Volume 5 (2022) Special Edition No.4 Pages S25-S33
Abstract
In acute leukemia, advances have been made in therapeutic strategies centered on allogeneic hematopoietic stem cell transplantation (allo-SCT), three of which are presented here. The indication of allo-SCT for acute myeloid leukemia (AML) in 1st complete remission (CR1) has been debated. Genomic medicine has helped us gain a deeper understanding of this disease, some of which may serve as prognostic factors. Such genetic abnormalities could also help measure minimal residual disease (MRD) and provide additional clues to estimate the efficacy of chemotherapy. Combined with existing prognostic factors, these data can be used to construct a more accurate prognostic model, providing an optimal indication of allo-SCT for AML in CR1. Furthermore, overall treatment algorithms for high-risk AML after allo-SCT should include prophylactic and pre-emptive treatment to prevent relapse. These include immunotherapy using donor lymphocyte infusion (DLI), FLT3 inhibitors in FLT3-mutated AML, hypomethylating agents, or a combination of DLI with these agents. Clinical trials are currently ongoing to elucidate the role of these strategies, which will lead to a risk-adapted treatment for preventing relapse in high-risk AML. CD19-targeted chimeric antigen receptor (CAR) T-cell therapy induces a remarkable response in B-acute lymphoid leukemia (B-ALL); however, relapse remains a major problem. In this regard, allo-SCT as a consolidation treatment after CAR-T cell therapy for B-ALL is recommended for pediatric and adult patients. Achieving complete remission (CR) with CAR-T cell therapy is considered a promising bridging therapy to allo-SCT. Novel CAR-T treatment techniques are being developed to change their role as a pre-transplant treatment.
Introduction
Although allogeneic stem cell transplantation (allo-SCT) is an important therapeutic modality for acute leukemia, its outcomes remain unsatisfactory. Thus, many attempts are still being made to improve outcomes. The indication for allo-SCT is influenced by the outcome of allo-SCT and alternative treatments; however, accurately predicting prognosis by disease is the most important determinant. Improved prognostic accuracy by elucidating genomic abnormalities and minimal residual disease (MRD) detection techniques will change the indications for allo-SCT. Additionally, elucidation of the genomic pathophysiology has led to the development of many molecularly-targeted drugs. Unlike in the case of chronic myeloid leukemia (CML), molecularly-targeted drugs are not expected to cure the disease by themselves. However, they may effectively increase the proportion of patients induced into deep remission before allo-SCT and prevent relapse after transplantation. CAR-T therapy is recognized as a major cell therapy, along with hematopoietic stem cell transplantation, and is spreading rapidly. Moreover, new technologies are being developed successively, and the indications and outcomes of CAR-T therapy are predicted to improve in the future. Attempts are being made to combine these two cell therapies to improve the outcome of acute lymphoid leukemia (ALL) treatments, which is challenging to treat. In this article, we provide updates on three fascinating topics: changes in transplantation indications for acute myeloid leukemia (AML) 1st complete remission (CR1) patients owing to the introduction of genomics, post-transplant prophylactic strategies in AML, and allogeneic transplantation after CAR-T therapy for ALL.
Allo-SCT for AML in CR1 in the Era of Genomics
The optimal selection of post-remission treatment for AML in CR1 has remained a topic of debate since the advent of allo-SCT as an example of personalized treatment. Many studies have been conducted to address this question based on the availability of an HLA-matched donor. A meta-analysis showed the advantage of allogeneic transplantation in CR1 over chemotherapy in intermediate and poor prognostic groups1. However, with improvements in transplantation technology and refinement of HLA typing, the difference in outcomes between HLA-matched related and unrelated donors has narrowed2. Furthermore, the prognostic classification has improved dramatically. The evaluation of disease status after induction/consolidation has been refined with the advent of genomic testing, and attempts are being made to incorporate these results to select the optimal post-consolidation treatment. This section reviews the latest evidence on the indications for allo-SCT in AML CR1 in the genomic era.
