Volume 6 (2023) Issue 3 No.5 Pages 87-94
Abstract
Introduction: Patients with relapsing or primary refractory Hodgkin lymphoma, still have unsatisfactory outcomes after high dose chemotherapy followed by autologous stem cell transplantation (ASCT). Brentuximab Vedotin (BV) is the only approved agent for maintenance therapy for up to one year in these patients, however, this agent is often not available or affordable for many patients, especially in the developing countries. In this study, we used Everolimus as maintenance therapy after ASCT among patients with relapsing/refractory Hodgkin lymphoma.
Materials and Methods: We collected the data of 20 patients with primary refractory or relapsing Hodgkin lymphoma who had undergone ASCT between June 2016 and June 2021. Everolimus was started at 10 mg daily on day +90 after ASCT for at least two years in patients with stable disease status, confirmed by imaging modalities. Patients were followed for disease status and drug side effects every 3 months.
Results: In our single-arm case-series study, the median duration of treatment was 22.95 months. Except for three patients, who had progression, others had no progression and are still receiving Everolimus (85%). No serious side effect was seen. We had no mortality due to disease recurrence.
Conclusion: Until now, results showed promising effects of Everolimus in patients with relapsing or primary refractory Hodgkin lymphoma as maintenance therapy after ASCT.
Introduction
Hodgkin lymphoma accounts for 10% of all lymphomas1. Despite the progress and advancements in treatment of Hodgkin lymphoma, which has led to approximate cure rate of 90%, there remains 10-15% of patients with early-stage and 15-20% of patients with advanced-stage who are at high risks of relapse. In addition, about 15% of patients do not respond to several lines of chemotherapy which is called primary refractory Hodgkin lymphoma2. Hematopoietic stem cell transplantation (HSCT) is recommended for primary Hodgkin lymphoma patients in complete response 2 (CR2), Partial response (PR1), and beyond. There are some patients with poor risk factors, including primary refractory disease, patients who are not in CR before ASCT, patients who had received more than one salvage chemotherapy, patients with positive positron emission tomography (PET) scan before HSCT and those who relapsed less than 12 months after the end of chemotherapy3. Brentuximab Vedotin is recommended after ASCT at the dose of 1.8 mg/kg every three weeks for up to sixteen cycles (one year) for these high-risk patients. Brentuximab Vedotin therapy results in increasing disease-free survival (DFS) but not overall survival (OS)4. Meanwhile, some other agents such as pembrolizumab 200 mg (every three weeks for eight cycles) are being studied and the progression free survival (PFS) and OS were calculated 78% and 100% respectively5.
Treatment with Brentuximab Vedotin for up to one-year results in high costs of health-care, especially in developing countries. Also, this drug may not always be available. Everolimus belongs to a class of kinase inhibitors that targets Mammalian Target of Rapamycin (mTOR). This protein kinase, regulates cell growth, metabolism and survival. It is also involved in many critical regulatory cell cycle functions, such as controlling the cellular response to DNA damage and hence, apoptosis6. The catalytic domain of mTOR is highly homologous to the lipid domain of phosphatidylinositol 3-kinase (PI3K)7. There are growing evidence about the role of PI3K/Akt/mTOR axis in the proliferation, and angiogenesis of lymphoproliferative disorders. Using mTOR inhibitors as a possible therapeutic regimen for controlling the progression of lymphoproliferative malignancies has been a main scientific focus in certain translational and clinical studies8–11. Everolimus has been suggested effective in treatment of several subtypes of non-Hodgkin lymphomas and HL, but there has been little data on its efficacy as a maintenance therapy for those of HL patients that undergo hematopoietic stem cell transplantation.
In this study, we evaluated the efficacy of Everolimus as maintenance therapy in patients with high-risk Hodgkin lymphoma who underwent autologous hematopoietic stem cell transplant (ASCT).
Materials and Methods
Ethical statement
This study was approved by the ethical committee of Shahid Beheshti University of Medical Sciences (SBMU) with the approval code of IR.SBMU.RETECH.REC.1400.185. This study was conducted based on the declaration of Helsinki. The institutional review board committee approved this study. All the patients were informed about the study method and Everolimus, and all their questions regarding this study were addressed before inclusion. Written informed consent was obtained from each of the participant.
