blood. 2021; 138: 2278-89.
24.Rice JB, White AG, Scarpati LM, Wan G, Nelson WW. Long-term Systemic Corticosteroid Exposure: A Systematic Literature Review. Clin Ther. 2017; 39: 2216-29.
25.Carpenter PA, Kitko CL, Elad S, Flowers ME, Gea-Banacloche JC, Halter JP, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: V. The 2014 Ancillary Therapy and Supportive Care Working Group Report. Biol Blood Marrow Transplant. 2015; 21: 1167-87.
Search
News