Volume 6 (2023) Issue 3 No.3 Pages 77-79
Abstract
Patients with relapsed or refractory acute myeloid leukemia (RR-AML) with mutations of FMS-like tyrosine kinase 3 (FLT3) have a poor prognosis even after allogeneic hematopoietic cell transplantation (allo-HCT). Multiple FLT3 inhibitors, including gilteritinib, have been developed and serve as treatment options for RR-AML. Here, we describe three cases of FLT3 mutated RR-AML that were successfully treated with gilteritinib administration before and after allo-HCT. Gilteritinib treatment before HCT was helpful in achieving remission. However, HCT often resulted in mild liver damage, and careful introduction of gilteritinib after HCT at a lower dose may be helpful for its safe usage. The three cases discussed had a successful clinical outcome in terms of disease control as well as the management of side effects associated with gilteritinib treatment.
Introduction
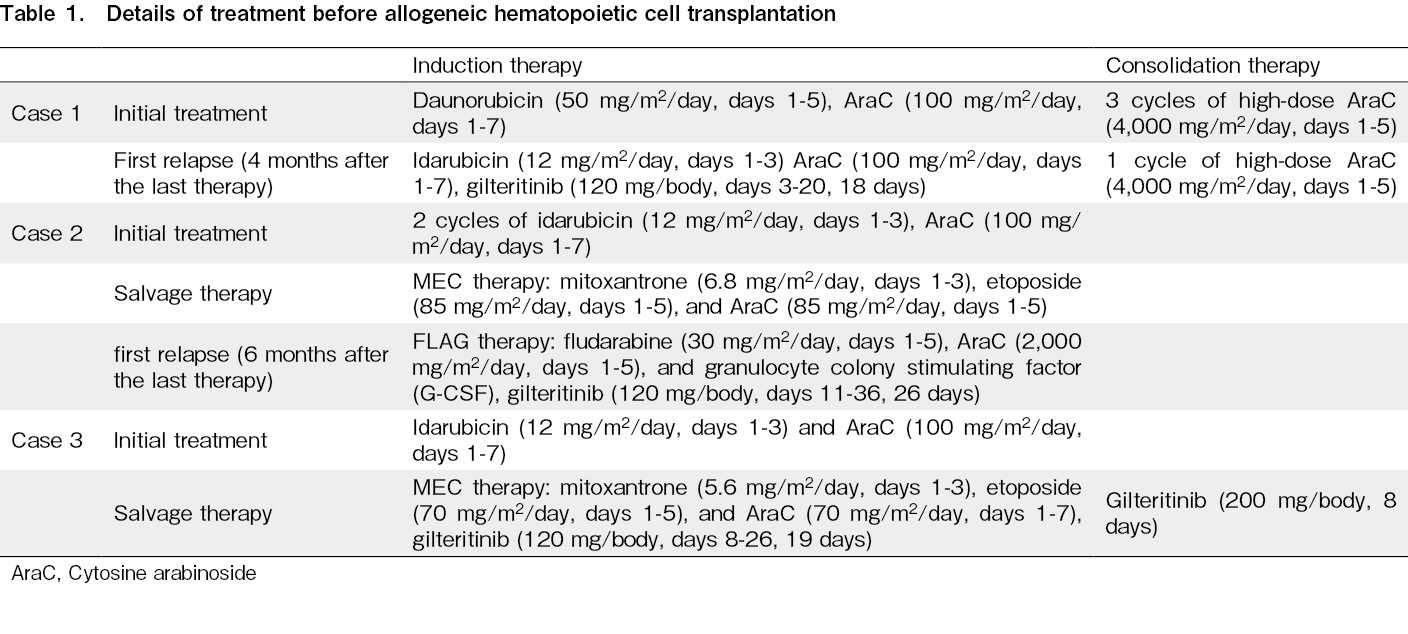
Patients with relapsed or refractory acute myeloid leukemia (RR-AML) with mutations of FMS-like tyrosine kinase 3 (FLT3), including FLT3-ITD and FLT3-TKD, cannot be fully treated with intensive chemotherapy alone. In such cases, allogeneic hematopoietic cell transplantation (allo-HCT) is the only feasible treatment option1, though it is not sufficiently effective. Multiple FLT3 inhibitors, including gilteritinib, have been developed and are now valid treatment options2–4. In the ADMIRAL study, gilteritinib administration was associated with higher response and transplant rates than that of salvage chemotherapy3. Due to the small sample size and lack of randomization, the effectiveness and safety of gilteritinib administration before and after HCT were not well established. Here, we describe three cases of FLT3 mutated RR-AML that were successfully treated with gilteritinib administration before and after allo-HCT at our institute. Detailed information on each case is summarized in Table 1.