Study design
The inclusion criteria for considering Everolimus as post-transplantation maintenance therapy were: Patients aged 18 years old or above, with primary refractory or relapsing Hodgkin lymphoma (As mentioned in the definition section). All patients had ECOG performance status (PS) 0 and 1, glomerular filtration rate (GFR) of more than 30 mL/min/1.73 m2, ejection fraction (EF) of more than 50% in the transthoracic echocardiography (TTE), alanine aminotransferase (ALT) and aspartate aminotransferase (AST) less than 3 times upper normal. All patients were first advised to receive standard post-transplantation treatment (Brentuximab Vedotin); those of patients were included only, who were unable to afford standard treatment (Brentuximab Vedotin). Stem cell mobilization and harvesting were done for all patients with standard ward protocols, followed by conditioning regimens. The protocol of conditioning regimen in autologous transplantation in Hodgkin lymphoma in our center is LEAM protocol (Lomustine + Etoposide + Cytarabine + Melphalan). In certain cases, Bendamustine, an alkylating agent, was chosen as a substitute for Lomustine (BeEAM regimen). Lomustine 200 mg/
For each patient, demographic and laboratory data were obtained. Demographic data included patient age, gender, stage of Hodgkin lymphoma at presentation, evaluation at the end of treatment, chemotherapeutic agents and targeted therapies, stage at relapse in patients with relapsing Hodgkin lymphoma, day of neutrophil engraftment, type of conditioning regimen, and infection history in BMT ward. Laboratory data included mononuclear cells (MNCs) and CD34+ counts in stem cell analysis. Patients were visited monthly in the first three months after transplantation, then every three months thereafter. Patients were assessed for disease status and drug side effects on each of the visits.
