Volume 6 (2023) Issue 3 No.6 Pages 95-103
Abstract
Background: The prognosis of Hodgkin lymphoma (HL) relapsing post autologous transplant (AuSCT) is poor. Even with novel therapies, only approximately 20%-25% of patients attain complete remissions, with a median progression-free survival (PFS) of approximately 5-15 months. Lenalidomide has been shown to have activity in relapsed HL. We retrospectively analyzed the outcomes of patients with relapsed HL post AuSCT treated with lenalidomide alone or in combination with dexamethasone at our center.
Patients and methods: Records of 143 patients transplanted from November 2007 to October 2021 were reviewed. Of these patients, 41 (28%) relapsed, and 16 (39%) received lenalidomide alone or in combination with dexamethasone. Data collected included demographic, pathological, staging, and prior therapy details. Lenalidomide was administered at 10-25 mg/day on an intermittent or continuous schedule alone or in combination with dexamethasone (20-40 mg weekly). Response was assessed using PET-CT scan in accordance with Lugano criteria. Standard definitions were used for response, PFS, and overall survival (OS). Toxicities were graded using Common Terminology Criteria for Adverse Events version 5.0. Statistical analysis was done using SPSS Version 21.
Results: The median age of the patients was 25.5 years, and 10 were males. Eleven (69%) had advanced disease, and 7 (44%) were refractory to last systemic therapy. Nine patients received lenalidomide alone and 7 with dexamethasone. Four (25%) had complete response, and another four (25%) had partial response, with an overall response rate of 50%. The 3-year PFS and OS were 31% and 38%, respectively. Grade III/IV toxicities were only hematological, neutropenia and thrombocytopenia in four and three patients, respectively. No therapy-related deaths were recorded.
Conclusions: Lenalidomide alone or in combination with dexamethasone is a safe and effective therapy for relapsed HL post AuSCT and results in durable response and long-term survival in approximately one-third of the patients. However, these results needs verification in larger prospective studies.
Introduction
Prognosis of patients with Hodgkin lymphoma (HL) with relapsed or refractory disease after autologous stem cell transplant (AuSCT) is poor. Such patients have limited therapeutic options and a long-term survival of approximately 20%-30%1, 2. Over the last decade, several new therapeutic options (e.g., anti-CD30 antibody brentuximab vedotin or immune checkpoint inhibitors such as nivolumab and pembrolizumab) have become available for these patients3–7. Although these agents have become available, an allogeneic transplant is considered the only curative option for patients with post AuSCT relapse of HL8. The availability of several of these novel agents and improved outcomes following allogeneic transplant have improved the outcomes of patients with relapsed or refractory HL (RRHL) post AuSCT in recent years2. However, all these treatments are extremely costly and/or morbid and are infeasible for the majority of patients in low- and middle-income countries, such as India. Lenalidomide is an anti-neoplastic drug with immunomodulatory and several other anti-neoplastic properties9 Apart from its approved indications for multiple myeloma and myelodysplastic syndrome (especially those with del 5q abnormality), lenalidomide has been used in several other hematological malignancies10–18. It is also effective in HL, either alone19–23 or in combination with other drugs24–26. However, data on the long-term outcomes of patients treated with lenalidomide, especially those from low- and middle-income countries, are scarce. In this study, we retrospectively analyzed the outcomes of patients at our center who had received lenalidomide with or without dexamethasone for RRHL after AuSCT.
Patients and Methods
Patient selection
The pharmacy and medical records of all patients who underwent an AuSCT for RRHL between November 2007 and October 2021 were reviewed. All patients who received lenalidomide with or without dexamethasone for relapsed (recurrence of HL after a previous complete remission) or progressive disease (disease progression without a previous complete remission) post AuSCT were included in this single-center retrospective analysis. The choice of treatment for post AuSCT relapse was at the discretion of the treating clinician, and no specific policy was followed for selecting patients for lenalidomide-based therapy. It may be noted that, checkpoint inhibitors were not available for a large part of the study period, and anti-CD30 therapies such as brentuximab were unavailable for the entire study period. Therefore, patients with post AuSCT relapse did not have significant treatment options available. Those who received lenalidomide as a maintenance strategy were excluded. Data were updated until November 30, 2021. The study was approved by the institutional ethics committee (IEC-III) of Tata Memorial Centre (protocol number 900946) and was carried out in accordance with the principles in the Declaration of Helsinki. The need for an informed consent was waived off, considering the nature of the study.
Prior management
At baseline diagnosis, all patients underwent biopsy for histopathological diagnosis. Staging work-up included PET-CT scan (or contrast-enhanced CT scan of the thorax and abdomen along with bone marrow biopsy). Risk stratification and management was based on the German Hodgkin study group guidelines27. The first-line therapy was a combination therapy of adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) with bleomycin replaced by etoposide (AEVD) in patients with poor lung function at baseline28, 29. Accordingly, patients with early favorable disease, early unfavorable disease, and advanced disease received 2, 4, and 6 cycles of ABVD, respectively27. Involved-field radiotherapy was administered to all patients with early-stage disease. It was administered to those with advanced stage disease only in selected situations, such as residual disease with inability/unwillingness to undergo immediate salvage therapy. At relapse, restaging was done with PET-CT in all patients. Relapse was confirmed with biopsy wherever indicated. After relapse, salvage therapy was at the discretion of the treating clinician. Gemcitabine, dexamethasone, and cisplatin (or carboplatin)30 was the preferred salvage option. However, other salvage regimens were also used. Response was assessed typically after 2-3 cycles using PET-CT scan. Stem cell mobilization was done using chemo-mobilization using the subsequent cycle of salvage chemotherapy. The conditioning chemotherapy for autologous transplant consisted of lomustine, cytarabine, cyclophosphamide, and etoposide (LACE) regimen or the BCNU, etoposide, cytarabine, and melphalan (BEAM) regimen31. Post-transplant, the patients remained under regular follow up with annual PET-CT scans until 5 years post-transplant. Whenever post-transplant relapse was suspected, PET-CT scan was repeated with biopsy performed when clinically indicated.
