Volume 6 (2023) Issue 3 No.2 Pages 72-76
Abstract
Plerixafor for peripheral blood hematopoietic stem cell (PB HSC) mobilization in children undergoing autologous hematopoietic stem cell transplantation is primarily used following failure of the initial mobilization attempt. Data on plerixafor use in pediatric patients are limited.
This retrospective study conducted at a single tertiary care center in India, details the efficacy and safety of plerixafor for 10 children with relapsed/refractory solid tumors or lymphomas. High risk neuroblastomas (HR NB) underwent autologous HSCT as part of consolidation. Plerixafor was administered at a dose of 240 μg/kg body weight of the recipient, subcutaneously, approximately 11-12 h prior to harvest. Ten patients (eight males, two females), with a median age of 8 years (range 2-18 years), received plerixafor prior to PB HSC harvest. All patients were administered granulocyte colony stimulating factor (GCSF) before the administration of plerixafor. The median CD 34 count for all patients pre-plerixafor was 29/μL, nine patients exhibited higher CD 34 post plerixafor (median of 148/μL). In nine patients, the values of the CD 34 count and total leukocyte count (TLC) of the harvested product were available, and in all cases, we achieved a good yield. All patients in this study were heavily chemotherapy pre-treated, and the use of plerixafor resulted in a satisfactory yield of peripheral blood stem cells. No side effects were observed.
Introduction
Autologous peripheral blood stem cell transplantation (A-PBSCT) has become the standard of care for many malignancies and its use is increasing in non-malignant conditions, such as autoimmune diseases1. For optimal outcomes post-A-PBSCT, it is imperative that an adequate number of hematopoietic stem cells (HSC) are infused. Optimal outcomes are achieved if more than 2.5 million HSC per kg body weight is administered post high-dose conditioning chemotherapy2.
Few strategies can be used for the mobilization of HSC into the peripheral blood to allow for collection. The most common method involves the use of granulocyte colony-stimulating factor (GCSF) alone or following the administration of a high-dose of cyclophosphamide or chemotherapy. However, in patients that have undergone significant chemotherapy, these methods may result in mobilization failure. Furthermore, in such patients, repeat apheresis may be required, causing delays in treatment and additional burdens on healthcare resources. It may also rarely necessitate bone marrow harvest, which is a more invasive procedure requiring the administration of general anesthesia3.
Plerixafor is an antagonist of the CXCR4 chemokine receptor, and its use in combination with GCSF to aid HSC mobilization has been approved in adults in the United States of America and Europe. Plerixafor reversibly inhibits the interaction of the CXCR4 receptor, present on the surface of stem cells, with the stromal cells in the bone marrow niches that carry the CXCL2 ligand. This prevents the attachment of HSC to stromal cells, allowing them to enter peripheral blood circulation4. The MOZAIC study was a phase I/II study conducted in children, which established the dose of plerixafor for use in phase II of the trial (n=45; plerixafor arm=30, standard arm=15). It was shown to be safe and effective for HSC mobilization in children5. The use of plerixafor in children, remains understudied and further evidence is required.
We performed this retrospective analysis of 10 pediatric patients diagnosed with lymphomas and solid tumors, in whom plerixafor was administered for the mobilization of HSC to enable harvesting. Here, we investigated the efficacy and safety of plerixafor administration in these children.
Methods
The records of patients who underwent A-PBSCT as part of their treatment were analyzed. The details of the patients who received plerixafor for the mobilization of HSC were collected. The demographic details, diagnosis, previous chemotherapy administered, doses of GCSF administered, CD 34 and total leukocyte count (TLC) pre- and post-harvest along with CD 34 and TLC of the harvested product were recorded. GCSF was administered subcutaneously at a dose of 5 μg/kg/dose twice a day or 10 μg/kg/dose once a day. Plain GCSF was used in all cases. The number of days GCSF was administered varied between patients, as admission for the apheresis procedure depended on the bed availability. Plerixafor was administered to the patient 11-12 hours prior to harvest at a dose of 240 μg/kg. Plerixafor was administered if the CD 34 count was less than 50/μL. We chose a CD 34 count of 50/μL, based on previous experience when apheresis was performed on patients with lower CD 34 counts as it yielded suboptimal doses of stem cells in the final product. In instances when the CD 34 count was not available on the day prior to the harvest, plerixafor was administered irrespective of the count. Enumeration of CD34 percentage was performed using flow cytometry. The absolute CD 34 count was obtained using TLC and CD 34 percentage.