Definitions
Primary refractory patients in Hodgkin lymphoma in our study were patients who had received multi-agent chemotherapy and may also had received targeted therapy such as Brentuximab Vedotin (BV) before the conditioning regimen. Patients must have had the status of stable disease or partial response or complete response. Patients with relapsing Hodgkin lymphoma are characterized as patients who had reached complete response but then relapsed. Complete response (CR) was defined as the disappearance of all lymphoma-related abnormality; partial response (PR) was defined as more than 50% reduction in size of lymph nodes. Stable disease (SD) was defined as no change in the imaging data, and progressive disease (PD) was defined as appearance of a new lesion or increasing in the size of previous lesions by 25% or more.
Results
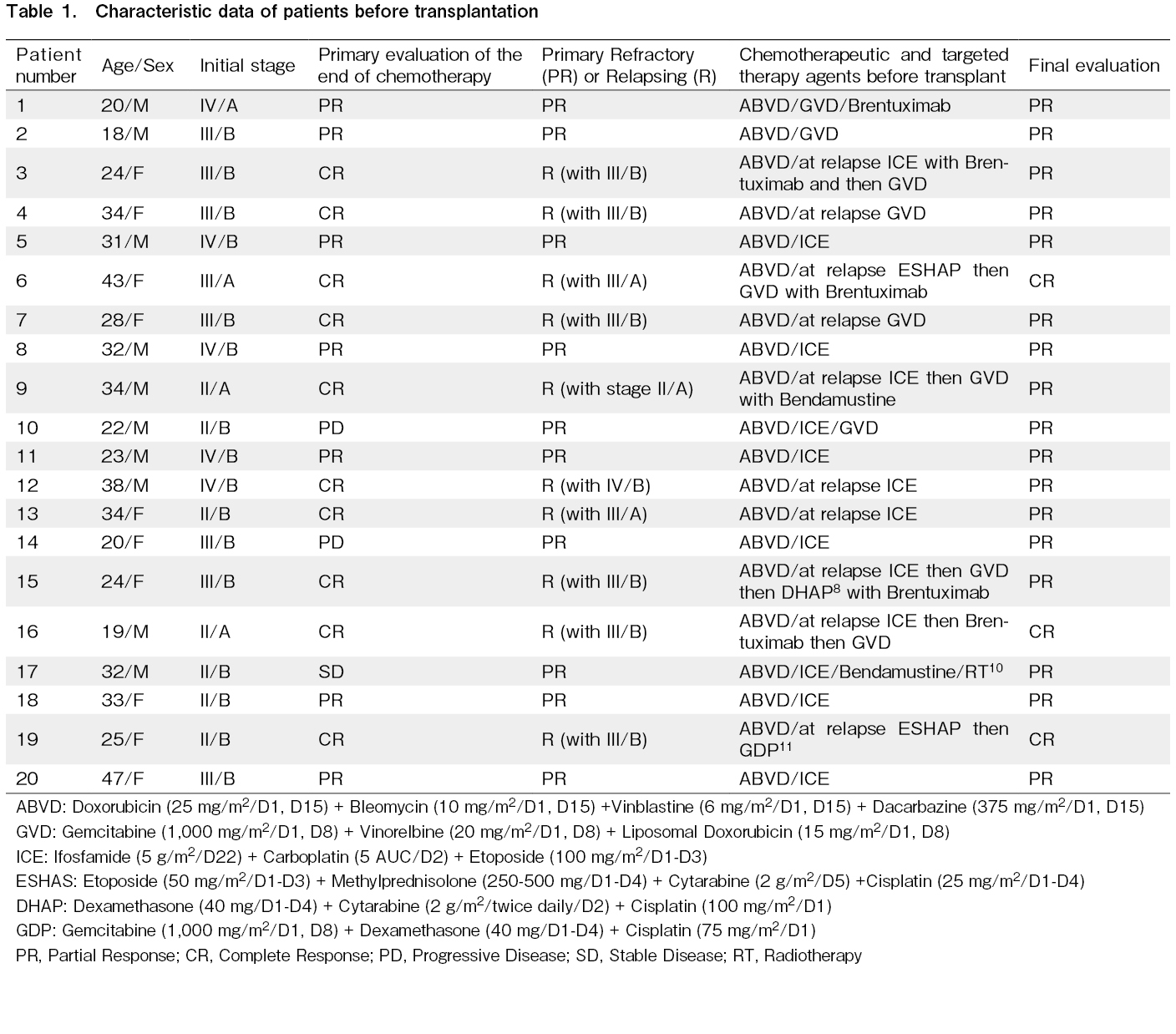
Data from 20 patients with high-risk Hodgkin lymphoma who had undergone autologous bone marrow transplantation between June 2016 and June 2021, were collected in this case series study. Based on the previously approved studies, these patients needed maintenance therapy to prevent relapse. 10 males and 10 females were enrolled in this study. Median age was 29.05 years (18-47 years old). Demographic data, disease status before and after transplant, and the type of chemotherapies before transplant are demonstrated in Table 1. Thirteen patients had advanced-stage Hodgkin lymphoma before transplant (stage III