Study treatment
Lenalidomide was administered at a dose of 10-25 mg per day either on a continuous or intermittent schedule as per the discretion of the treating clinicians. In addition, those who received dexamethasone along with lenalidomide received dexamethasone at a dose of 20-40 mg per week. All patients received aspirin 75 mg per day for prophylaxis against thrombosis. All patients who received dexamethasone along with lenalidomide also received cotrimoxazole twice weekly for prophylaxis against Pneumocystis carinii pneumonia.
Study endpoints and outcome measures
Responses were assessed using PET-CT scans in accordance with Lugano criteria32. PET-CT scans were generally done at 3 months post start of therapy and subsequently as per the treating clinician. Complete and partial responses, stable disease, and progressive disease were defined as per Lugano criteria32. Overall response rate (ORR) was complete response (CR) + partial responses (PR). For evaluation of ORR, responses at a specific time point were not considered because of the retrospective design of this study. Best responses at any time until initiation of a new therapy for HL was considered. Additionally, as described in a couple of previous papers, clinical benefit rate included patients with stable disease for > 6 months in addition to ORR. Progression-free survival (PFS) was defined from the start of lenalidomide treatment until disease progression. Those patients who were progression free at their last follow up were censored. Overall survival (OS) was counted from the start of lenalidomide treatment until death due to any cause, and those patients who were alive on their last follow up were censored. For patients undergoing allogeneic stem cell transplant after lenalidomide-based therapy, the data for OS were not censored at transplant because lenalidomide may increase the risk of post allogeneic transplant graft versus host disease33, 34. Toxicity was graded using Common Terminology Criteria for Adverse Events Version 5.0.
Statistics
For all analyses, data were updated until November 30, 2021. Patient characteristics, responses, and toxicities were described using descriptive statistics. PFS and OS were calculated using Kaplan-Meier curves. Statistical analysis was performed using SPSS software Version 21.
Results
A total of 143 patients underwent AuSCT for HL from November 2007 until October 2021. Of these 143 patients, 41 (28%) experienced relapse post AuSCT. Sixteen of the 41 (39%) patients received lenalidomide-based therapy post AuSCT for relapse/refractory disease and were included in the analysis.
Patient characteristics
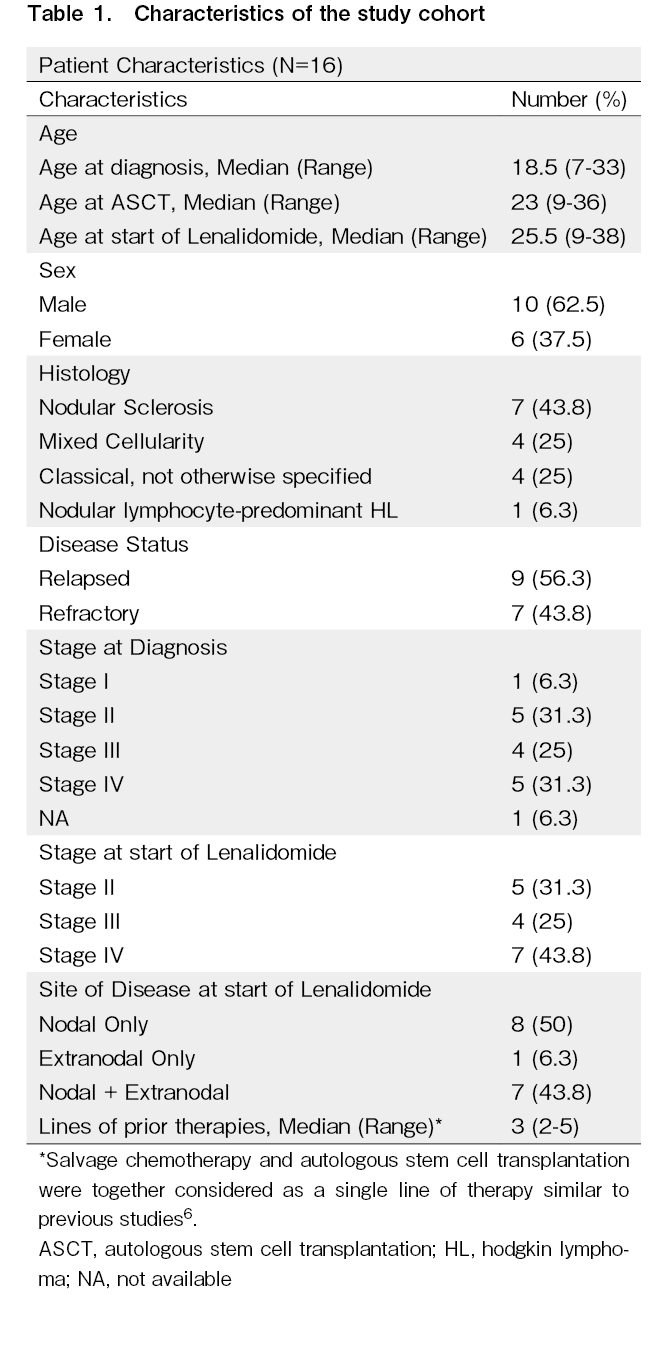
Patient characteristics are shown in Table 1. The median ages at diagnosis and at the start of lenalidomide treatment were 18.5 and 25.5 years, respectively. Majority of patients were males (n = 10, 62.5%). Nodular sclerosis was the most common histology (43.8%). Seven patients (43.8%) were refractory to their last line of therapy, and 11 patients had advanced disease (69%). The patients were heavily pretreated with a median of three lines of prior therapy (see footnote below Table 1). All patients had received ABVD/AEVD as the first-line therapy. Salvage therapies included gemcitabine, dexamethasone, and cisplatin in nine; ifosfamide, gemcitabine, vinorelbine, dexamethasone/dexamethasone, etoposide, cisplatin, cytarabine35 in four; and mitoxantrone, ifosfamide, etoposide36 in three patients. The conditioning regimen for transplant included LACE in 10 and BEAM in 6 patients. The median time to relapse post AuSCT was 8 months. No patient had received prior immunotherapy (nivolumab/pembrolizumab) or brentuximab vedotin. Nine patients received lenalidomide alone, and seven patients received lenalidomide with dexamethasone.
