Volume 6 (2023) Issue 2 No.3 Pages 49-53
Abstract
Patients who have undergone hematopoietic cell transplantation (HCT) are at a higher risk of severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection than the general population. Therefore, early vaccination is recommended for post-transplant patients. Although exacerbation of chronic graft-versus-host disease (cGVHD) after the initial vaccination has been reported, it is unknown whether severe cGVHD occurs when different RNA vaccines are combined. We treated a patient who developed severe oral mucosal cGVHD after receiving two different RNA vaccines. Visual inspection showed that the patient presented with typical mucocutaneous cGVHD, and cGVHD in this case responded well to low-dose steroids compared to common oral GVHD exacerbations. Histopathological findings revealed T cell, B cell, and conspicuous neutrophil infiltration. Multiple doses of SARS-Cov2 vaccination are required in post-transplant recipients. In conclusion, it is essential to obtain the vaccination history of allo-HSCT recipients with cGVHD exacerbation. Furthermore, reviewing the pathological findings may help treat patients with lower doses of steroids.
Introduction
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may seriously affect patients who have undergone allogeneic hematopoietic cell transplantation (HCT)1. Nanoparticle-encapsulated messenger RNA (mRNA) SARS-CoV-2 vaccines, such as mRNA-1273 (Moderna) and BNT162b2 (Pfizer), exhibit immunogenicity in not only the general population but also in HCT patients2. The European Group for Blood and Marrow Transplantation (EBMT) and the American Society for Transplantation and Cellular Therapy (ASTCT) currently recommend that allogeneic HCT patients receive these vaccinations three months post-transplant (ASH-ASTCT General Principles of COVID-19 Vaccines for Immunocompromised Patients, Version 5.0; March 22, 2022; EBMT COVID VACCINE INFORMATION, Version 8.0, January 3, 2022). Meanwhile, the worsening of chronic graft-versus-host disease (cGVHD) has been reported after vaccination, and vaccination should be avoided in cases of poorly controlled cGVHD. Herein, we report the case of a patient who developed severe post-HSCT oral mucocutaneous cGVHD after receiving different types of SARS-CoV-2 RNA vaccines.
Case Report
A 61-year-old man developed anemia and was referred to a local hospital in December 2019, where he was diagnosed with myelodysplastic syndrome (MDS). The patient was followed-up while receiving blood transfusions. In June 2020, the MDS progressed to acute myeloid leukemia (AML) with myelofibrosis, and he received CAG therapy (cytarabine, aclarubicin, and granulocyte colony-stimulating factor). Blast cells in the peripheral blood (PB) decreased, but with no recovery of neutrophils, and febrile neutropenia persisted, possibly due to MDS-associated fever. The patient was referred to our hospital for HCT. In October 2020, due to profound neutropenia with persistent blast cells in the PB, he underwent urgent cord blood transplantation (CBT) following a conditioning regimen consisting of fludarabine (30 mg/m2 days −7 to −2), melphalan (40 mg/m2, days −3 and −2), and total body irradiation (4 Gy, day −1). Tacrolimus and mycophenolate mofetil (2,000 mg/day) were administered to prevent GVHD. Neutrophil engraftment was achieved on day 29. On day 36, the patient developed stage 3 skin GVHD and stage 1 acute gastrointestinal GVHD (total grade II). Prednisolone (0.5 mg/kg/day) in combination with topical steroids was initiated. On day 39, the patient received defibrotide for late-onset veno-occlusive disease (VOD). Acute GVHD and VOD gradually improved. We carefully tapered and discontinued tacrolimus by December 2021. By January 2022, we discontinued the low-dose steroids that had been administered to prevent corticosteroid withdrawal. Fortunately, there was not recurrence of AML after CBT. The patient received the BNT162b2 mRNA vaccine twice during the clinical course; however, no emergence of cGVHD occurred.