and IV), of whom, 5 patients had stage IV and 8 patients had stage III disease. Seven patients had early-stage Hodgkin lymphoma (stage I and II). Five patients had B symptoms as an unfavorable risk factor for early-stage disease. Two patients had mediastinal mass larger than 10 cm (patients no 13, 17) as another unfavorable risk factor for early-stage disease. As shown in Table 1, patients had received multi-agent chemotherapy. Ten patients had primary refractory disease, in which, they received further salvage treatments (as shown in Table 1) and at the end of treatment before transplantation, they had a partial response to the treatment. Ten patients had relapsing Hodgkin lymphoma. At relapse, they received salvage chemotherapy, and at the end of the treatment before transplantation, seven and three patients had a partial response and complete response to the treatment, respectively. Five of our patients had received Brentuximab Vedotin as a targeted agent without favorable response before autologous hematopoietic transplantation.
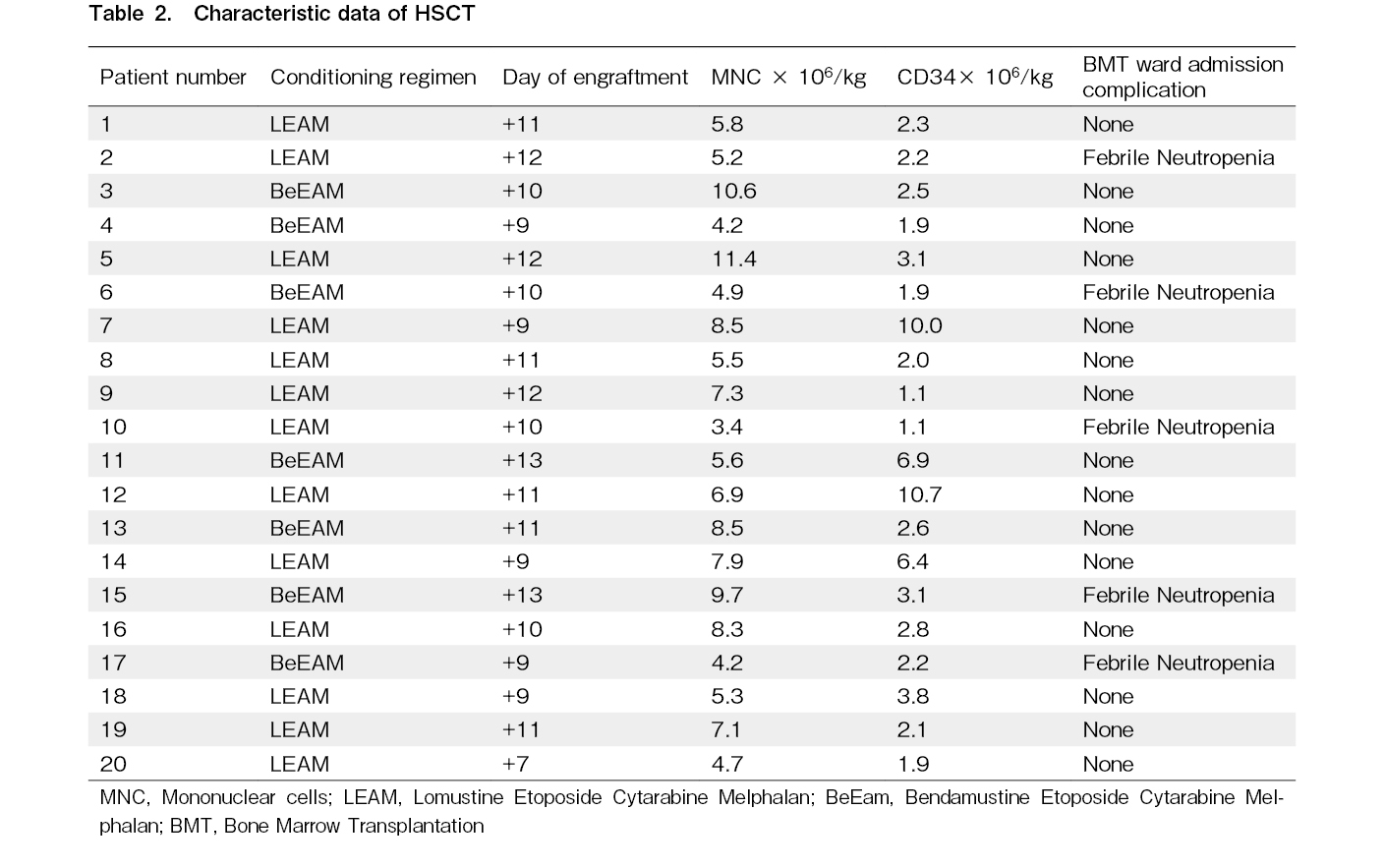
Thirteen and seven patients had received LEAM and BeEAM as conditioning regimen respectively. The median day of engraftment was 10.4 days for neutrophil engraftment. The median MNC count was 6.75 cells×106 per kilogram of body. The median CD34+ count was 3.53 cells×106 per kilogram of body. We had not any mortality at the time of admission. Five patients developed febrile neutropenia at the day of (+6, +6, +8,
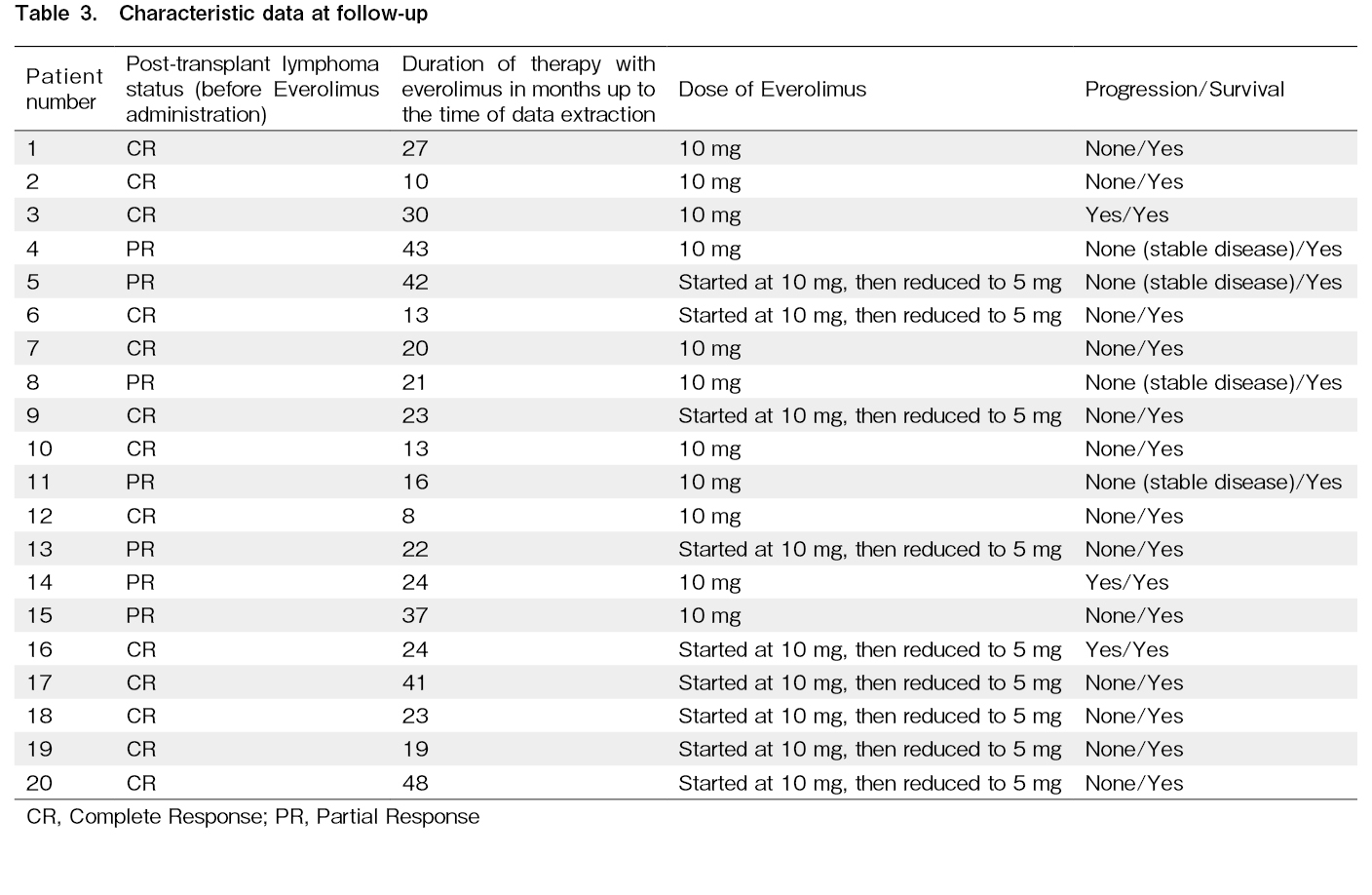