Responses, PFS, and OS
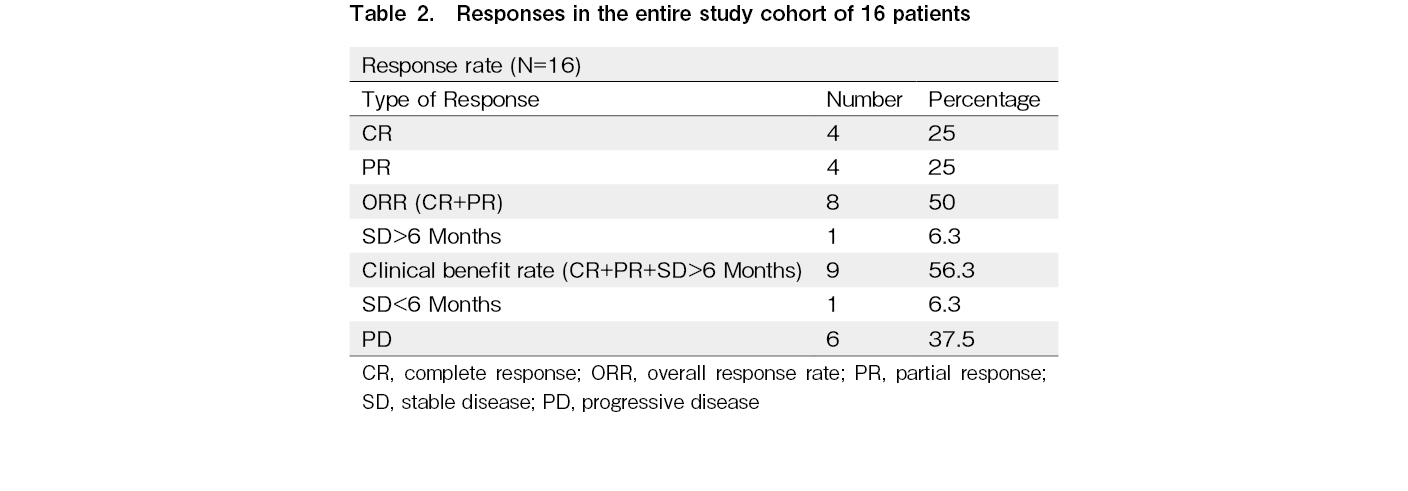
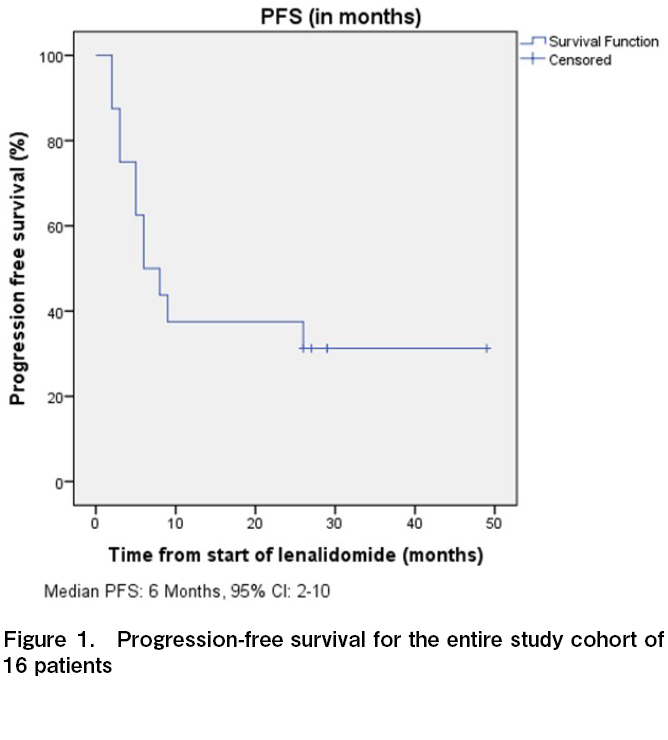
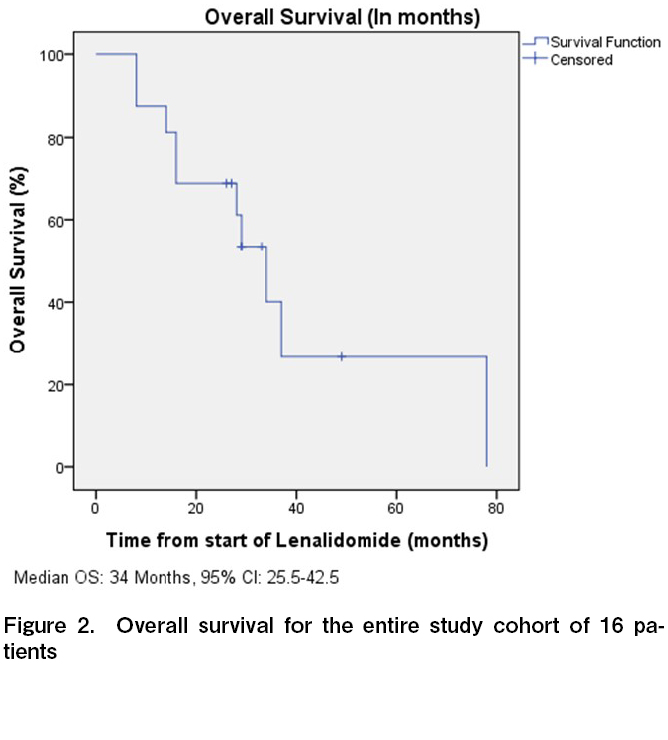
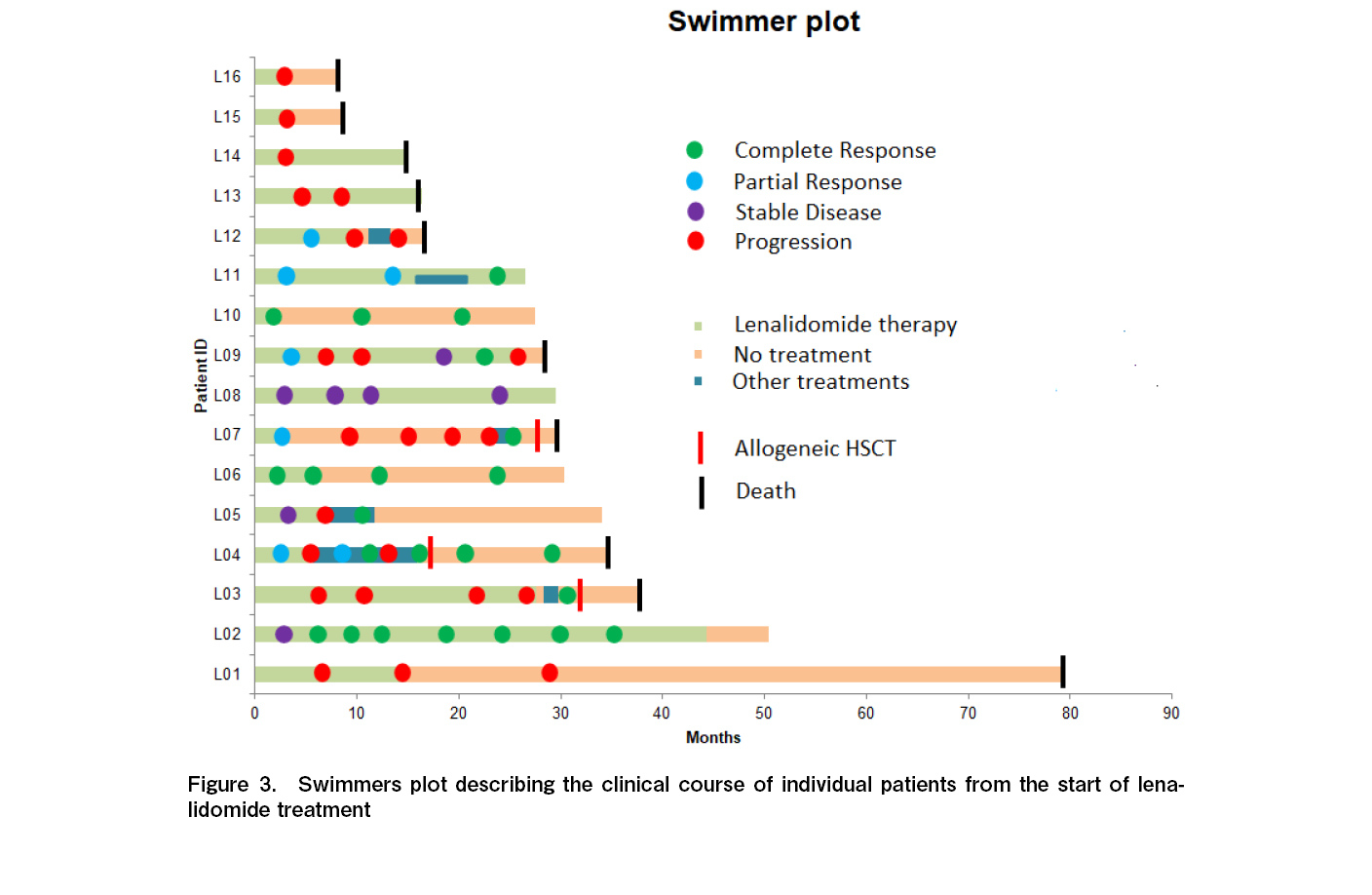
All 16 patients were evaluable for response, and the ORR was 50% with four CR and four PR (Table 2). One additional patient had stable disease for > 6 months, with a clinical benefit rate (CR + PR + SD > 6 months) of 56.3%. Among the four patients who attained CR, three continued to remain in CR at the time of last follow up. The median time to response and duration of response among the responders were 3 and 14.5 months, respectively. With a median follow up time of 28.5 months, the median PFS and OS were 6 months (95% CI, 2-10 months) and 34 months (95% CI, 25.5-42.5 months), respectively (Figures 1 and 2). The 3-year PFS and OS were 31 and 38%, respectively (Figures 1 and 2). Of the 11 patients who progressed, 10 (91%) were within 12 months of the start of therapy. Consistent with this result, the PFS curve flattened after the first year of therapy. The clinical course of individual patients is shown in a swimmer plot in Figure 3.
