Volume 6 (2023) Issue 1 No.4 Pages 18-22
Abstract
Assessing acute and chronic graft-versus-host disease (GVHD) is challenging because there are several classification systems. The European Society for Blood and Marrow Transplantation and the Center for International Bone Marrow Transplantation Registry task force recommends using the eGVHD application (App) to score acute GVHD according to the Mount Sinai Acute GvHD International Consortium (MAGIC) criteria and chronic GVHD according to the National Institutes of Health 2014 criteria. We prospectively used the eGVHD App at each follow-up visit in a large-volume bone-marrow transplant center in India from 2017 to 2021. We retrospectively evaluated the discrepancy in scoring GVHD severity by physicians not using the App from the same patient charts. The App user satisfaction and experience were recorded using the technology acceptance model (TAM) and the Post-Study System Usability Questionnaire (PSSUQ). In 100 consecutive allogeneic hematopoietic cell transplantation recipients, there was more discrepancy in scoring the severity of chronic GVHD (38%) than acute GVHD (9%) without using the App. The median TAM and PSSUQ scores were six (IQR:1) and two (IQR:1), respectively, indicating high perceived usefulness and user satisfaction. The eGVHD App is an excellent learning tool for hematology/BMT fellows and helps manage GVHD in high-volume BMT centers.
Introduction
In clinical practice, assessing the severity of acute and chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic cell transplantation (allo-HCT) is challenging. There has been an evolution in the guidelines for assessing acute GVHD from the Original Glucksberg criteria1 to the Modified Glucksberg criteria, International Bone Marrow Transplantation Registry (IBMTR) criteria2, and the most recent Mount Sinai Acute GvHD International Consortium (MAGIC) criteria3. Similarly, the chronic GVHD assessment has evolved from the Original Seattle criteria4 to the Revised Seattle criteria to the 2005 National Institutes of Health (NIH) criteria5 and the most recent 2014 NIH criteria6. The European Society for Blood and Marrow Transplantation-NIH-Center for International Bone Marrow Transplantation Registry (EBMT-NIH-CIBMTR) task force recommends using the MAGIC and NIH 2014 criteria, in addition to providing consensus definitions for the onset, response, and status of GVHD to facilitate future clinical and translational research7. They also recommend using the eGVHD App to improve the quality of data collection. The App-based practice to score GVHD has shown better accuracy and inter-physician agreement8.
Methods
This single-center study was performed at a high-volume bone marrow transplant (BMT) center in India. The eGVHD smartphone application (App) was used prospectively at each follow-up clinic visit by a group of BMT physicians at the bedside to score the severity of GVHD in allo-HCT recipients from 2017 to 2021. The eGVHD App was developed by UZ Leuven (Belgium) in collaboration with the EBMT Complications and Quality of Life Working Party and NIH and is available on both android and apple stores9. The discrepancy in GVHD scoring of the same patients by physicians not using the App was evaluated retrospectively using patient charts. For each patient, different evaluators recorded the eGVHD and non-eGVHD scores. The App and non-App evaluators included either trained BMT physicians or BMT physicians-in-training. In addition, the standard terminology for the onset of GVHD and response to therapy was recorded. App user satisfaction and experience were administered to the BMT team members, including the BMT faculty and fellows-in-training. This was recorded using the validated perceived usefulness subscale of the technology acceptance model (TAM) and the Post-Study System Usability Questionnaire (PSSUQ)9.