Case Descriptions
Case 1
The first case was that of a 43-year-old woman who had no significant medical history and was initially diagnosed with AML with an FLT3-TKD mutation. She received conventional induction chemotherapy and achieved hematologic complete remission (hCR). Four months after the last consolidation cycle, she experienced a recurrence. Reinduction therapy was initiated with cytoreduction, followed by gilteritinib treatment (120 mg/day, 18 days), and hCR was achieved. She was referred to our hospital for allo-HCT and underwent peripheral blood (PB) stem cell transplantation (
Case 2
The second case was that of a 57-year-old woman with a history of asthma who was diagnosed with AML; hCR was not achieved after 2 courses of conventional induction therapy but was attained after salvage chemotherapy. Initially, she underwent a cord blood transplantation (CBT) and achieved mCR with undetectable levels of WT1 mRNA in PB. Six months later, WT1 mRNA was tested positive in PB, and 5-azacytidine (AZA) monotherapy was initiated as a preemptive therapy. After 15 cycles of AZA therapy, she experienced hematological recurrence of AML with an FLT3-ITD mutation. She received another round of salvage chemotherapy followed by gilteritinib treatment (120 mg/day, 26 days). hCR was achieved but WT1 mRNA in PB remained positive (1×104 copies/μg RNA). She subsequently underwent a second CBT (
Case 3
The third patient was a 56-year-old man diagnosed with AML with an FLT3-ITD mutation. His medical history included hypertension, diabetes mellitus, and depression. Since he did not achieve hCR after conventional induction chemotherapy, he received salvage chemotherapy followed by gilteritinib treatment (120 mg/day). Furthermore, a lumbar puncture revealed AML cell infiltration into the cerebrospinal fluid. The dose of gilteritinib was increased to 200 mg/body, which finally led to hCR. However, WT1 mRNA in PB remained positive (1.3×104 copies/μg RNA). He subsequently underwent CBT, (
Discussion
We report three patients with FL3 mutated RR-AML who underwent allo-HCT with gilteritinib treatment before and after HCT. In the ADMIRAL study, 63 patients who were randomized to receive gilteritinib underwent allo-HCT, and the proportion of patients who underwent allo-HCT was greater than that of salvage therapy. Forty patients resumed gilteritinib treatment after HCT, and 16 among them remained in remission after 2 years of gilteritinib treatment. Remission rates were higher among patients who received allo-HCT and gilteritinib treatment after the procedure5. It has been suggested that FLT3 inhibitors induce graft-versus-leukemia (GVL) effects, in addition to cytotoxicity. Although a post-transplant GVL effect has been clinically observed following treatment with sorafenib (another FLT3 inhibitor)6, this effect has not been confirmed for the more specific FLT3 inhibitors, gilteritinib and quizartinib. The phase 3 MORPHO study was initiated to evaluate the efficacy of gilteritinib treatment as a maintenance therapy after transplantation (NCT02997202).
The safety of gilteritinib treatment in post-transplant therapy was also addressed in the ADMIRAL trial. Although gilteritinib carries a lower risk of myelosuppression than other FLT3 tyrosine kinase inhibitors, transaminitis occurred more frequently in the gilteritinib-administered arm than in the control arm, especially in the first year of treatment. Monitoring liver function is imperative as liver injury often results from other causes, such as drugs, GVHD, and iron overload. In addition, reducing the dose of gliteratinib should be considered when administered with drugs that inhibit the CYP3A4 system, including azole antifungal agents. In the cases reported in our study, we started gilteritinib treatment at a low dose under conditions of stable blood cell count and lack of severe GVHD symptoms. If the lower doses were tolerated, the doses were increased gradually. Nonetheless, it would be beneficial to establish an optimal standard strategy in the future, for gilteritinib introduction after allo-HCT.
In summary, we presented the clinical experiences of three patients who were administered gilteritinib before and after allo-HCT, without serious complications. As of the date of submission, there have been no relapses. Further evidence is needed to establish the optimal approach to gilteritinib treatment before and after allo-HCT.
Funding Statement
This study was partly supported by a Health, Labor, and Welfare Sciences Research Grants (20FF1002).
Author Contributions
FM and SF designed the research and wrote the paper. KS, SK, KT, YT, YS, TY, and JI were involved in patient treatment. All authors contributed to the final version of the manuscript and approved it for publication.
Ethics approval
No approval from any IRB was required.
Consent for publication
Informed consent was obtained from each participant.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Kurosawa S, Yamaguchi H, Yamaguchi T, Fukunaga K, Yui S, Kanamori H, et al. The prognostic impact of FLT3-ITD, NPM1 and CEBPa in cytogenetically intermediate-risk AML after first relapse. Int J Hematol. 2020; 112: 200-9.
2.Daver N, Schlenk RF, Russell NH, Levis MJ. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019; 33: 299-312.
3.Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019; 381: 1728-40.
4.Wang J, Jiang B, Li J, Liu L, Du X, Jiang H, et al. Gilteritinib versus salvage chemotherapy for relapsed/refractory FLT3-mutated acute myeloid leukemia: A Phase 3, Randomized, Multicenter, Open-Label Trial in Asia. Blood. 2021; 138 (Suppl 1): 695.
5.Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, et al. Follow-up of patients with R/R FLT3-mutation-positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood. 2022; 139: 3366-75.
6.Sorà F, Chiusolo P, Metafuni E, Bellesi S, Giammarco S, Laurenti L, et al. Sorafenib for refractory FMS-like tyrosine kinase receptor-3 (FLT3/ITD+) acute myeloid leukemia after allogenic stem cell transplantation. Leuk Res. 2011; 35: 422-3.
Search
News