Volume 6 (2023) Issue 2 No.4 Pages 54-60
Abstract
Hyperglycemia in the early days following allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-known risk factor for acute graft-versus-host disease (GVHD) and non-relapse mortality. The FreeStyle Libre Pro, a factory calibrated continuous glucose monitoring (CGM) device, has been used for the retrospective analysis of glucose testing in patients with diabetes. We assessed the safety and accuracy of the device in patients undergoing allo-HSCT. We recruited eight patients who underwent allo-HSCT between August 2017 and March 2020. They wore the FreeStyle Libre Pro on the day before or on the day of transplantation until 28 days after transplantation. Adverse events, especially bleeding and infection, were monitored to assess safety, and blood glucose levels were measured and compared with the device values. None of the eight participants experienced bleeding that was difficult to stop from the sensor site or local infection that required antimicrobial administration. The device value was well correlated with blood glucose (correlation coefficient r=0.795, P<0.01); however, the overall mean absolute relative difference was 32.1%±16.0%. Our study demonstrated the safety of FreeStyle Libre Pro in allo-HSCT patients. However, the sensor results tended to be lower than the blood glucose levels.
Introduction
Although survival is prolonged following allogeneic hematopoietic stem cell transplantation (allo-HSCT), several post-transplantation complications can be fatal. The risk of non-relapse mortality (NRM) has decreased over the past few years1 and has been reported in Japan2, 3. Allo-HSCT recipients are also at a higher risk of hyperglycemia due to the use of steroids, total parenteral nutrition (TPN), immunosuppressive drugs, elevations in insulin resistance, and infectious complications4–6. Post-transplant hyperglycemia during neutropenia has been reported as a risk factor for infectious diseases, acute graft-versus-host disease (GVHD), and NRM4.
Glucose control is crucial in the days following allo-HSCT. While self-monitoring of blood glucose (SMBG) requires frequent fingertip puncture, continuous glucose monitoring (CGM) does not require frequent fingertip puncture, thus, reducing the stress on the patient. There are two types of CGM: 1) CGM, which is used as an adjunct to SMBG, and 2) retrospective CGM, which is used for retrospective analysis of glucose excursion. The FreeStyle Libre Pro (Abbott Diabetes Care) is a retrospective CGM that does not require calibration by SMBG7. The sensor patch of the CGM that is worn on the upper arm is 5 mm thick and 35 mm in diameter. It was calibrated at the factory and did not require calibration during the 14 days that it was worn. It is used only in patients with diabetes mellitus. Several adverse events related to wearing this sensor have been reported; however, these are not critical and have improved gradually7. Mean absolute relative difference (MARD) is used as an accuracy indicator for CGM devices. Several studies comparing the device with blood glucose values have reported MARDs of 12.1%7, 8.2%8, and 18.7%9, indicating much variability. Since these studies were conducted in patients with diabetes mellitus, the safety and accuracy of the device in allo-HSCT recipients is still unclear. Therefore, we aimed to evaluate the safety and efficacy of the CGM prospectively in patients undergoing allo-HSCT.
Materials and Methods
Study participants
This was a single-center, phase I study to evaluate the safety of FreeStyle Libre Pro in patients undergoing allo-HSCT. Allo-HSCT recipients who had not used the Libre Pro device previously and were between 20 and 70 years of age were enrolled in the study from August 2017 to March 2020. All patients provided voluntary written consent for inclusion in this study. The exclusion criteria were as follows: (1) haploidentical hematopoietic stem cell transplantation; (2) requirement of radiographs and computed tomography (CT) scans at least once a week; (3) previous infection at the device site; (4) contact dermatitis; (5) inability to be disinfected with alcohol or chlorhexidine gluconate; (6) other implantable medical devices such as pacemakers; and (7) patients who were considered inappropriate by the investigator owing to inability to perform the test accurately.
