Volume 6 (2023) Issue 2 No.5 Pages 61-65
Abstract
Introduction: The role of fluoroquinolone (FQ) prophylaxis in preventing gram-negative bacilli (GNB) bacteremia, graft-versus-host disease (GVHD), and overall survival (OS) after allogeneic hematopoietic cell transplantation (allo-HCT) is debatable and may differ in settings with low and high prevalences of FQ resistance. In this study, we aimed to answer this question in regions with high FQ resistance.
Methods: This single-center retrospective study included all consecutive allo-HCT recipients aged ≥12 years from 2012 to 2021. Allo-HCT recipients until 2016 were administered FQ prophylaxis (levofloxacin). After 2016, the institutional protocol was modified to no antibiotic prophylaxis. Data were retrieved from patient records for disease and transplant characteristics, the incidence of GNB bacteremia, duration of parenteral antibiotics, hospitalization duration, acute GVHD, and OS.
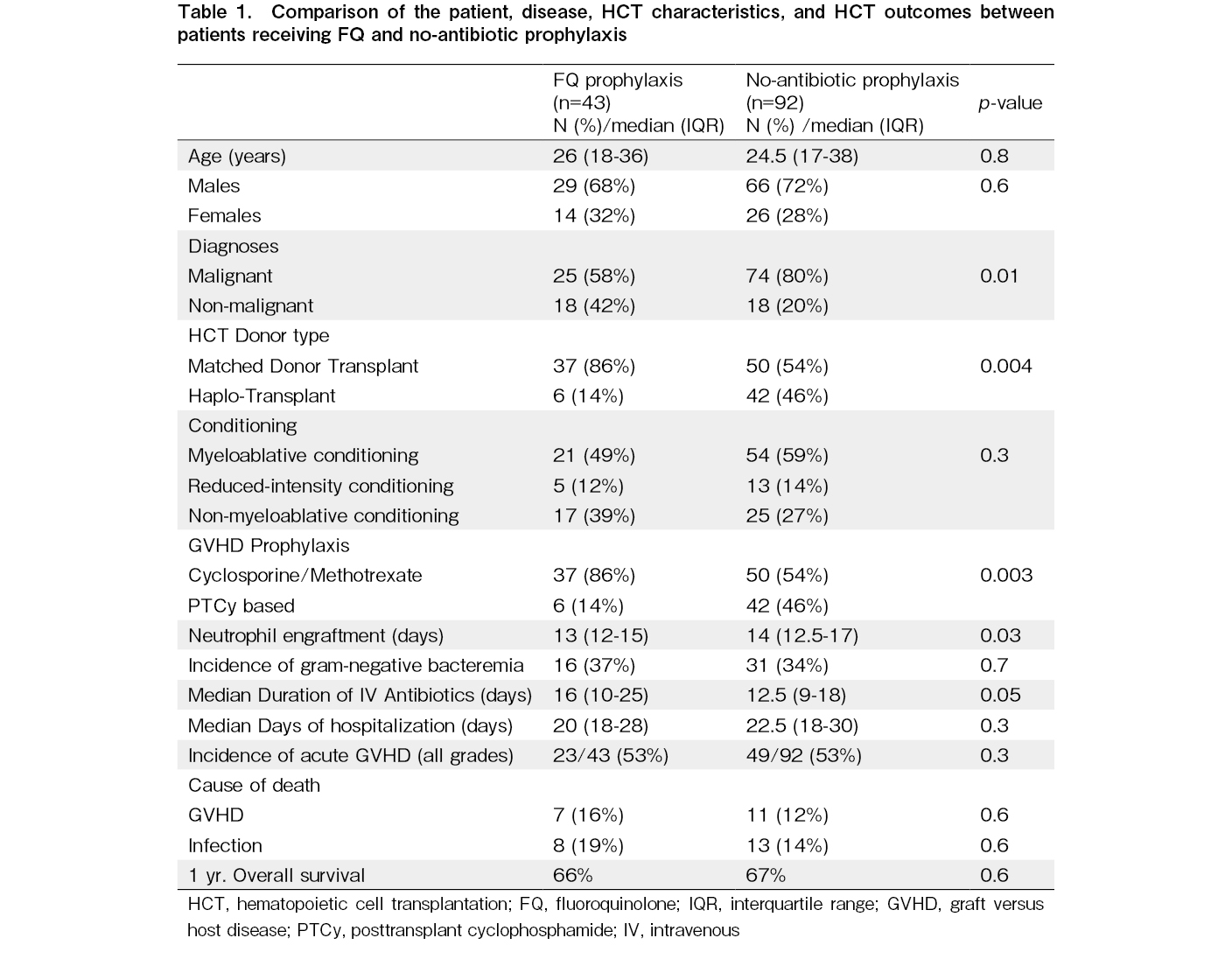
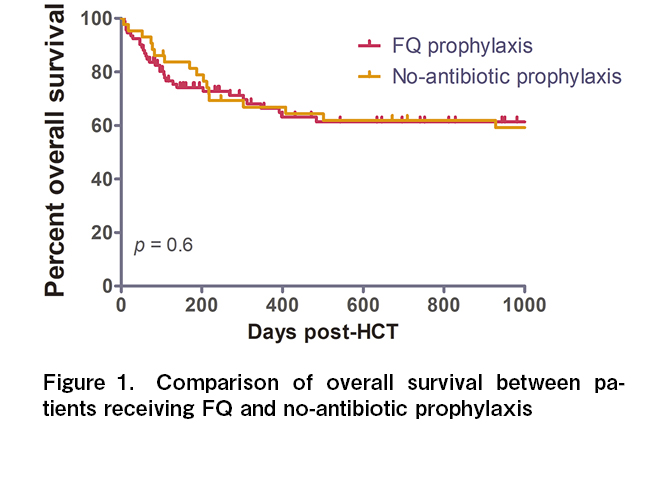
Results: A total of 135 allo-HCT recipients (43 in the FQ-prophylaxis cohort and 92 in the no-antibiotic prophylaxis cohort) were analyzed in this study. The two cohorts were matched for age (median, 26 vs. 24.5 years; p = 0.8). The no-antibiotic prophylaxis cohort had a higher proportion of malignant diagnoses (80% vs. 58%, p = 0.01), haploidentical transplants (46% vs. 14%, p = 0.004), and posttransplant cyclophosphamide exposure (46% vs. 14%, p = 0.003) than did the FQ cohort. Despite this, the incidence of GNB bacteremia was not significantly different between the two cohorts (37% vs. 34%, p = 0.6). There were no differences in parenteral antibiotic use or hospitalization duration, as well as the incidence of acute GVHD (53% vs. 53%, p = 0.3). The 1-year OS was similar between the two cohorts (66% vs. 67%, p = 0.6).
Conclusion: This study shows that FQ prophylaxis did not affect the incidence of GNB bacteremia, parenteral antibiotic use, hospitalization duration, acute GVHD, and OS post-allo-HCT.