Adverse effects after the administration of plerixafor were recorded, including allergic and hypersensitivity reactions, thrombocytopenia, respiratory difficulties, bone pain, and splenomegaly. Vital signs and laboratory parameters were recorded for all patients at regular intervals after the administration of plerixafor.
The data were analyzed using descriptive statistics. Continuous variables were analyzed using the mean, standard deviation (SD), median (range), and categorical variables were summarized using numbers and proportions.
Results
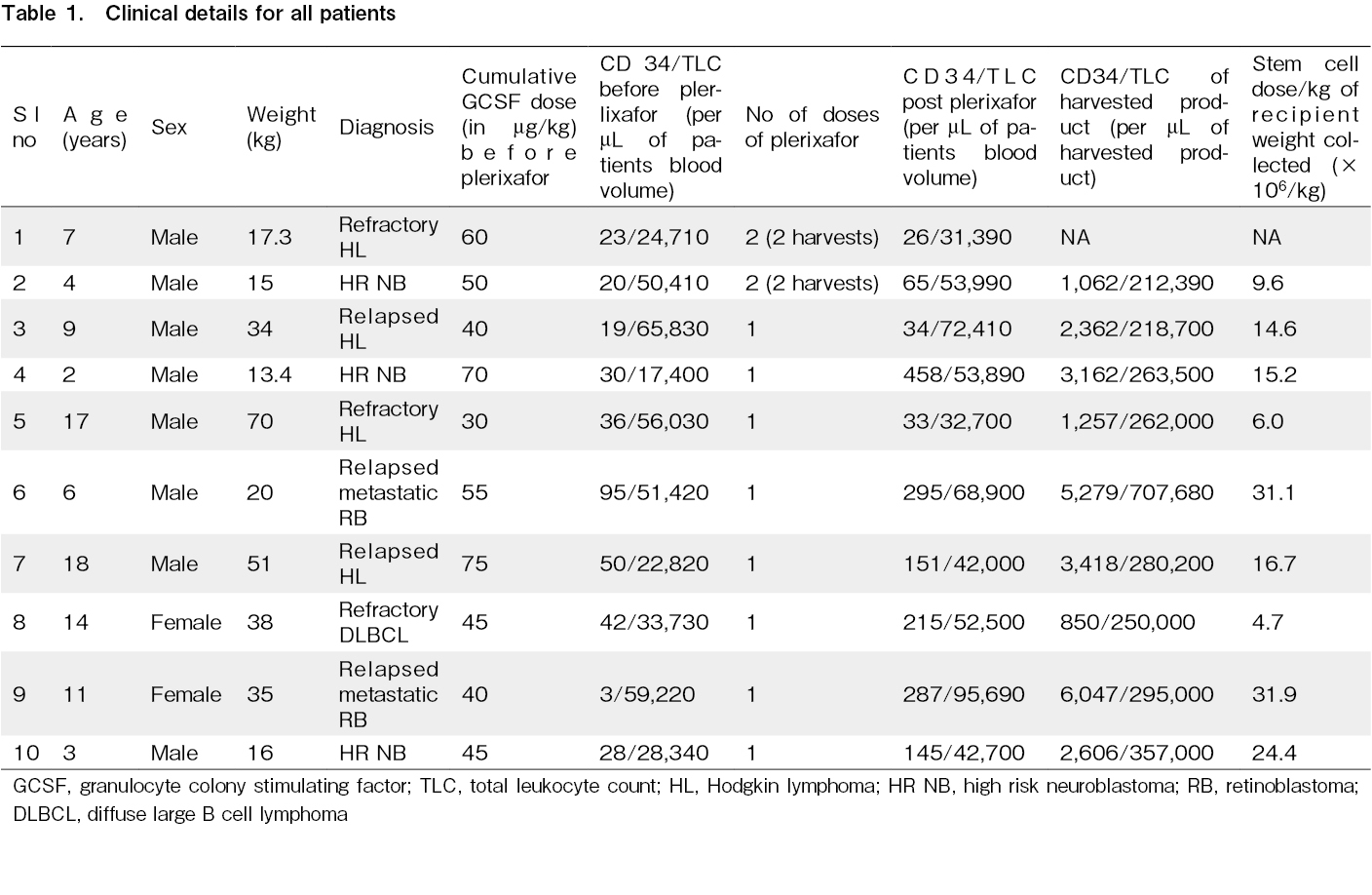
Ten patients received plerixafor before the peripheral blood hematopoietic stem cell (PB HSC) harvest was performed. There were 8 male and 2 female patients, with a median age of 8 y (2-18 y).