Treatment beyond progression
Considering the immunomodulatory effect of lenalidomide, we also evaluated the patients who had been continued on therapy beyond progression (similar to what has been evaluated for checkpoint inhibitors)6. Five patients (L01, L03, L09, L13, and L14; Figure 3) were continued on therapy beyond the PET-CT scan-defined progression because they were clinically benefiting from therapy. In these patients, the duration of therapy after progressive disease was between 8 and 24 months. One of these patients subsequently achieved a CR (patient L09).
Toxicities and study therapy discontinuation
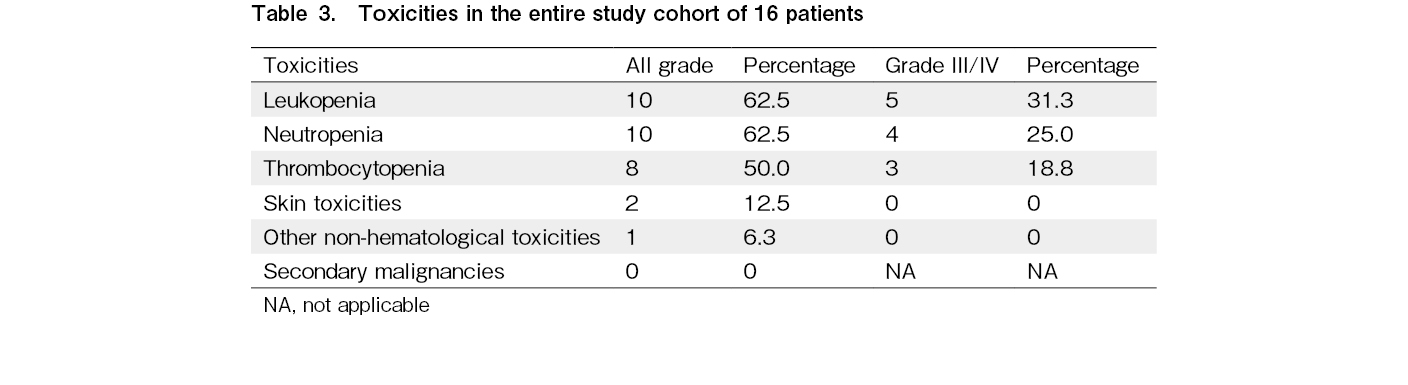
Overall, the treatment was well tolerated. The most common grade 3 or 4 toxicities (Table 3) included neutropenia (n = 4), leukopenia (n = 5), and thrombocytopenia (n = 3). Two patients had grade 2 skin toxicities. One patient experienced a tumor flare reaction (grade 2). No episodes of febrile neutropenia were observed. Lenalidomide was interrupted and restarted with reduced dose in two patients (because of cytopenia in one patient and skin toxicity in the other patient). The patient with tumor flare required interruption, but therapy was subsequently restarted at the same dose. The median duration of lenalidomide therapy was 11.5 months (range, 1-43+ months). Overall, 15 patients (94%) discontinued therapy due to progressive disease (n = 10) and unrelated causes (n = 5). Among these five patients, one (L02) discontinued because of the non-availability of the drug during the COVID pandemic, one (L10) because of disseminated tuberculosis, one (L06) discontinued while undergoing spinal surgery, one (L11) after achieving CR (with total lymphoid irradiation), and one (L07) after achieving PR. No patient discontinued therapy due to toxicities, and no therapy-related deaths were recorded.
Subsequent allogeneic stem cell transplant (allo-SCT) and post allo-SCT outcomes
Three patients (19%; L03, L04, and L07) underwent allogeneic stem cell transplant (Figure 3). Among these three patients, the times from last dose of lenalidomide to allogeneic HSCT were 4, 11, and 23 months respectively. Two (L03 and L04) of these three received nivolumab either alone (L03) or sequentially with conventional chemotherapy (L04) after failure of lenalidomide-based therapy. One patient (L07) received only conventional chemotherapy. All three were in complete remission at the time of allogeneic stem cell transplant. All three succumbed because of transplant-related complications – acute GVHD and subsequent bacterial, fungal, and viral infections.