Introduction
Current global guidelines for preventing bacterial infections among adult hematopoietic cell transplantation (HCT) recipients recommend the use of fluoroquinolone (FQ) (i.e., levofloxacin) prophylaxis1, 2. It also mentions that the local epidemiological data should be carefully considered before applying FQ prophylaxis. It also recommends monitoring for FQ resistance in gram-negative bacilli (GNB) if used as prophylaxis1. A systematic review3 and meta-analysis4, which included studies up to a decade ago, showed that FQ prophylaxis decreased bacteremia in allogeneic HCT (allo-HCT) recipients. Subsequently, several recent studies from developed regions with a lower prevalence of FQ resistance have confirmed this5–7. However, there is a weak recommendation against routine FQ prophylaxis in pediatric HCT recipients8, 9. Despite this, an intercontinental study involving 65 centers from 25 countries showed that 75% of the centers used FQ prophylaxis. This global study also showed that approximately 55% of GNB isolates were FQ-resistant and that using FQ prophylaxis was associated with a higher incidence of multidrug-resistant isolates10. Recent data also suggest that antibiotic prophylaxis increases the risk of acute graft-versus-host disease (GVHD) by modifying the gut microbiome, leading to questioning the role of FQ prophylaxis11. There are little data on the incidence of GNB bacteremia in regions with a high prevalence of FQ resistance. One study showed that FQ prophylaxis was ineffective in patients with pre-existing colonization by FQ-resistant Enterobacterales12. In this study, we aimed to investigate the impact of levofloxacin prophylaxis on GNB bacteremia and acute GVHD in allo-HCT recipients in India, where the susceptibility of GNB isolates to FQ is <30% in hospital settings (institutional antimicrobial susceptibility data).