Four patients had relapsed/refractory Hodgkin lymphoma (2 relapsed/2 refractory), one patient had refractory diffuse large B-cell lymphoma (DLBCL), three patients had high-risk neuroblastoma, and two patients had relapsed retinoblastoma. The mean weight of the patients was 30.9±17.6 kg. Patients were administered either 5 μg/kg twice a day or 10 μg/kg once a day of GCSF. The patients received variable cumulative doses of GSCF before receiving plerixafor prior to admission for apheresis, and the mean dose given was 51.0±13.3 μg/kg.
The peripheral blood CD 34 count on the day prior to the harvest was less than 50/μL in nine cases, and in one child (patient no. 6), the CD 34 count was not available.
The median CD 34 count for all patients on the day prior to harvest was 29/μL (3-95/μL) and the corresponding TLC was 40,991/μL (17,400-65,830/μL). Following plerixafor administration, there was a significant increase in the CD 34 count and TLC in all patients, except one (patient no. 5). In the nine patients in whom there was a rise in counts after plerixafor, the CD 34 count post plerixafor increased to a median of 148/μL (26-458/μL) and the TLC rose to a median of 53,195/μL (31,390-95,690/μL). In nine cases, we were able to obtain the CD 34 count of the harvested product, the median CD 34 count was 2,606/μL (850-6,047/μL). The stem cell doses collected for all patients are shown in Table 1.
None of the patients experienced any side effects related to plerixafor.
Discussion
In our retrospective study, we found that the use of plerixafor post-GCSF led to an increase in the CD 34 counts in the peripheral blood, allowing for an improved yield of CD 34 positive HSC. The use of plerixafor was not associated with any adverse effects.
In pediatric autologous HSCT, a good stem cell dose is important. A stem cell count of at least 2-5 million cells per kg is recommended, As higher stem cell doses are associated with faster engraftment and better overall outcomes6. In cases of tandem transplants, larger numbers of stem cells need to be collected for use in more than one rescue after high-dose chemotherapy.
Mobilization using GCSF has been predominantly used for stem cell harvest, but it carries a 5-30% risk of failure, and this could be more in patients treated with multiagent chemotherapy. Poor mobilization may lead to the abandonment of A-PBSCT as a treatment option. Remobilization carries the risk of morbidity, inconveniences for the patient, and increased utilization of resources7.