The study protocol was approved by the Kobe University Clinical Research Ethical Committee (No. C180063) and registered as a phase I trial titled
Study design
We used FreeStyle Libre Pro for CGM, wherein, the sensor was attached to the upper arm of the patient, to enable continuous measurement of glucose levels in the tissue interstitial fluid by the filament for up to 14 days, without the need for frequent punctures. Baseline laboratory tests, including glucose and glycoalbumin levels, were performed a day prior to the initiation of the conditioning regimen. The patients wore the device on the day either before or on the day of transplantation for 28 days. Alcohol and chlorhexidine gluconate were used to disinfect the fitting area prior to attachment of the sensors. Laboratory tests, measurement of sensor glucose levels by the device were repeated at least once weekly until a week after sensor removal. Adverse events were evaluated daily. If no neutrophil engraftment was achieved within 28 days from the start of sensor attachment, sensor attachment was continued until neutrophil engraftment. On the 14th day of sensor attachment, the sensor was removed, the puncture site was observed, and a new sensor was attached to a different site. The test sensor had to be removed before imaging procedures such as magnetic resonance imaging (MRI), CT or radiographical examination, and a new sensor applied to a different site after evaluation. Serum blood glucose monitoring was performed as part of routine medical care and the time of blood collection was determined using the electronic medical charts.
Study outcomes
The primary endpoints were major bleeding and antimicrobial administration due to infection at the Libre Pro sensor attachment site.
Adverse event monitoring
The National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was used for evaluation of the severity of adverse events in this study.
Statistical analysis
Pearson's product-moment correlation was used to assess the association between serum glucose levels and sensor glucose values. Clarke error grid (CEG) analysis was used to assess the clinical accuracy of sensor glucose values as compared to a reference value10. The grid is divided into five zones―A, B, C, D, and E. Zone A and B values represent glucose results that are accurate or acceptable while a Zone E value represents
Results