Table 3 demonstrates the follow-up data on disease status after transplantation. Up to now, all patients are alive. At three months after transplantation, 13 patients had no residual disease (CR) in the following imaging evaluation which is mostly due to the conditioning regimen. Seven patients had residual disease even after the conditioning regimen. Two patients (patient no. 4 and 15) had 3 cm and 1.9 cm lymphadenopathy, both in the right para-tracheal region. Patient number 5 BMT, Bone Marrow Transplantation had lymphadenopathy in the left supraclavicular region. Patients 8 and 11 had some lymphadenopathies with the largest diameter of 15 mm in the retroperitoneal region, plus mild splenomegaly (15.5 cm) and lymphadenopathies (17 mm and 18 mm) in the abdominal region, respectively. Patient number 13 had 1.5 cm lymphadenopathy in the para-aortic region. The patient number 14 had a 2.0 cm lymphadenopathy in the mediastinum. The median duration of follow-up for these patients was 25.9 months (9-51 months) after the transplantation. The median duration of treatment with Everolimus was 22.95 months (6-48 months). These patients were followed by clinical examination and laboratory data every three months and by imaging modalities (CT-Scan or PET-Scan) every three months for the first two years and then every six months thereafter. Only three patients (patient no 3, 14 and 16) relapsed 40, 36, 30 months after administration of everolimus respectively. All three of these patients received at least 2 years of everolimus maintenance therapy post transplantation. Two patients (no 8 and no 20) had positive PET Scans on follow up, but biopsies didn't show disease relapse. We administered Everolimus 10 mg once daily. In seven patients, there were some adverse effects that led to reducing the dose to 5 mg daily.