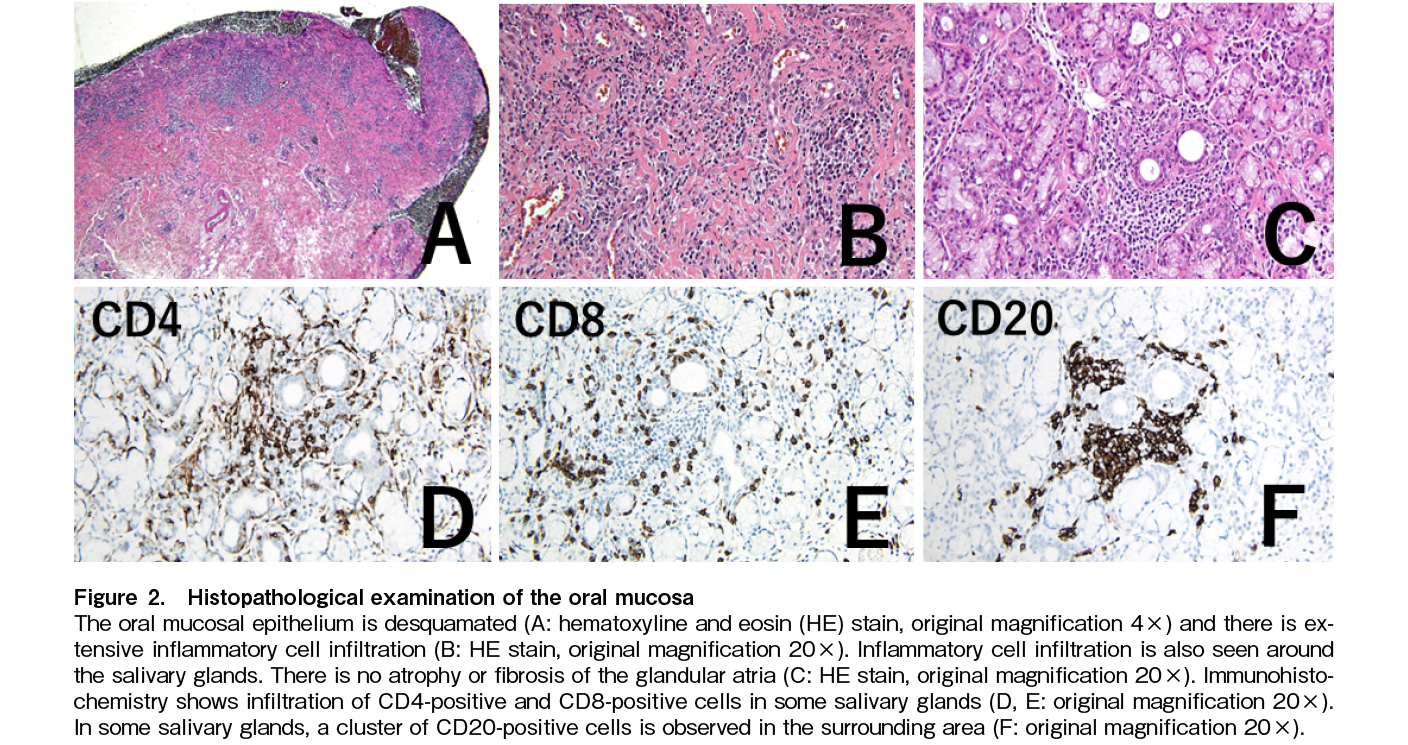
On day 581, he received a third SARS-CoV-2 vaccine (mRNA-1273). One week later, the patient noticed mouth ulcers. They gradually worsened, and he visited our hospital on day 601. Mucositis was observed throughout the oral cavity, with fused ulcers on the buccal mucosa, tongue margin, and gingiva, accompanied by hyperkeratotic white lines and a lacy appearance, so-called lichen planus-like changes (Figure 1A and 1B). These findings are consistent with the clinical features of oral cGVHD. We performed mucosal biopsy and microbiological assessment. Examination revealed only oral commensal bacteria. Candida and other fungi were not detected. Pathology at diagnosis revealed the infiltration of CD4-positive, CD8-positive, and CD20-positive cells, in addition to neutrophils (Figure 2A, 2B, 2C, 2D, 2E, 2F). Oral intake was mildly restricted, but with no weight loss. No other systemic symptoms such as dry eyes, skin rashes, or joint contractures were observed. No abnormalities were found in laboratory tests (Table 1). Respiratory function tests showed a decrease in FEV1.0, which remained unchanged from pre-transplantation, and no lung lesions due to cGVHD were noted after transplantation. As the lesion was confined to the oral cavity, a topical oral steroid was prescribed; however, it was ineffective, and maculopapular eruptions appeared on over 20% of the body surface. Therefore, we initiated systemic betamethasone treatment at 1 mg/day for cGVHD exacerbation. Mucositis gradually improved within a week. The oral mucosa was epithelialized. Ulcers on the tongue also exhibited mucosal epithelial repair, although some erosions remained. We were able to taper the systemic steroids.
Discussion
Several studies have reported a low risk of exacerbation when the BNT162b2 mRNA COVID-19 vaccine was administered multiple times to patients after allogeneic stem cell transplantation3. Ali et al. reported that the incidence of cGVHD onset or exacerbation in patients who received the first or second vaccine after allogeneic transplantation was 12.2%4. Few studies have reported the onset or worsening of cGVHD after the third and subsequent vaccinations, but Kimura et al. reported a frequency of 5.4%5. The skin is the most common site, followed by the oral mucosa. In these reports, cGVHD may have been associated with multiple organs, but there were also several single-organ cases. The development of severe, new-onset oral mucosal cGVHD > 100 days after cord blood transplantation is rare6. Therefore, our patient's symptoms were considered vaccine-induced new-onset cGVHD. The onset and exacerbation of cGVHD after vaccination varies widely, with reports ranging from within a week to several weeks later. In these reports, GVHD exacerbations and new-onset GVHD occurred more frequently in relatively elderly patients over 60 years of age who received transplants, suggesting a possible risk, although this has not been statistically validated4, 5. Pabst et al. reported that angiopoietin-2 levels were elevated in patients with exacerbations of cGVHD after mRNA vaccination, which was not evaluated in this case report and is expected to be a biomarker7. Many reported cases of post-vaccination cGVHD were treated with 0.5-1 mg/kg of steroids, increased doses of calcineurin inhibitors, or both. Some patients were treated with relatively low doses of tacrolimus and topical steroids4.