Methods
This single-center retrospective study included all consecutive allo-HCT recipients aged ≥12 years who underwent HCT between 2012 and 2021. The study was approved by the Institutional Ethics Committee and adhered to the principles of the Declaration of Helsinki. Allo-HCT recipients until 2016 were administered FQ prophylaxis (levofloxacin 750 mg orally once daily) starting with conditioning chemotherapy. This treatment was continued until parenteral antibiotics were initiated for febrile neutropenia. After 2016, the protocol was modified to avoid the use of antibiotic prophylaxis. For febrile neutropenia, the first-line antibiotic at our center was cefoperazone-sulbactam. The escalation of next-line antibiotics (carbapenems and colistin) was at the discretion of the transplant physician in the absence of positive cultures. Antibiotics were modified according to susceptibility in the event of isolation of any organism. Antibiotics were continued until engraftment or defervescence, whichever occurred later. An automated BACTEC 9240 (BD Becton Dickinson, USA) system was used for blood culture. Gram-stained smears were prepared from beep-positive bottles and subcultured. All differentiated colonies were identified by matrix-assisted laser desorption ionization-time-of-flight mass spectrometry using Biotyper 3.0. Isolation of GNB bacilli was documented as evidence of GNB bacteremia. Myeloablative conditioning (MAC) regimens included busulfan 12.8 mg/kg or treosulfan or total body irradiation of 12 Gy. Reduced-intensity conditioning regimens included fludarabine-melphalan 140 mg/m2. Non-myeloablative regimens include fludarabine-cyclophosphamide-based regimens. GVHD prophylaxis included cyclosporine/methotrexate (CSA/MTX) for matched donor HCT and posttransplant cyclophosphamide/cyclosporine/mycophenolate (PTCy/CSA/MMF) for haplo-HCT. We analyzed patient records for the incidence of GNB bacteremia, duration of parenteral antibiotics during the peri-transplant period, hospitalization duration, acute GVHD, and overall survival (OS). Acute GVHD was diagnosed and graded according to the Mount Sinai Acute GVHD International Consortium (MAGIC) criteria13. OS was defined as the time from HCT to death from any cause. Statistical analyses were performed using GraphPad Prism, version 5.0. Categorical variables were compared using the chi-square or Fisher exact test. Continuous variables were compared using the independent t-test for normally distributed variables and the Mann-Whitney test for skewed variables. Statistical significance was set at p
Results
A total of 135 allo-HCT recipients (43 in the FQ prophylaxis cohort and 92 in the no-antibiotic prophylaxis cohort) were analyzed in this study. The two cohorts were matched for age (median, 26 years [IQR, 18-36] vs. 24.5 years [IQR, 17-38]; p = 0.8) and sex (Table 1). The FQ cohort had a lower proportion of malignant diagnoses (58% vs. 80%, p = 0.01). In both cohorts, the most common diagnoses included acute leukemia (51% vs. 66%), followed by aplastic anemia (37% vs. 17%). The conditioning intensity was also matched between cohorts (MAC, 49% vs. 59%; p = 0.3). The details of the disease diagnoses and conditioning regimens are listed in