Patient characteristics
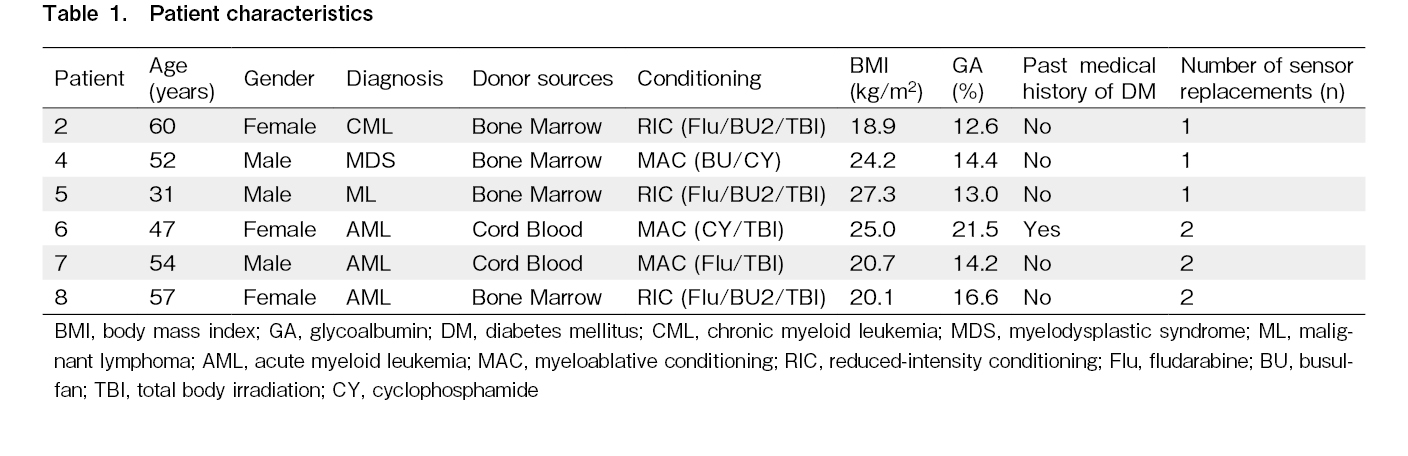
A total of eight patients were enrolled in the study, two of whom withdrew eventually. Patient 1 was a 59-year-old male with acute myeloid leukemia withdrew from the study and discontinued on day 12. Patient 3 was a 36-year-old male with malignant lymphoma was admitted to the intensive care unit for management of sinusoidal obstruction syndrome on day 12 post-transplantation. Frequent radiographs were required, and it was judged that continuing to wear the device would be too cumbersome for the patient; hence, he was excluded from the study. The remaining six patients completed the study. The patient characteristics are shown in Table 1. Most patients were male (62.5%), and the median age was 53 years (range, 31-60 years). Mycophenolate mofetil (MMF) and tacrolimus (TAC) were administered to all patients for GVHD prophylaxis. In all patients, the nutrition support team established target energy intake based on basal energy consumption, activity coefficient, and stress coefficient, and adjusted diet with intravenous nutrition. All patients were treated with TPN. Patient 4 had an engraftment syndrome and was treated with hydrocortisone (100 mg) for 5 days. Patient 6 was diagnosed with type 2 diabetes prior to study entry and was taking linagliptin and patient 7 had a high level of glycoalbumin at screening. Therefore, both patients 6 and 7 were treated with insulin under the direction of the diabetic physician. Steroids and insulin were not used for the other patients. Three patients had their sensors changed twice, and the other three had them replaced three times.
Safety assessment
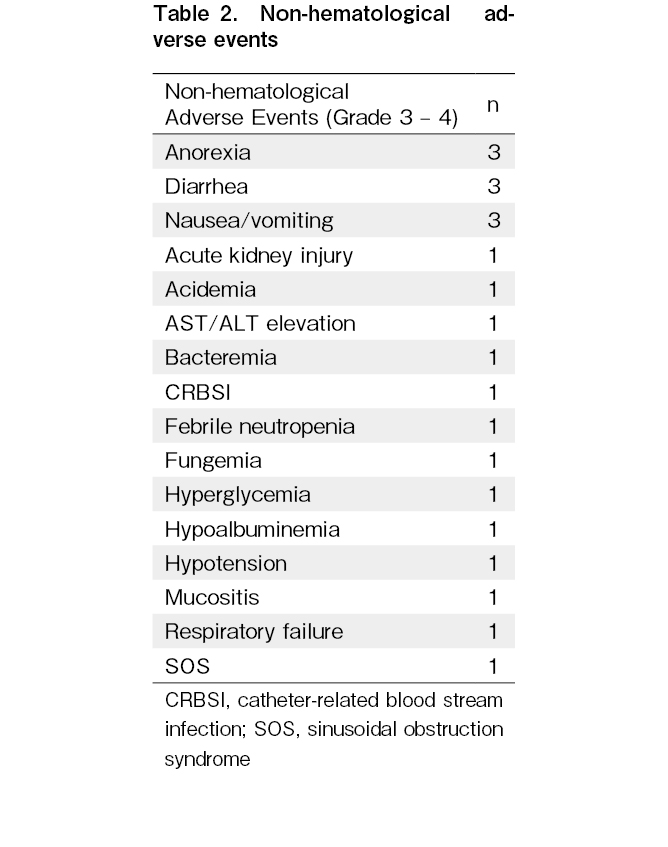
Hematological and gastrointestinal toxicities and infections associated with conditioning regimen and transplantation were reported in all patients; however, these were predictable events and did not cause serious illness (Table 2). There were no instances of bleeding from the sensor site that were difficult to stop or local infection that required antimicrobial administration at the site. Mild adverse events (grade 1) associated with the sensor application or insertion sites were experienced in two participants: one patient had purpura at the device site, which resolved spontaneously, and another patient experienced minor bleeding when the device was attached.