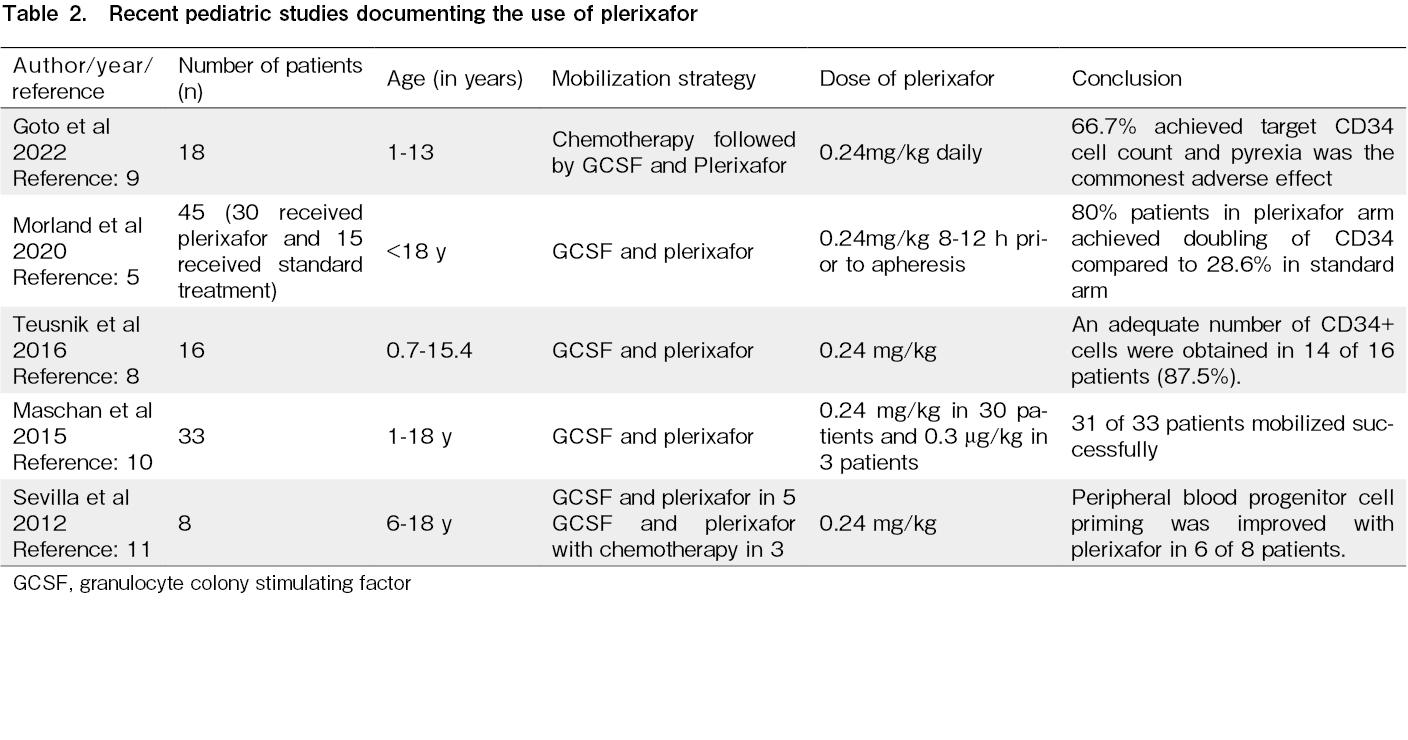
Plerixafor alone or in combination with GCSF has been reported to significantly increase the number of PB HSC cells and is being increasingly used as a modality to avoid mobilization failure. However, evidence in the pediatric population is still emerging. Teusink et al (2016) have reported the use of plerixafor in 16 children undergoing A-PBSCT, following failed initial mobilization attempts, and demonstrated that 14 patients were able to achieve optimal CD 34 counts for autologous HSCT8.
The phase I/II MOZAIC trial in children between 1-18 years with solid tumors, lymphomas, and brain tumors showed that with plerixafor and standard mobilization, the proportion of patients with doubled PB CD 34 HSC counts significantly increased when compared to standard mobilization alone5. In 18 patients with solid tumors, plerixafor was found to be effective in increasing PB HSC counts, permitting a successful harvest9. In this study, the use of plerixafor led to an increase in PB HSC counts in nine out of ten children. In eight children who received plerixafor, one dose was sufficient to achieve an acceptable HSC yield for A-PBSCT, and a second apheresis procedure was avoided.