Results
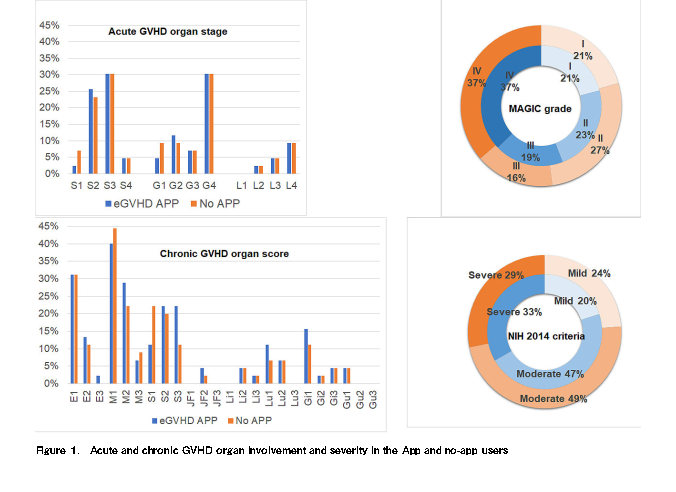
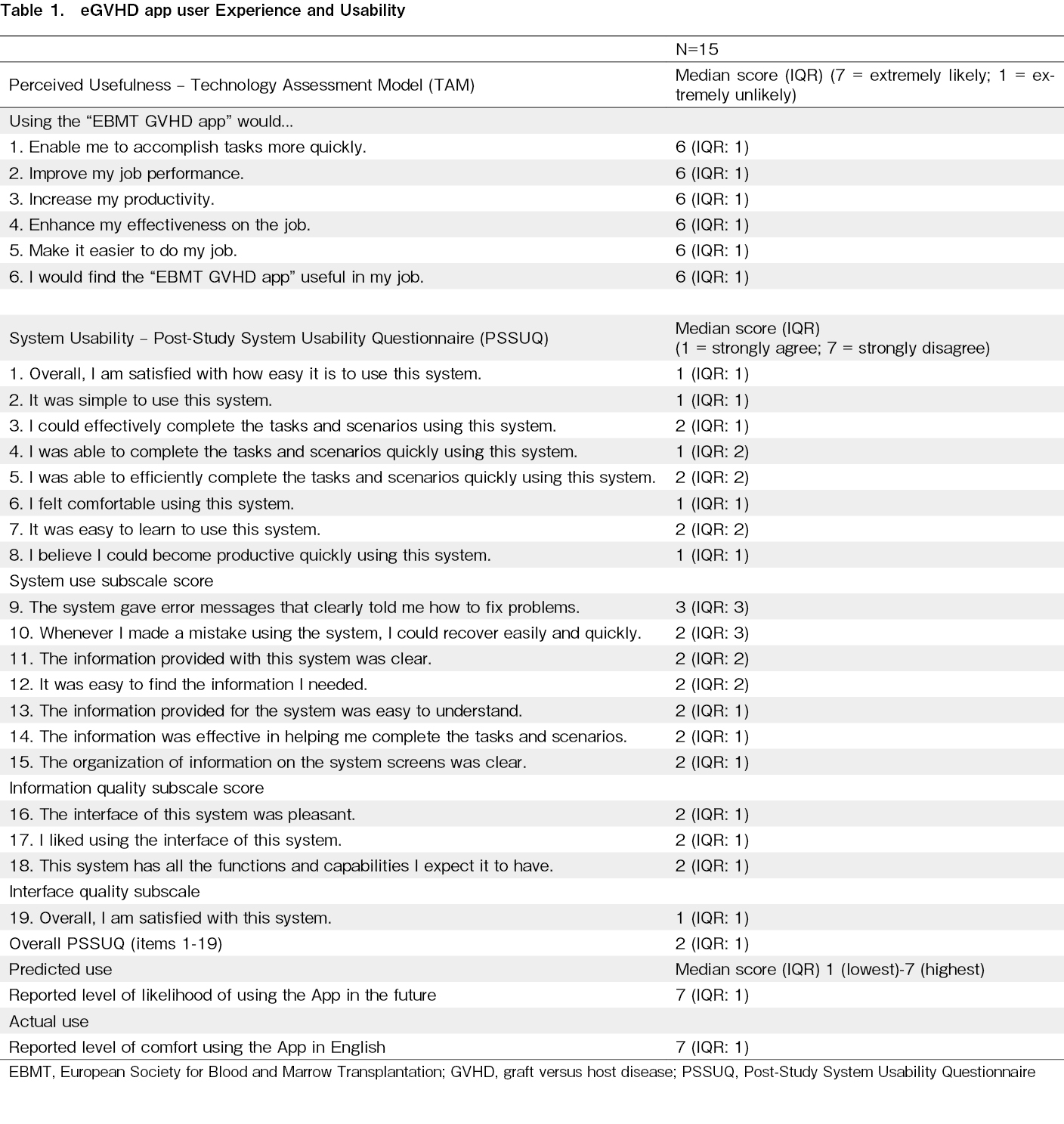
One hundred consecutive allo-HCT recipients were included (male, 70, female, 30) with a median age of 23 years (17-39 Years). The median follow-up period was 295 days (109-981 days). The underlying diagnoses included hematological malignancies (81%) and aplastic anemia (19%). The conditioning was myeloablative (54%) and reduced intensity/non-myeloablative (46%). Peripheral blood was the source of stem cells in all recipients. The types of HCT were matched related donor (57%), matched unrelated donor (9%), and haploidentical (34%). Acute GVHD occurred in 43% of patients, with onset at a median of 38 days (25-67 days). Onset was classic in 33 (77%) patients, late-onset in six (14%), and recurrent in four (9%). Thirty percent of the patients with acute GVHD had skin stage 3 and gut stage 4 involvement. GVHD severity using the eGVHD App was MAGIC grade I in nine (21%), grade II in ten (23%), grade III in eight (19%), and grade IV in 16 (37%) patients. There was a discrepancy in scoring the severity in four (9%) patients without using the App, with severity being downgraded in two and upgraded in two. This was mainly observed in skin and gut stages 1 and 2 (Figure 1). Seventy-seven percent of patients with acute GVHD were in-patients at the time of acute GVHD diagnosis. Acute GVHD was steroid-responsive in 28 patients (65%) and steroid-refractory in 15 (35%). Chronic GVHD occurred in 45 patients (45%), with onset occurring at a median of 168 days (117-212 days). The onset was de novo in 20 (44%) patients, quiescent in 21 (47%), and progressive in four (9%). Most patients had either mouth, eye, and/or skin GVHD. The severity was mild in nine (20%), moderate in 21 (47%), and severe in 15 (33%) patients. There was a discrepancy in scoring chronic GVHD in 17 (38%) patients without the use of the App, with severity being downgraded in 13 (29%) and upgraded in four (9%) patients. This was mainly seen in the skin, mouth, eye, and lung GVHD, where a mix of objective and subjective symptom/sign scoring is required (Figure 1). All patients were steroid-responsive; however, 11 (25%) were steroid-dependent. A total of 15/18 (83%) App users answered the TAM and PSSUQ. The median TAM score was six (IQR:1), with a score of seven indicating extremely likely perceived usefulness. The median PSSUQ was 2 (IQR:1), with a score of one indicating strong agreement with system usability. The median predicted and actual use scores were highest at seven (IQR:1) (Table 1).
Discussion
There was more discrepancy in scoring the severity of chronic GVHD (38%) than acute GVHD (9%) without using the App. The better agreement in scoring acute GVHD may be due to most of them getting GHVD as in-patients and mainly grading only three organ systems. The poor agreement in scoring chronic GVHD may be due to less time for outpatient evaluation and the need for multiple organ system assessments. In addition, scoring in some organ systems is complex, combining objective and subjective symptoms/signs. (For example, skin sclerosis has a minimum score of two; some skin features are not