Discussion
Lenalidomide is an immunomodulatory drug with several effects on the immune system. At a broader level, lenalidomide can alter cytokine production, activate T cells, augment the natural killer cell function, exert anti-angiogenic effect, and even directly affect tumor cells in several hematological malignancies9. At the molecular level, cereblon has been identified as the target for lenalidomide37. Cereblon is a component of the E3 ubiquitin ligase complex. When lenalidomide binds to cereblon, it alters the substrate specificity of the E3 ubiquitin ligase complex. This phenomenon increases the ubiquitination of different proteins (individual proteins differ in different diseases) and thus enhances their proteosomal degradation. For example, lenalidomide enhances the binding of Ikaros 3 (IKZF3) to this ubiquitin ligase complex and promotes its degradation. IKZF3 is a transcriptional repressor of interleukin-2 (IL-2); thus, its degradation increases IL-2 production, leading to increased proliferation of NK cells and certain subsets of T cells37. Similarly, lenalidomide decreases the expression of PDL-1 on the surface of lymphoma cells38. Although the precise targets in HL are unclear, PDL-1 is overexpressed in Reed-Sternberg cells in HL39.
Although a biological rationale exists, clinical data on the outcomes of patients with RRHL treated with lenalidomide are scarce. From a clinical perspective, patients with RRHL after AuSCT have few effective treatment options, and their prognosis is generally poor1, 2. Although conventional chemotherapies have been used, their outcomes are far from satisfactory. Currently, brentuximab vedotin and check-point inhibitors (nivolumab and pembrolizumab) are considered the “standard of care” in such scenarios. However, even these treatment options fail to cure the majority of patients, and an allogeneic HSCT is often considered the only curative option8.
The outcomes of immunotherapies and brentuximab merit a discussion. With brentuximab, an ORR of approximately 75% and a CR rate of 34% have been reported. Additionally, in the pivotal study of brentuximab, a median PFS of 5.6 months and median OS of 22 months have been reported3, 4. Similarly, as reported in the CheckMate 205 trial5, the ORR and CR rates with nivolumab were 69% and 16%, respectively. The 1-year survival with nivolumab was approximately 90%, and the median duration of response in responding patients was 16 months. Results with pembrolizumab have been similar to those with nivolumab7. Other treatment options for RRHL post autologous HSCT include conventional chemotherapies. However, the results of conventional chemotherapy have either been poor or similar40–42.
In the present study, we obtained an ORR of 50%, CR rate of 25%, 1-year OS of 82%, and median duration of response approximately 15 months. Additionally, similar to checkpoint inhibitors, we used lenalidomide beyond PET-CT-defined progression; 1 of 5 patients (20%) attained CR with continued lenalidomide. Although these results cannot be compared with either of these novel therapies because of the small numbers and the retrospective nature of the study, they suggest that lenalidomide is active and safe in this scenario.
A few previous studies have assessed the role of lenalidomide in RRHL, either alone19–23 or in combination with other drugs24–26. In these studies, ORRs were 15%-50% and CR rates were approximately 5%-30%. The median PFS and OS in these studies were also similar to those in the present study. However, these studies are limited by their small sample sizes, which would likely explain the differences in the results in these studies. Therefore, whether adding agents, such as panabinostat or temsirolimus, or others confers any additional benefit over and above that of lenalidomide alone remains unclear to date. Currently, trials combining lenalidomide with nivolumab (ClinicalTrials.gov identifier: NCT03015896)43, brentuximab vendotin (ClinicalTrials.gov identifier: NCT03302728)44, and bendamustine (ClinicalTrials.gov identifier: NCT01412307)45 in RRHL are ongoing. A clinical trial also evaluated the combination of lenalidomide and pembrolizumab in RRHL (ClinicalTrials.gov identifier: NCT02875067)46. However, this study was terminated prematurely when none of the six participants who had been enrolled completed the treatment, which made the safety and efficacy of this combination in RRHL difficult to assess. A trial combining pembrolizumab and lenalidomide – dexamethasone in multiple myeloma was terminated prematurely by the US FDA because of an unfavorable risk benefit profile47. Thus, drugs for combining with lenalidomide in RRHL must be selected carefully.
At this juncture, discussing the impact of lenalidomide on the outcomes of subsequent allogeneic stem cell transplant is important because, as mentioned earlier, this procedure is the only curative option in this scenario8. Some reports suggested that lenalidomide increases the risk of acute graft versus host disease after an allogeneic stem cell transplant48. It should also be noted that immunotherapies like nivolumab increases the risk of acute graft versus host disease when used prior to transplant49. Given some similarities between lenalidomide and nivolumab from an immunological perspective, lenalidomide use prior to allogeneic transplant may also increase the risk of acute GVHD. In the present study, all three patients who underwent allogeneic HSCT in complete remission died of transplant-related complications. In our patients, lenalidomide treatment was stopped > 3 months prior to allogeneic transplant, and two of the three patients had received nivolumab after lenalidomide but before allogeneic transplant. Thus, whether lenalidomide had any impact on the post allogeneic HSCT course was difficult to determine. Such patients who are subsequently receiving an allogeneic transplant may be allowed a washout period of few weeks to months. Whether this would help or not and the length of the washout period warrant further investigation.
Our study has some limitations, such as small sample size and retrospective design. Nevertheless, this study is probably one of the largest single-center cohorts for lenalidomide in RRHL. In addition, the data were complete because no patient was lost to follow up. However, we could not evaluate the impact of sequencing of lenalidomide because no patients had received nivolumab/pembrolizumab or brentuximab prior to lenalidomide and only two patients received nivolumab after. Therefore, this study cannot provide a particular sequence of lenalidomide in the current era even with the availability of several new molecules. However, in patients where these novel agents are unavailable or infeasible, lenalidomide could be considered a treatment option.