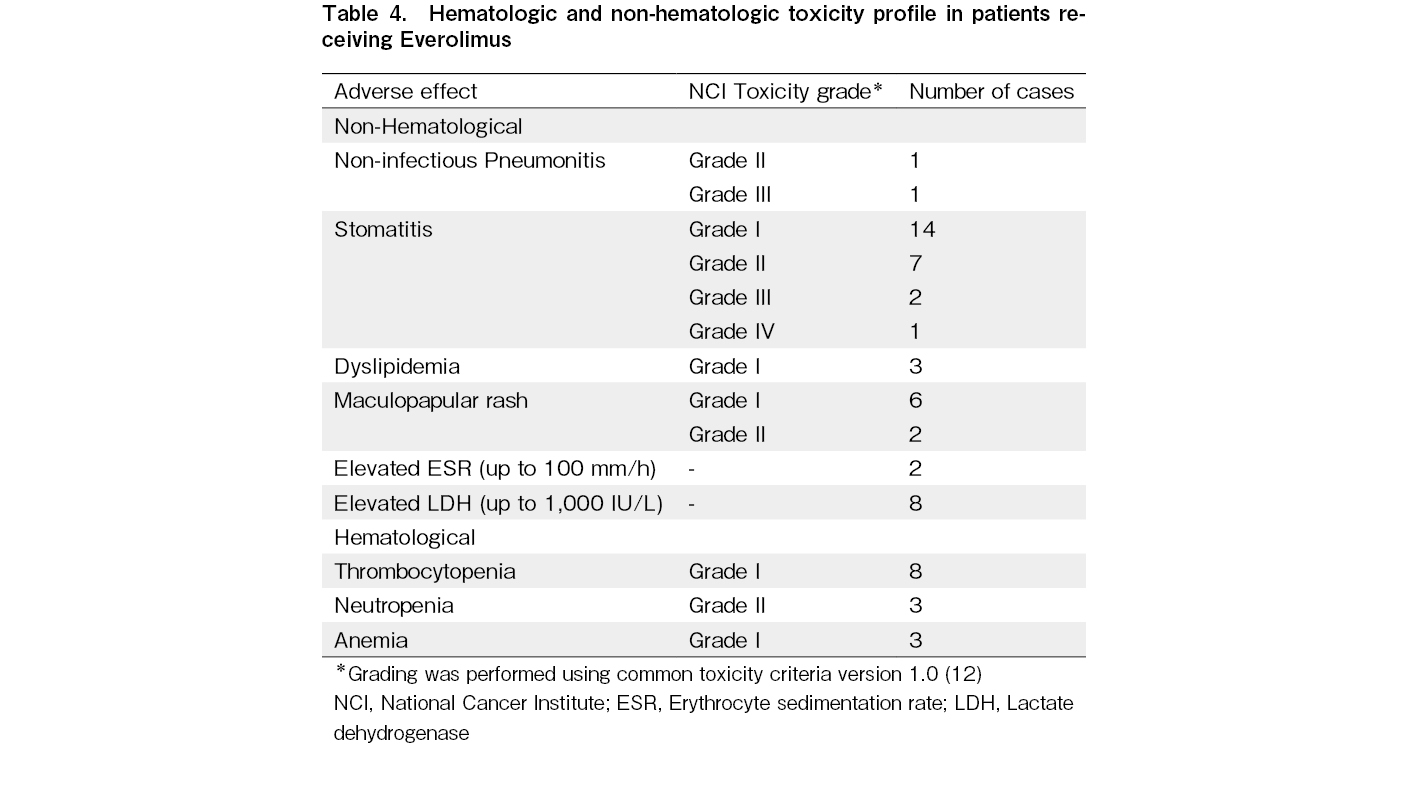
The most notable adverse effects in these patients were as follows: Non-infectious pneumonitis (1 patient grade II and 1 patient grade III), stomatitis (2 patients grade III and 1 patient grade IV), neutropenia (3 patients with grade II). Table 4, demonstrates detected adverse effects related to everolimus during the course of this study.
Discussion
Suggestions for maintenance therapy in HL
In this study, we followed Everolimus efficacy and safety profile as an agent for post-ASCT maintenance therapy among 20 patients with high-risk Hodgkin lymphoma. To our knowledge this is one of the first phase II clinical studies to evaluate the efficacy and safety profile of Everolimus for HL patients in post-ASCT setting. In this study we recognized favorable outcomes. Only three patients out of the twenty high risk patients relapsed (15%), in other words, 85% did not show any disease progression. Relapse occurred after Everolimus discontinuation in all of these three patients. None of the patients who did not stop the drug, relapsed or progressed.
The AETHERA study was the first study that established a role for maintenance therapy for patients with primary refractory or relapsing Hodgkin lymphoma (HL) after autologous stem cell transplantation (ASCT)4. In the AETHERA study, Brentuximab Vedotin was administered within 30 to 45 days after ASCT in one arm and the results were compared to a control placebo arm with no maintenance therapy. The results were promising and PFS was improved to 42.9 months in the Brentuximab Vedotin arm in comparison with 24.1 months in the placebo arm4. A remaining question is whether if the patients who had received Brentuximab Vedotin as a salvage regimen besides chemotherapeutic agents would also benefit from this maintenance therapy, however, it may depend on how they responded to the initial treatment. In another study conducted by Jeries Kort et al. 20 patients with relapsed or refractory Hodgkin lymphoma received Brentuximab Vedotin post-ASCT as a consolidation therapy every 3 weeks for 4 cycles13. After a median follow-up of 26.5 months, 3 patients progressed. The median time till relapse was 6 months among these 3 patients. The median PFS and OS at two years were 72% and 100% respectively with no significant toxicities. The PFS in this study was comparable to the AETHERA study in 2 years.