In our case, histopathological examination revealed predominantly neutrophilic mixed-cell infiltration and a lack of fibrosis, which differed from the typical features of cGVHD exacerbations8, 9. In the present case, gross findings showed severe mucosal cGVHD, but histopathological examination was atypical. Post-vaccination mucocutaneous reactions in healthy individuals are characterized by the mixed inflammatory cell infiltration, similar to the pathological results in the present study10. In healthy people, heterologous vaccination reportedly has greater immunogenicity than those of homologous schedules, resulting in an increase in neutralizing antibodies in the serum11. Inflammatory cytokines such as IL-18 and IFN-γ are higher in patients with worsening cGVHD after COVID-19 vaccination than in those without cGVHD, and a similar mechanism was suggested in the present case, although cytokines were not measured7. In the present case, the administration of two different RNA vaccines might have caused stronger immune activation, triggered inflammation, and resulted in the development of cGVHD.
Conclusion
Routine vaccination is necessary for transplant recipients, and opportunities for vaccination with different RNA vaccines should be provided, as in this case. When cGVHD exacerbations are observed in post-transplant patients, pathological examination and the confirmation of vaccination history are important when deciding to treat cGVHD.
Author Contributions
K.S. and S.F. designed, performed research, and wrote the paper. H.S. and M.K. provided pathology images and advice regarding pathology findings. N.N. provided imaging data and advised on mucosal findings. Y.T., Y.S., Y.S., T.Y., J.I. were involved in the care of this patient.
Ethics Approval
This report needs no approval from the relevant institutional or equivalent review board.
Consent for Publication
The authors agree with this publication.
Funding Statement
This study was partly supported by Health, Labor and Welfare Sciences Research Grants (20FF1002).
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani MA, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia. 2021; 35: 2885-94.
2.Ni B, Yanis A, Dee K, Chappell JD, Dulek DE, Kassim AA, et al. SARS-CoV-2 vaccine safety and immunogenicity in patients with hematologic malignancies, transplantation, and cellular therapies. Blood Rev. 2022; 56: 100984.
3.Shem-Tov N, Yerushalmi R, Danylesko I, Litachevsky V, Levy I, Olmer L, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in haematopoietic stem cell transplantation recipients. Br J Haematol. 2022; 196: 884-91.
4.Ali H, Ngo D, Aribi A, Arslan S, Dadwal S, Marcucci G, et al. Safety and tolerability of sars-cov2 emergency-use authorized vaccines for allogeneic hematopoietic stem cell transplant recipients. Transplant Cell Ther. 2021; 27: 938e1-6.
5.Kimura M, Ferreira VH, Kothari S, Pasic I, Mattsson JI, Kulasingam V, et al. Safety and immunogenicity after a three-dose sars-cov-2 vaccine schedule in allogeneic stem cell transplant recipients. Transplant Cell Ther. 2022; 28: 706e1-10.
6.Isobe M, Konuma T, Masuko M, Uchida N, Miyakoshi S, Sugio Y, et al. Single cord blood transplantation for acute myeloid leukemia patients aged 60 years or older: a retrospective study in Japan. Ann Hematol. 2021; 100: 1849-61.
7.Pabst C, Benning L, Liebers N, Janssen M, Caille L, Speer C, et al. Humoral responses and chronic GVHD exacerbation after COVID-19 vaccination post allogeneic stem cell transplantation. Vaccines (Basel). 2022; 10: 330.
8.Cooke KR, Luznik L, Sarantopoulos S, Hakim FT, Jagasia M, Fowler DH, et al. The biology of chronic graft-versus-host disease: A task force report from the National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2017; 23: 211-34.
9.Santos PS, Coracin FL, Barros JC, Gallottini MH. Histopathologic diagnosis of chronic graft-versus-host disease of the oral mucosa according to the National Institutes of Health Consensus. Einstein (Sao Paulo). 2014; 12: 204-10.
10.Seirafianpour F, Pourriyahi H, Gholizadeh Mesgarha M, Pour Mohammad A, Shaka Z, Goodarzi A. A systematic review on mucocutaneous presentations after COVID-19 vaccination and expert recommendations about vaccination of important immune-mediated dermatologic disorders. Dermatol Ther. 2022; 35: e15461.
11.Stuart ASV, Shaw RH, Liu X, Greenland M, Aley PK, Andrews NJ, et al. Immunogenicity, safety, and reactogenicity of heterologous COVID-19 primary vaccination incorporating mRNA, viral-vector, and protein-adjuvant vaccines in the UK (Com-COV2): a single-blind, randomised, phase 2, non-inferiority trial. Lancet. 2022; 399: 36-49.
Search
News