The findings of our study support the efficacy and feasibility of lenalidomide with acceptable toxicity in patients with RRHL post AuSCT. This treatment results in long-term survival in approximately one-third of the patients. Larger and prospective studies are warranted to confirm these findings and identify the subgroup of patients who are likely to benefit the most from lenalidomide treatment.
Author Contributions
KK and SP designed the study. KK, SP, AG, LN, AC, SM, NJ and LM did the data collection. KK and SP did the analysis. KK wrote the first draft of the manuscript. SP, AG, LN, AC, SM, NJ and NK – critical revision of the manuscript.
Ethics approval
This study was approved by the institutional ethics committee. The protocol number is mentioned in the manuscript.
Consent for publication
Given the retrospective nature of the study, no contact with the patient in any form, analysis of de-identified patient data, and no risk to the patients, the institutional ethics committee waived off the need for a consent.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Bazarbachi A, Boumendil A, Finel H, Khvedelidze I, Romejko-Jarosinska J, Tanase A, et al. The outcome of patients with Hodgkin lymphoma and early relapse after autologous stem cell transplant has improved in recent years. Leukemia. 2022; 36: 1646-53.
2.Moskowitz AJ, Perales MA, Kewalramani T, Yahalom J, Castro-Malaspina H, Zhang Z, et al. Outcomes for patients who fail high dose chemoradiotherapy and autologous stem cell rescue for relapsed and primary refractory Hodgkin lymphoma. Br J Haematol. 2009; 146: 158-63.
3.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012; 30: 2183-9.
4.Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016; 128: 1562-6.
5.Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for relapsed/refractory classic Hodgkin lymphoma after failure of autologous hematopoietic cell transplantation: extended follow-up of the multicohort single-arm phase ii checkmate 205 trial. J Clin Oncol. 2018; 36: 1428-39.
6.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin's lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016; 17: 1283-94.
7.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II study of the efficacy and safety of pembrolizumab for relapsed/refractory classic Hodgkin lymphoma. J Clin Oncol. 2017; 35: 2125-32.
8.Iqbal M, Kharfan-Dabaja MA. Relapse of Hodgkin lymphoma after autologous hematopoietic cell transplantation: a current management perspective. Hematol Oncol Stem Cell Ther. 2021; 14: 95-103.
9.Kotla V, Goel S, Nischal S, Heuck C, Vivek K, Das B, et al. Mechanism of action of lenalidomide in hematological malignancies. J Hematol Oncol. 2009; 2: 36.
10.Leonard JP, Trneny M, Izutsu K, Fowler NH, Hong X, Zhu J, et al. AUGMENT: a phase iii study of lenalidomide plus rituximab versus placebo plus rituximab in relapsed or refractory indolent lymphoma. J Clin Oncol. 2019; 37: 1188-99.
11.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006; 355: 1456-65.
12.List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005; 352: 549-57.
13.Santini V, Almeida A, Giagounidis A, Gröpper S, Jonasova A, Vey N, et al. Randomized phase III study of lenalidomide versus placebo in RBC transfusion-dependent patients with lower-risk non-del(5q) myelodysplastic syndromes and ineligible for or refractory to erythropoiesis-stimulating agents. J Clin Oncol. 2016; 34: 2988-96.
14.Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007; 357: 2123-32.
15.Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007; 357: 2133-42.
16.Fehniger TA, Uy GL, Trinkaus K, Nelson AD, Demland J, Abboud CN, et al. A phase 2 study of high-dose lenalidomide as initial therapy for older patients with acute myeloid leukemia. Blood. 2011; 117: 1828-33.
17.Blum W, Klisovic RB, Becker H, Yang X, Rozewski DM, Phelps MA, et al. Dose Escalation of lenalidomide in relapsed or refractory acute leukemias. J Clin Oncol. 2010; 28: 4919-25.
18.Punatar S, Gokarn A, Nayak L, Bonda A, Mirgh S, Chichra A, et al. Human leukocyte antigen (HLA) alleles as predictive factors for benefit from lenalidomide in acute myeloid leukemia (AML). Am J Blood Res. 2021; 11: 564-70.
19.Fehniger TA, Larson S, Trinkaus K, Siegel MJ, Cashen AF, Blum KA, et al. A phase 2 multicenter study of lenalidomide in relapsed or refractory classical Hodgkin lymphoma. Blood. 2011; 118: 5119-25.
20.Ma H, Cheng B, Montanari F, Lue JK, Deng C, Marchi E, et al. Low dose continuous lenalidomide in heavily pretreated patients with relapsed or refractory classical Hodgkin lymphoma: a retrospective case series. Ther Adv Hematol. 2020; 11: 2040620720947340.
21.Kuruvilla J, Taylor D, Wang L, Blattler C, Keating A, Crump M. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2008; 112: 3052.
22.Böll B, Borchmann P, Topp MS, Hänel M, Reiners KS, Engert A, et al. Lenalidomide in patients with refractory or multiple relapsed Hodgkin lymphoma. Br J Haematol. 2010; 148: 480-2.
23.Mandac I, Kolonic SO. Lenalidomide induced good clinical response in a patient with multiple relapsed and refractory Hodgkin's lymphoma. J Hematol Oncol. 2010; 3: 20.
24.Rueda A, García-Sanz R, Pastor M, Salar A, Labrador J, Quero-Blanco C, et al. A phase II study to evaluate lenalidomide in combination with metronomic-dose cyclophosphamide in patients with heavily pretreated classical Hodgkin lymphoma. Acta Oncol. 2015; 54: 933-8.