In addition to Brentuximab Vedotin, anti-PD-1 (programmed death 1) therapies had some effects on relapsing or refractory Hodgkin lymphoma. In a single-arm phase II study, patients received Pembrolizumab at the dose of 200 mg every 3 weeks for 8 cycles. Among 27 patients, 6 patients relapsed at a median of 8 months. The PFS and OS at the 18th– month evaluation was 78% and 100% respectively, with no mortality reported5. Another trial, NCT02362997, is now conducted for evaluating the efficacy of pembrolizumab as adjuvant treatment in these categories of patients.
Mammalian Target of Rapamycin (mTOR) inhibitors as an alternative maintenance therapy
mTOR is a serine kinase and a key member of phosphatidylinositol 3-kinase (PI3K/AKT) signaling stream. It consists of two distinct subunits of mTOR complex 1 and 2 (mTORC1 and mTORC2). These subunits interact with molecular input signals that are induced by growth factors and nutritional supplies, and play a central role in cellular growth, proliferation and survival. Activated mTORC1 induces a downstream signaling that leads to phosphorylation of an inhibitory binding protein called 4E-BP1 (eukaryotic translation initiation factor 4E binding protein 1), and subsequently, to activation of two transcription promoting molecules, eI-F4E (eukaryote initiation factor 4E) and S6K1 (ribosomal S6 protein kinase 1). These all lead to reinforced cellular protein synthesis. mTORC2 interacts with several kinases as well and is mostly involved in cytoskeletal structure. Numerous mechanisms can cause an unfavorable increase in PI3K/AKT/mTOR pathway activity and subsequently, cancer. In this regard, mTOR inhibition has been a strategic suggestion for cancer treatments. Sirolimus and Everolimus inhibit ribosomal protein S6 and also 4E-BP1 protein which leads to inhibition of mRNAs translation that leads to cell cycle arrest6, 14.
Furthermore, PI3K/Akt/mTOR pathway is an axis that is related closely to vascular endothelial growth factor (VEGF) and hypoxia-induced factor-1α (HIF-
Everolimus is a member of first-generation mTOR inhibitor with immunosuppressive and anti-proliferative activity. This drug increase G0 and G1 cells and decrease G2 and M phases in cell cycles20.
From a clinical point of view, Johnston et al, studied patients with relapsed Hodgkin lymphoma after ASCT or patients with relapsed disease who were ineligible for ASCT. Of nineteen patients, the overall response rate (ORR) was 47%. Eight partial responses (PR), one complete response (CR) and eight stable diseases (SD) were observed11.
In another study that was conducted by Patrick B. Johnston et al., they evaluated 57 patients from fourteen centers between 2009 and 2014. These patents were patients with Hodgkin lymphoma with progression after ASCT and or Gemcitabine, Vinblastine or Vinorelbine chemotherapy regimens. Everolimus was administered 10 mg in these patients. The ORR was 45.6% with five patients achieving CR and twenty-one patients achieving PR and twenty patients with stable disease21.
Nagler et al. randomized patients with post-ASCT Hodgkin lymphoma to receive Interleukin-2 (IL-2) and Interferon-α versus observation. There was no difference in OS and disease-free survival (DFS) between both arms22.
In this study, we evaluated patients with primary refractory or relapsing Hodgkin lymphoma who had undergone ASCT and received Everolimus thereafter as maintenance therapy. We have seen favorable results. Only 3 patients relapsed after a median of 22.95 months of follow-up.
This study was a single-arm study, hence, the efficacy of Everolimus should be evaluated in randomized control clinical trials with larger population studies. The important point is, that we had not any serious side effects of Everolimus. The mucocutaneous side effects were common; however, they were controlled easily.
In conclusion, we reported a case-series of patients with primary refractory or relapsing Hodgkin lymphoma who had undergone ASCT and had received Everolimus as maintenance therapy. The results in our study were promising, however, it needs studies with larger populations and different groups of maintenance therapy.
Author Contributions
MM drafted original manuscript, and HAT, AH, HR and SZ reviewed it. SS helped to revise it, and all authors approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69: 7-34.
2.Collins GP, Parker AN, Pocock C, Kayani I, Sureda A, Illidge T, et al. Guideline on the management of primary resistant and relapsed classical Hodgkin lymphoma. Br J Haematol. 2014; 164: 39-52.
3.Isidori A, Piccaluga PP, Loscocco F, Guiducci B, Barulli S, Ricciardi T, et al. High-dose therapy followed by stem cell transplantation in Hodgkin's lymphoma: past and future. Expert Rev Hematol. 2013; 6: 451-64.
4.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015; 385: 1853-62.
5.Armand P, Chen Y Bin, Redd RA, Joyce RM, Bsat J, Jeter E, et al. PD-1 blockade with pembrolizumab for classical Hodgkin lymphoma after autologous stem cell transplantation. Blood. 2019; 134: 22-9.