Discussion
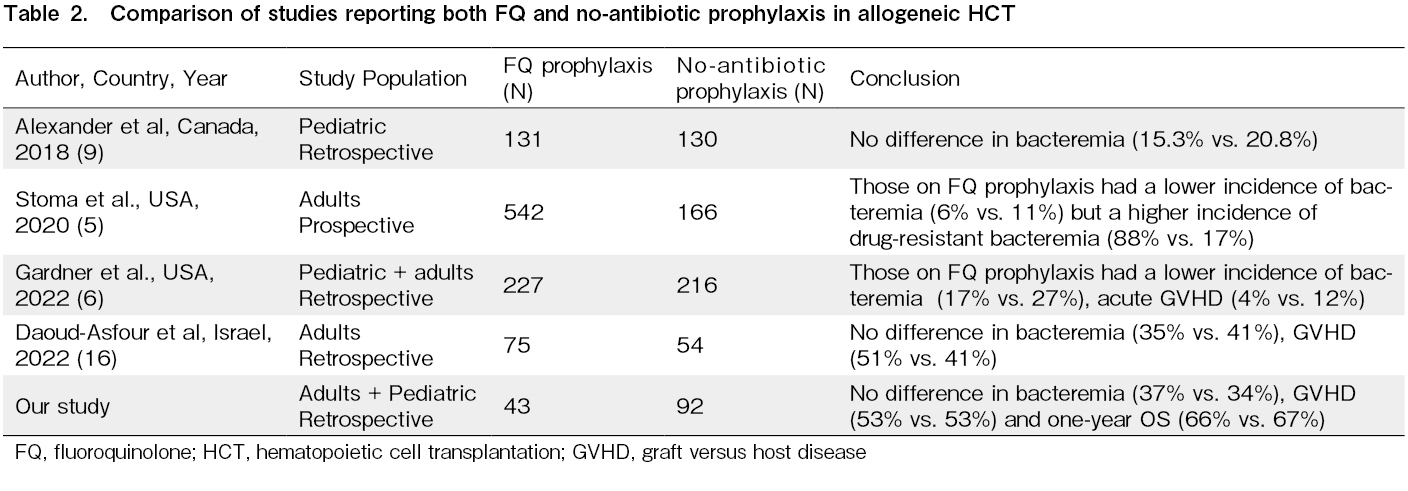
Most studies from high-income countries with a low prevalence of FQ resistance have found a beneficial effect of FQ prophylaxis on reducing the incidence of GNB bacteremia in the adult allo-HCT setting5–7. There is always weak evidence of FQ prophylaxis in the pediatric allo-HCT settings8. However, recently, there has been interest in antibiotic-mediated modification of the gut microbiome and its adverse impact on GVHD and survival outcomes14, 15. Additionally, the role of FQ in bacterial prophylaxis in HCT, specifically in regions with a wide prevalence of FQ and multidrug resistance, is debatable10, 12, 16 (Table 2). At our center, the susceptibility of common GNB isolates (Escherichia coli and Klebsiella pneumoniae) to FQ is <30% (institutional antimicrobial susceptibility data). Our study shows that FQ prophylaxis did not cause a difference in the incidence of GNB bacteremia, subsequent parenteral antibiotic duration, hospitalization duration, acute GVHD, infection-attributable mortality, and OS outcomes after HCT. This was despite the fact that more recipients in the no-antibiotic prophylaxis cohort underwent transplantation for malignant conditions with alternative donors and PTCy exposure. The major limitation of this study is its retrospective nature, and the two cohorts were transplanted at different periods. Although we included pediatric and adult patients in our study, most of them were adults. We also did not have data for pre-HCT colonization by FQ-resistant GNB or post-HCT drug susceptibility data for both cohorts. Gut microbiome data were also unavailable in this study. In conclusion, the advantages and disadvantages of FQ prophylaxis should be evaluated based on the prevalence of FQ resistance in the allo-HCT settings.
Author Contributions
AN, SK, PM, and DPL conceived the study. AN, SK, and DPL collected and analyzed the data and drafted the manuscript. All authors were involved in patient care, reviewed the manuscript, and approved the final version. AN, SK, and DPL confirm full access to the study's data and final responsibility for the manuscript.
Funding
Part of this work was sponsored by the Indian Council of Medical Research (ICMR) IRIS ID 2020-0664 grant to DPL.
Ethics Approval
The study was cleared by the institutional ethics committee; Postgraduate Institute of Medical Education and Research, Institutional Ethics Committee, Approval reference no: NK/7610/PhD/468.
Informed Consent
Informed consent was obtained from all participants included in the study.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Tomblyn M, Chiller T, Einsele H, Gress R, Sepkowitz K, Storek J, et al. Guidelines for preventing infectious complications among hematopoietic cell transplantation recipients: a global perspective. Biol Blood Marrow Transplant. 2009; 15: 1143-238.
2.Taplitz RA, Kennedy EB, Bow EJ, Crews J, Gleason C, Hawley DK, et al. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA Clinical Practice Guideline Update. J Clin Oncol. 2018; 36: 3043-54.