Correlation between blood glucose level and glucose level in tissue interstitial fluid
A positive correlation was observed between blood glucose levels and those measured by the sensor (r=0.795, P<0.01). The median difference between the measurements (
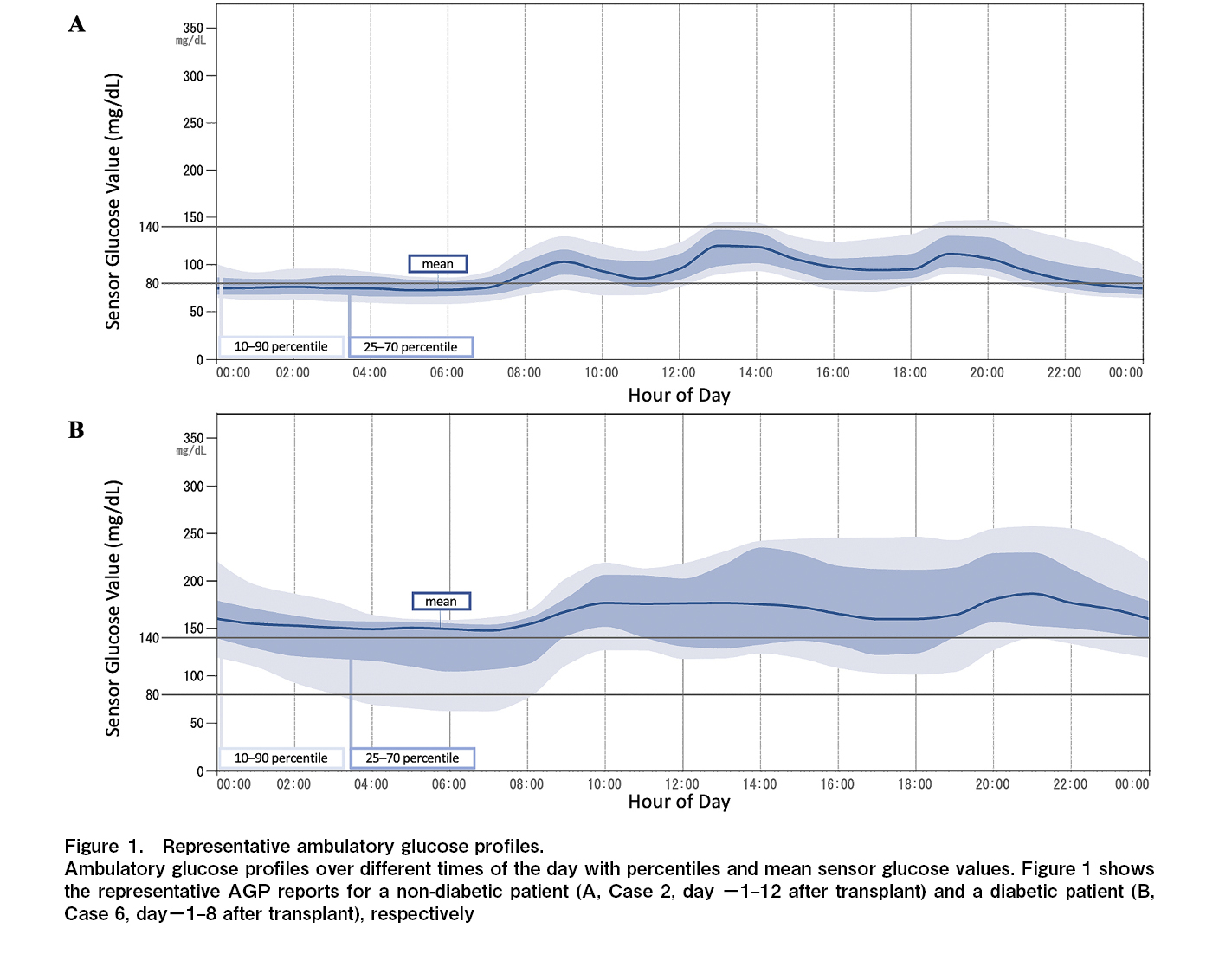
Ambulatory glucose profiles
The ambulatory glucose profile (AGP) reports produced by the FreeStyle Libre Pro for two representative patients are shown in Figure 1. The blood glucose fluctuations in the diabetic Case 6 were larger than those in the non-diabetic Case 2.