6.Janku F, Park H, Call SG, Madwani K, Oki Y, Subbiah V, et al. Safety and Efficacy of Vorinostat Plus Sirolimus or Everolimus in Patients with Relapsed Refractory Hodgkin Lymphoma. Clin Cancer Res. 2020; 26: 5579-87.
7.Rule S. Everolimus in relapsed Hodgkin's lymphoma: something exciting or a case of caveat mTOR?. Am J Hematol. 2010; 85: 313-4.
8.Bennani NN, LaPlant BR, Ansell SM, Habermann TM, Inwards DJ, Micallef IN, et al. Efficacy of the oral mTORC1 inhibitor everolimus in relapsed or refractory indolent lymphoma. Am J Hematol. 2017; 92: 448-53.
9.Witzig TE, Reeder C, Han JJ, LaPlant B, Stenson M, Tun HW, et al. The mTORC1 inhibitor everolimus has antitumor activity in vitro and produces tumor responses in patients with relapsed T-cell lymphoma. Blood. 2015; 126: 328-35.
10.Ghobrial IM, Gertz M, LaPlant B, Camoriano J, Hayman S, Lacy M, et al. Phase II trial of the oral mammalian target of rapamycin inhibitor everolimus in relapsed or refractory Waldenstrom macroglobulinemia. J Clin Oncol. 2010; 28: 1408-14.
11.Johnston PB, Inwards DJ, Colgan JP, Laplant BR, Kabat BF, Habermann TM, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010; 85: 320-4.
12.NIH, Division of Allergy and Infectious Diseases, Regulatory Support Center. Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, v. 1.0. https://rsc-prod.niaid.nih.gov/clinical-research-sites/table-grading-severity-adult-pediatric-adverse-events-version-one [Accessed: 8 April 2023]
13.Kort J, Chidiac A, El Sayed R, Massoud R, Nehme R, Bazarbachi A, et al. Safety and efficacy of four cycles of Brentuximab Vedotin as consolidation after autologous peripheral stem cell transplantation in relapsed/refractory Hodgkin lymphoma. Leuk Lymphoma. 2020; 61: 1732-5.
14.Fasolo A, Sessa C. Targeting mTOR pathways in human malignancies. Curr Pharm Des. 2012; 18: 2766-77.
15.Guarini A, Minoia C, Giannoccaro M, Rana A, Iacobazzi A, Lapietra A, et al. mTOR as a target of everolimus in refractory/relapsed Hodgkin lymphoma. Curr Med Chem. 2012; 19: 945-54.
16.Korkolopoulou P, Thymara I, Kavantzas N, Vassilakopoulos TP, Angelopoulou MK, Kokoris SI, et al. Angiogenesis in Hodgkin's lymphoma: a morphometric approach in 286 patients with prognostic implications. Leukemia. 2005; 19: 894-900.
17.Mainou-Fowler T, Angus B, Miller S, Proctor SJ, Taylor PRA, Wood KM. Micro-vessel density and the expression of vascular endothelial growth factor (VEGF) and platelet-derived endothelial cell growth factor (PdEGF) in classical Hodgkin lymphoma (HL). Leuk Lymphoma. 2006; 47: 223-30.
18.Sápi Z, Füle T, Hajdu M, Matolcsy A, Moskovszky L, Márk Á, et al. The activated targets of mTOR signaling pathway are characteristic for PDGFRA mutant and wild-type rather than KIT mutant GISTs. Diagn Mol Pathol. 2011; 20: 22-33.
19.Matsuo M, Yamada S, Koizumi K, Sakurai H, Saiki I. Tumour-derived fibroblast growth factor-2 exerts lymphangiogenic effects through Akt/mTOR/p70S6kinase pathway in rat lymphatic endothelial cells. Eur J Cancer. 2007; 43: 1748-54.
20.Buglio D, Georgakis GV, Hanabuchi S, Arima K, Khaskhely NM, Liu YJ, et al. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008; 112: 1424-33.
21.Johnston PB, Pinter-Brown LC, Warsi G, White K, Ramchandren R. Phase 2 study of everolimus for relapsed or refractory classical Hodgkin lymphoma. Exp Hematol Oncol. 2018; 7: 12.
22.Nagler A, Berger R, Ackerstein A, Czyz JA, Diez-Martin JL, Naparstek E, et al. A randomized controlled multicenter study comparing recombinant interleukin 2 (rIL-2) in conjunction with recombinant interferon alpha (IFN-alpha) versus no immunotherapy for patients with malignant lymphoma postautologous stem cell transplantation. J Immunother. 2010; 33: 326-33.
Search
News