Maschan et al. reported mild side effects in approximately 25% of children aged 1-18 years receiving plerixafor10. Sevilla et al. also found mild adverse effects in two of eight patients11. Serious side effects are rare, but some, such as spontaneous pneumomediastinum after the use of plerixafor, have been described in the literature12. None of the patients in this study experienced any side effects and plerixafor was well tolerated. A low rate of adverse effects has been reported in 18 Japanese children who received plerixafor9. The MOZAIC study also demonstrated that plerixafor was safe for use in children, and all the adverse effects were attributable to mobilizing chemotherapy5.
The findings of recent pediatric studies on the use of plerixafor is presented in Table 2.
Conclusion
Plerixafor was found to be effective in increasing PB HSC counts in chemotherapy pre-treated pediatric patients and led to successful mobilization. In nine out of the ten patients, we achieved an adequate HSC count in the final product harvested post plerixafor. Plerixafor did not cause any side effects in the ten patients.
Author Contributions
AKG drafted the original manuscript. AKG, JPM, HCP, PC and RS contributed to patient care. All authors revised the manuscript. All authors approved the final manuscript.
Consent
Informed consent was obtained by all participants in this study.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019; 54: 1525-52.
2.Snowden JA, Sánchez-Ortega I, Corbacioglu S, Basak GW, Chabannon C, de la Camara R, et al. Indications for haematopoietic cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2022. Bone Marrow Transplant. 2022; 57: 1217-39.
3.Gertz MA, Wolf RC, Micallef IN, Gastineau DA. Clinical impact and resource utilization after stem cell mobilization failure in patients with multiple myeloma and lymphoma. Bone Marrow Transplant. 2010; 45: 1396-403.
4.Fricker SP. Physiology and pharmacology of plerixafor. Transfus Med Hemother. 2013; 40: 237-45.
5.Morland B, Kepak T, Dallorso S, Sevilla J, Murphy D, Luksch R, et al. Plerixafor combined with standard regimens for hematopoietic stem cell mobilization in pediatric patients with solid tumors eligible for autologous transplants: two-arm phase I/II study (MOZAIC). Bone Marrow Transplant. 2020; 55: 1744-53.
6.Duong HK, Savani BN, Copelan E, Devine S, Costa LJ, Wingard JR, et al. Peripheral blood progenitor cell mobilization for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2014; 20: 1262-73.
7.Gertz MA. Current status of stem cell mobilization. Br J Haematol. 2010; 150: 647-62.
8.Teusink A, Pinkard S, Davies S, Mueller M, Jodele S. Plerixafor is safe and efficacious for mobilization of peripheral blood stem cells in pediatric patients. Transfusion. 2016; 56: 1402-5.
9.Goto H, Kanamori R, Nishina S, Seto T. Plerixafor stem cell mobilization in Japanese children: A post-marketing study. Pediatr Int. 2022; 64: e15106.
10.Maschan AA, Balashov DN, Kurnikova EE, Trakhtman PE, Boyakova EV, Skorobogatova EV, et al. Efficacy of plerixafor in children with malignant tumors failing to mobilize a sufficient number of hematopoietic progenitors with G-CSF. Bone Marrow Transplant. 2015; 50: 1089-91.
11.Sevilla J, Schiavello E, Madero L, Pardeo M, Guggiari E, Baragaño M, et al. Priming of hematopoietic progenitor cells by plerixafor and filgrastim in children with previous failure of mobilization with chemotherapy and/or cytokine treatment. J Pediatr Hematol Oncol. 2012; 34: 146-50.
12.Hong KT, Kang HJ, Kim NH, Kim MS, Lee JW, Kim H, et al. Successful mobilization using a combination of plerixafor and G-CSF in pediatric patients who failed previous chemomobilization with G-CSF alone and possible complications of the treatment. J Hematol Oncol. 2012; 5: 14.
Search
News