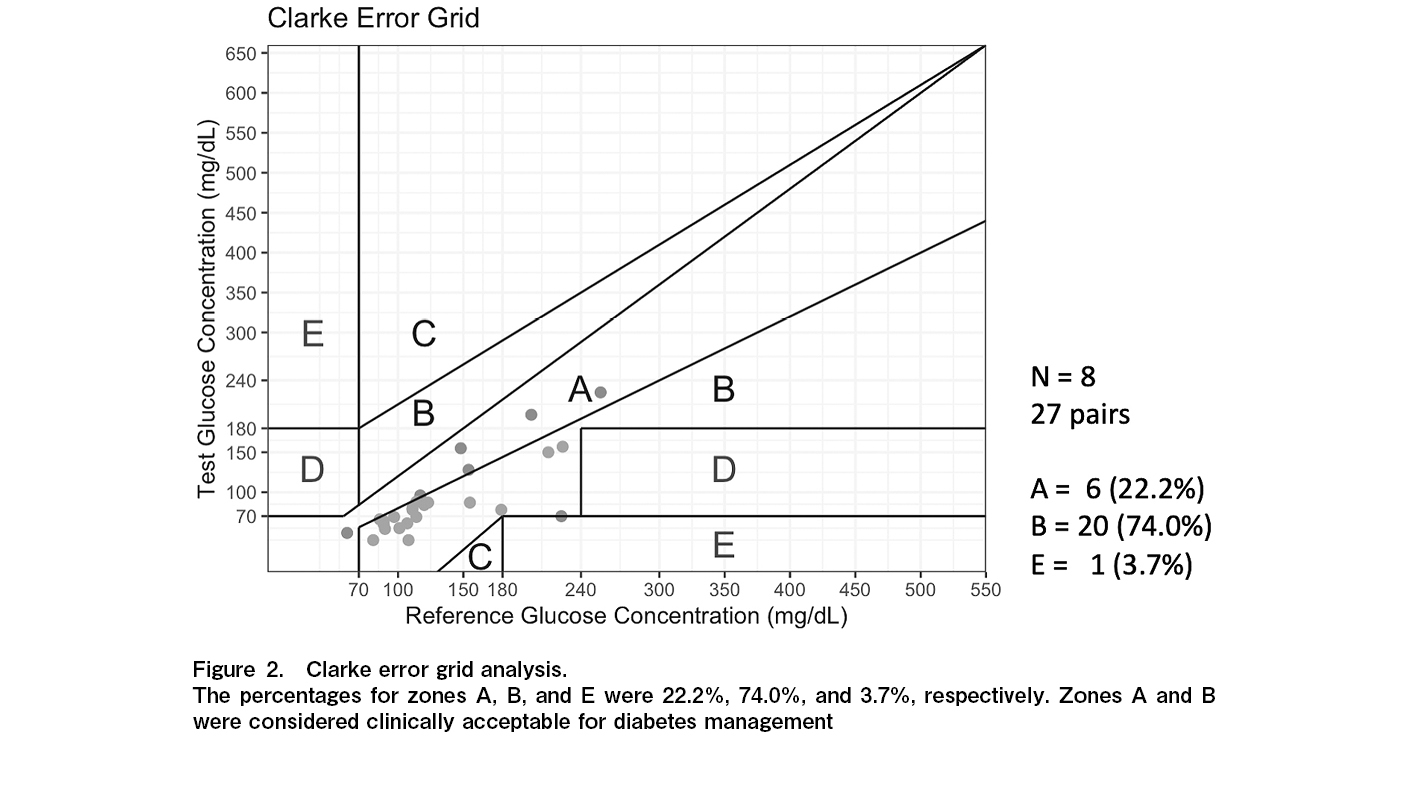
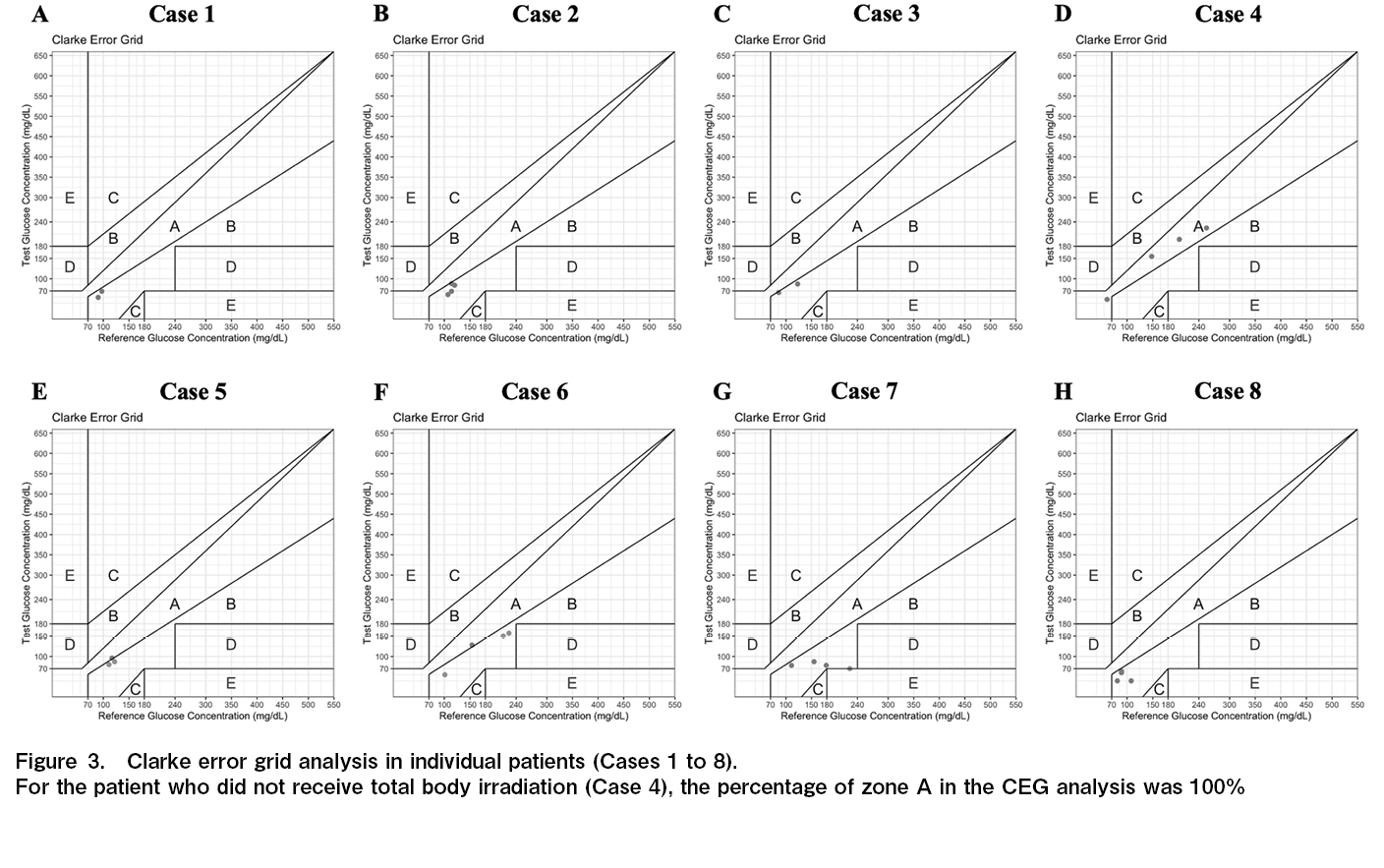
Clarke error grid analysis
The results of the CEG analysis are shown in Figure 2. The respective percentages were 22.2%, 74.0%, and 3.7% for zones A, B, and E, respectively. The individual results are shown in Figure 3. Seven of the eight patients in this study underwent total body irradiation (TBI). For the patient who did not receive TBI (Case 4), the percentage of zone A in the CEG analysis was 100%.