3.Egan G, Robinson PD, Martinez JPD, Alexander S, Ammann RA, Dupuis LL, et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials. Cancer Medicine. 2019; 8: 4536-46.
4.Kimura S-i, Akahoshi Y, Nakano H, Ugai T, Wada H, Yamasaki R, et al. Antibiotic prophylaxis in hematopoietic stem cell transplantation. A meta-analysis of randomized controlled trials. Journal of Infection. 2014; 69: 13-25.
5.Stoma I, Littmann ER, Peled JU, Giralt S, van den Brink MRM, Pamer EG, et al. Compositional flux within the intestinal microbiota and risk for bloodstream infection with gram-negative bacteria. Clin Infect Dis. 2021; 73: e4627-35.
6.Gardner JC, Courter JD, Dandoy CE, Davies SM, Teusink-Cross A. Safety and efficacy of prophylactic levofloxacin in pediatric and adult hematopoietic stem cell transplantation patients. Transplant Cell Ther. 2022; 28: 167.e1-5.
7.Miles-Jay A, Butler-Wu S, Rowhani-Rahbar A, Pergam SA. Incidence rate of fluoroquinolone-resistant gram-negative rod bacteremia among allogeneic hematopoietic cell transplantation patients during an era of levofloxacin prophylaxis. Biol Blood Marrow Transplant. 2015; 21: 539-45.
8.Lehrnbecher T, Fisher BT, Phillips B, Alexander S, Ammann RA, Beauchemin M, et al. Guideline for antibacterial prophylaxis administration in pediatric cancer and hematopoietic stem cell transplantation. Clin Infect Dis. 2020; 71: 226-36.
9.Alexander S, Fisher BT, Gaur AH, Dvorak CC, Villa Luna D, Dang H, et al. Effect of levofloxacin prophylaxis on bacteremia in children with acute leukemia or undergoing hematopoietic stem cell transplantation: A randomized clinical tria. JAMA. 2018; 320: 995-1004.
10.Averbuch D, Tridello G, Hoek J, Mikulska M, Akan H, Yanez San Segundo L, et al. Antimicrobial resistance in gram-negative rods causing bacteremia in hematopoietic stem cell transplant recipients: Intercontinental Prospective Study of the Infectious Diseases Working Party of the European Bone Marrow Transplantation Group. Clin Infect Dis. 2017; 65: 1819-28.
11.Gavriilaki M, Sakellari I, Anagnostopoulos A, Gavriilaki E. The impact of antibiotic-mediated modification of the intestinal microbiome on outcomes of allogeneic hematopoietic cell transplantation: systematic review and meta-analysis. Biol Blood Marrow Transplant. 2020; 26: 1738-46.
12.Satlin MJ, Chen L, Douglass C, Hovan M, Davidson E, Soave R, et al. Colonization with fluoroquinolone-resistant enterobacterales decreases the effectiveness of fluoroquinolone prophylaxis in hematopoietic cell transplant recipients. Clin Infect Dis. 2021; 73: 1257-65.
13.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, Multicenter Standardization of Acute Graft-versus-Host Disease Clinical Data Collection: A Report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant. 2016; 22: 4-10.
14.Kaundal S, Jandial A, Singh H, Chopra M, Kasudhan KS, Khaire N, et al. Impact of broad-spectrum antibiotic exposures and multidrug-resistant gram-negative bacteremia on hematopoietic cell transplantation outcomes. Transpl Infect Dis. 2021; 23: e13717.
15.Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, et al. Microbiota as predictor of mortality in allogeneic hematopoietic-cell transplantation. N Engl J Med. 2020; 382: 822-34.
16.Daoud-Asfour H, Henig I, Ghersin I, Rakedzon S, Stern A, Pitashny M, et al. Omitting ciprofloxacin prophylaxis in patients undergoing allogeneic hematopoietic stem cell transplantation and its impact on clinical outcomes and microbiome structure. Transplant Cell Ther. 2022; 28: 168.e1-8.
17.Signorelli J, Zimmer A, Liewer S, Shostrom VK, Freifeld A. Incidence of febrile neutropenia in autologous hematopoietic stem cell transplant (hsct) recipients on levofloxacin prophylaxis. Transpl Infect Dis. 2020; 22: e13225.
Search
News