scored, scoring per skin surface area involved, frequency of eye drops/day used, % weight loss, FEV1 values, and using the range of motion pics for joints and fascia). The eGVHD App can assist in documenting GVHD severity at the bedside. We believe that adding the GVHD onset, response, and status criteria to the eGVHD App will be helpful as a single source for all-in-one GVHD assessment. The major limitation of this study is its inability to address the accuracy of scoring by either method due to the prospective retrospective study design, and the lack of independent-expert validated measures. However, this has been addressed previously in a randomized controlled trial using ten expert-validated clinical vignettes8. The benefit of accurate scoring of GVHD cannot be overstated, as this affects the choice of immunosuppression and the duration of therapy. This study's high perceived usefulness and user satisfaction translated into high predicted and actual acceptability of the App among end users in a real-world high-volume BMT center. Having used the App for the past several years in an academic setting, we feel that it is an excellent learning tool for hematology/BMT fellows, and the assessment time decreases with experience. Overall, the eGVHD application is a helpful tool for managing allo-HCT survivors.
Author Contributions
DPL and PC conceived the study. PC, KSK, and DPL analyzed the data and drafted the manuscript. All authors were involved in patient recruitment, clinical care of the patients, and manuscript writing. DPL and KSK confirm full access to the data in the study and final responsibility for the manuscript.
Financial Support
This work was done as a part of the American Society of Hematology Global Research Award to DPL.
Ethics Approval
The study was cleared by the institutional ethics committee. Postgraduate Institute of Medical Education and Research, Institute Ethics Committee, INT/IEC/2021/SPL-982
Informed Consent
Informed consent was obtained from all participants included in the study.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18: 295-304.
2.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997; 97: 855-64.
3.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute GVHD international consortium. Biol Blood Marrow Transplant. 2016; 22: 4-10.
4.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980; 69: 204-17.
5.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11: 945-56.
6.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21:389-401.e1.
7.Schoemans HM, Lee SJ, Ferrara JL, Wolff D, Levine JE, Schultz KR, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018; 53: 1401-15.
8.Schoemans HM, Goris K, Van Durm R, Fieuws S, De Geest S, Pavletic SZ, et al. The eGVHD App has the potential to improve the accuracy of graft-versus-host disease assessment: a multicenter randomized controlled trial. Haematologica. 2018; 103: 1698-707.
9.Schoemans H, Goris K, Durm RV, Vanhoof J, Wolff D, Greinix H, et al. Development, preliminary usability and accuracy testing of the EBMT ‘eGVHD App' to support GvHD assessment according to NIH criteria-a proof of concept. Bone Marrow Transplant. 2016; 51: 1062-5.
Search
News