Discussion
This prospective study investigated the safety and accuracy of FreeStyle Libre Pro in patients undergoing allo-HSCT. The results showed that FreeStyle Libre Pro can be used safely, even in allo-HSCT patients. Although interstitial glucose measurements by FreeStyle Libre Pro showed a correlation with venous blood glucose levels, the sensor glucose levels tended to be low.
Numerous studies have demonstrated the safety and accuracy of FreeStyle Libre and FreeStyle Libre Pro in adult patients with diabetes7–9, 11–14. In addition, one report on the safety and accuracy of FreeStyle Libre Pro in pediatric allo-HSCT recipients has demonstrated its safety15. Similar to the aforementioned study, no serious adverse events related to the device placement were observed in this study.
This study demonstrated the safety of FreeStyle Libre Pro during the neutropenic phase following allo-HSCT. There were two cases of bacteremia; however, no signs of local infection such as redness or swelling at the device site were observed. A central venous catheter was placed, and it was determined that the bacteremia was not caused due to the device. AGP is an analysis method developed by Mazze et al.16 and is used to evaluate glucose levels. It is useful for identifying hypoglycemic and hyperglycemic episodes, as well as wide blood glucose level fluctuations as shown in Figure 1. Upon CEG analysis, zones A and B were considered clinically acceptable for diabetes management. In this study, 97% of zones A and B in CEG analysis were identified. There was one pair in zone E. Because the detected sensor value was significantly lower than the blood glucose level, there was no concern that a false low value would cause a serious error, such as administering insulin by mistake. The percentage of zone A was lower compared to the results of the aforementioned studies11–13. A study examining the accuracy of Freestyle Libre Pro in patients with type 2 diabetes mellitus undergoing hemodialysis reported a trend toward lower values compared to SMBG, similar to the present study17. This could be due to the alterations in the water content of the subcutaneous interstitial fluid owing to hemodialysis. A study of pediatric HSCT patients also reported edema in a patient as a cause of negative bias15. In our study, only one patient did not undergo TBI, and the percentage of zone A in the CEG analysis was 100% in the patient without TBI. Thus, the possibility that changes in the subcutaneous environment due to TBI had a certain effect cannot be ruled out. Another possible reason is that hydration, including TPN, was used in all patients, leading to a decrease in laboratory values due to increased water content in the subcutaneous interstitial fluid cannot be ruled out. Additionally, there could be a difference in the pattern at the timing after allo-HSCT. The alteration of the interstitial environment caused by cytokine release during neutrophil engraftment or systemic infection might impact the error in the glucose level determined by CGM.
This study had several limitations. Although the safety of Libre Pro was confirmed in this study, the sample size and the number of scheduled measurement points for glucose levels were small to verify the accuracy of Libre Pro in HSCT patients. Therefore, it is necessary to conduct a similar study using a larger number of specimens to evaluate the accuracy of the sensor. Further, this study defined infection associated with the device as
In conclusion, this phase 1 study suggested that the FreeStyle Libre Pro was safe to use even in allogeneic HSCT patients. However, the measurements tended to be lower than the blood glucose levels and may need to be interpreted with caution.
Acknowledgments
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Author Contributions
SN and KYak designed the study and wrote the manuscript. SN, AH, KK, MW, RS, YM, HG, KK, and AK submitted the data. SN, YI and KYak analyzed the data. YH, MT and HMi supervised the research. All authors participated in interpretation of the results and approval of the manuscript.
Acknowledgments
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Ethical approval
The study protocol was approved by the Kobe University Clinical Research Ethical Committee (No.
Acknowledgments
We would like to thank Editage [http://www.editage.com] for editing and reviewing this manuscript for English language.