25.Maly JJ,, Christian BA, Zhu X, Wei L, Sexton JL, Jaglowski SM, et al. A phase i/ii trial of panobinostat in combination with lenalidomide in patients with relapsed or refractory Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk. 2017; 17: 347-53.
26.Major A, Kline J, Karrison TG, Fishkin PAS, Kimball AS, Petrich AM, et al. Phase I/II clinical trial of temsirolimus and lenalidomide in patients with relapsed and refractory lymphomas. Blood. 2020; 136 (Suppl 1): 43-4.
27.Allen PB, Gordon LI. Frontline Therapy for classical Hodgkin lymphoma by stage and prognostic factors. Clin Med Insights Oncol. 2017; 11: 1179554917731072.
28.Bonfante V, Santoro A, Viviani S, Valagussa P, Bonadonna G. ABVD in the treatment of Hodgkin's disease. Semin Oncol. 1992; 19 (2 Suppl 5): 38-44; discussion 44-5.
29.Lacerda MP de, Oliveira JC de, Jungles S, Macedo GS, Tomazelli AP, Baptista JPR, et al. Doxorubicin, etoposide, vinblastine and dacarbazine (AEVD) for newly diagnosed classic Hodgkin lymphoma. Blood. 2021; 138 (Suppl 1): 2473.
30.Moccia AA, Hitz F, Hoskins P, Klasa R, Power MM, Savage KJ, et al. Gemcitabine, dexamethasone, and cisplatin (GDP) is an effective and well-tolerated salvage therapy for relapsed/refractory diffuse large B-cell lymphoma and Hodgkin lymphoma. Leuk Lymphoma. 2017; 58: 324-32.
31.Khattry N, Gupta A, Jain R, Gore A, Thippeswamy R, Jeevangi N, et al. LACE versus BEAM conditioning in relapsed and refractory lymphoma transplant: retrospective multicenter analysis of toxicity and efficacy. Int J Hematol. 2016; 103: 292-8.
32.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32: 3059-68.
33.Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012; 97: e34-5.
34.Zhong J, Zhang X, Liu M, et al. The efficacy and safety of lenalidomide in the treatment of multiple myeloma patients after allo-hematopoietic stem-cell transplantation: a systematic review and meta-analysis. Ann Palliat Med. 2021; 10: 7736-46.
35.Pandey A, Srinivasan S, Moulik N, Dhamne C, Chicchra A, Shah S, et al. Outcome of pediatric relapsed refractory Hodgkin lymphoma experience from Tata Memorial Hospital, Mumbai. Pediatric Hematology Oncology Journal. 2022; 7: S3.
36.Chang SH, Kim YS, Eo WK. MINE (mesna, ifosfamide, mitoxantrone, etoposide) chemotherapy as a treatment of relapsed or refractory aggressive non-Hodgkin's lymphoma. Cancer Res Treat. 2002;
34: 145-52.
37.Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood. 2015; 126: 2366-9.
38.Gribben JG, Fowler N, Morschhauser F. Mechanisms of action of lenalidomide in B-cell non-Hodgkin lymphoma. J Clin Oncol. 2015; 33: 2803-11.
39.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010; 116: 3268-77.
40.Johnston PB, Pinter-Brown L, Rogerio J, Warsi G, Graham A, Ramchandren R. Open-label, single-arm, phase ii study of everolimus in patients with relapsed/refractory classical Hodgkin lymphoma. Blood. 2011; 118: 2717.
41.Bartlett NL, Niedzwiecki D, Johnson JL, Friedberg JW, Johnson KB, van Besien K, et al. Gemcitabine, vinorelbine, and pegylated liposomal doxorubicin (GVD), a salvage regimen in relapsed Hodgkin's lymphoma: CALGB 59804. Ann Oncol. 2007; 18: 1071-9.
42.Moskowitz AJ, Hamlin PA Jr, Perales MA, Gerecitano J, Horwitz SM, Matasar MJ, et al. Phase II study of bendamustine in relapsed and refractory Hodgkin lymphoma. J Clin Oncol. 2013; 31: 456-60.
43.Nivolumab and lenalidomide in treating patients with relapsed or refractory non-Hodgkin or Hodgkin lymphoma – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03015896 [Accessed: 7 May 2022]
44.Brentuximab vedotin and lenalidomide in patients with relapsed/ refractory t-cell lymphoma or Hodgkin lymphoma – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03302728 [Accessed: 7 May 2022]
45.A phase 1/2 study of lenalidomide in combination with bendamustine in relapsed and primary refractory Hodgkin lymphoma – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT01412307 [Accessed: 7 May 2022]
46.Safety & efficacy study of combination of pembrolizumab and lenalidomide, in patients with relapsed non-Hodgkin and Hodgkin lymphoma – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02875067 [Accessed: 7 May 2022]
47.Usmani SZ, Schjesvold F, Oriol A, Karlin L, Cavo M, Rifkin RM, et al. Pembrolizumab plus lenalidomide and dexamethasone for patients with treatment-naive multiple myeloma (KEYNOTE-185): a randomised, open-label, phase 3 trial. Lancet Haematol. 2019; 6: e448-58.
48.Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, et al. Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): Results of the LENAMAINT trial. Haematologica. 2012; 97: e34-5.
49.Ijaz A, Khan AY, Malik SU, Faridi W, Fraz MA, Usman M, et al. Significant Risk of Graft-versus-Host Disease with Exposure to Checkpoint Inhibitors before and after Allogeneic Transplantation. Biol Blood Marrow Transplant. 2019; 25: 94-9.
Search
News