Consent for Publication
Informed consent was obtained from each participant.
Conflicts of Interest
YH discloses the following relationships: lecture fees from Abbott. The other authors declare no potential conflicts of interest. Disclosure forms provided by the authors are available on the website.
References
1.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010; 363: 2091-101.
2.Kurosawa S, Yakushijin K, Yamaguchi T, Atsuta Y, Nagamura-Inoue T, Akiyama H, et al. Changes in incidence and causes of non-relapse mortality after allogeneic hematopoietic cell transplantation in patients with acute leukemia/myelodysplastic syndrome: an analysis of the Japan Transplant Outcome Registry. Bone Marrow Transplant. 2013; 48: 529-36.
3.Fuji S, Kim S-W, Kamiya S, Nakane T, Matsumoto K, Onishi Y, et al. A multi-center prospective study randomizing the use of fat emulsion in intensive glucose control after allogeneic hematopoietic stem cell transplantation using a myeloablative conditioning regimen. Clin Nutr. 2018; 37: 1534-40.
4.Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation. 2007; 84: 814-20.
5.Sheean PM, Freels SA, Helton WS, Braunschweig CA. Adverse clinical consequences of hyperglycemia from total parenteral nutrition exposure during hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006; 12: 656-64.
6.Fuji S, Yakushijin K, Kim SW, Yoshimura K, Kurosawa S, Fukuda T. Dynamic change of glycemic status during the early phase after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2015; 50: 1473-5.
7.Administration UFaD. PMA P150021: FDA Summary of Safety and Effectiveness Data. https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150021B.pdf [Accessed: 22 October 2022]
8.Sato T, Oshima H, Nakata K, Kimura Y, Yano T, Furuhashi M, et al. Accuracy of flash glucose monitoring in insulin‐treated patients with type 2 diabetes. J Diabetes Investig. 2019; 10: 846-50.
9.Murata T, Nirengi S, Kawaguchi Y, Sukino S, Watanabe T, Sakane N. Accuracy of a novel “factory-calibrated” continuous glucose monitoring device in normal glucose levels: a pilot study. Biomed Sci. 2017; 3: 109-13.
10.Clarke WL. The original Clarke Error Grid Analysis (EGA). Diabetes Technol Ther. 2005; 7: 776-9.
11.Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015; 17: 787-94.
12.Fokkert MJ, van Dijk PR, Edens MA, Abbes S, de Jong D, Slingerland RJ, et al. Performance of the FreeStyle Libre Flash glucose monitoring system in patients with type 1 and 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2017; 5: e000320.
13.Ólafsdóttir AF, Attvall S, Sandgren U, Dahlqvist S, Pivodic A, Skrtic S, et al. A clinical trial of the accuracy and treatment experience of the flash glucose monitor FreeStyle Libre in adults with type 1 diabetes. Diabetes Technol Ther. 2017; 19: 164-72.
14.Ida S, Goto H, Ida S, Watanabe M, Okuda M, Takeuchi T, et al. Accuracy of a factory-calibrated retrospective CGM device and the comparison to a conventionally calibrated retrospective CGM device: A pilot study. Biomed Sci. 2018; 4: 32-6.
15.Sopfe J, Vigers T, Pyle L, Giller RH, Forlenza GP. Safety and accuracy of factory-calibrated continuous glucose monitoring in pediatric patients undergoing hematopoietic stem cell transplantation. Diabetes Technol Ther. 2020; 22: 727-33.
16.Mazze RS, Lucido D, Langer O, Hartmann K, Rodbard D. Ambulatory glucose profile: representation of verified self-monitored blood glucose data. Diabetes Care. 1987; 10: 111-7.
17.Toyoda M, Murata T, Saito N, Kimura M, Takahashi H, Ishida N, et al. Assessment of the accuracy of an intermittent-scanning continuous glucose monitoring device in patients with type 2 diabetes mellitus undergoing hemodialysis (AIDT2H) study. Ther Apher Dial. 2021; 25: 586-94.
Search
News