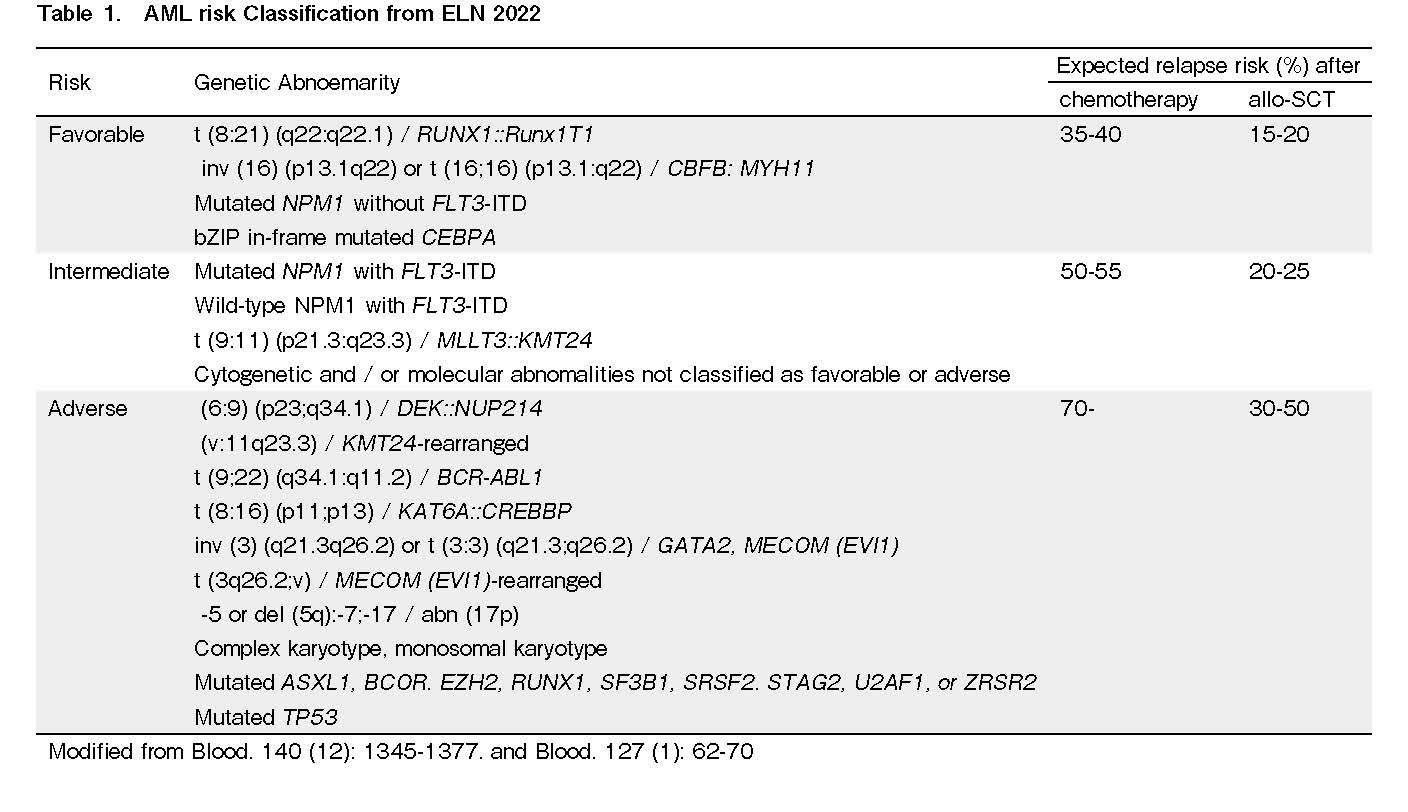
According to the European LeukemiaNet (ELN), favorable risk AML with core binding factor (CBF) translocations has not been considered a candidate for allo-SCT in CR1 based on their low relapse rate, especially after consolidation with high-dose cytarabine (Table 1). However, approximately 30% of the cases still relapse without allo-SCT, and several factors predictive of recurrence have been identified. For instance, the AML05 trial showed that patients with positive minimum residual disease (MRDpos) after the second consolidation had a better prognosis with allo-SCT, while those with MRDneg had a better prognosis with autologous transplantation or chemotherapy alone3. Furthermore, Zheng et al. reported that MRDpos cases with CBF leukemia after 2nd consolidation (n=69) had a better prognosis when treated with allo-SCT than those treated with chemotherapy alone4. Thus, MRD-based risk stratification after 2nd consolidation in CBF leukemia works well in determining the indication for allo-SCT. At diagnosis, the co-occurring mutational profile of leukemia also predicts relapse, with the KIT mutation being the most notorious for CBF leukemia. The Japanese group has revealed that KIT mutations have distinct impacts depending on the genetic background5. Specifically, KIT mutations had a negative impact on RUNX1::RUNX1T1 cases but not on CBFB::MYH11 cases. This negative impact on relapse-free survival (RFS) is explained by exon 17 mutations (including
In ELN intermediate-risk cases, the criteria for this risk group included FLT3-ITD irrespective of NPM1 mutation, MLLT3::KMT2A, and any other abnormalities that were not included in the favorable/adverse risk groups. Therefore, this class includes a wide variety of patients with heterogeneous genetic backgrounds. Overall, allo-SCT is recommended for this risk group based on the results of a meta-analysis1, but this kind of garbage-like class categorization inevitably entails the problem of whether it can be divided more precisely into subclasses based on risk. In this regard, the assessment of MRD markers and refinement of basic disease risk evaluation using genomic profiling may help identify a subset of this class that does not require upfront allo-SCT. Ahn et al. explored the role of MRD in AML CR1 patients with normal karyotype (n=124), which would significantly overlap with the ELN intermediate prognosis group6. They showed that allo-SCT recipients had significantly longer overall survival (OS) compared with those who received chemotherapy alone in the MRDpos group, while the outcome was almost the same for the MRDneg group, irrespective of allo-SCT. This result indicates that the MRDneg group might be used as a marker to identify patients who can spare allo-SCT among CR1 patients with normal karyotype AML. A clue to advanced personalized medicine was also provided by Gertung et al., who developed a model to predict individual patient prognosis (knowledge-based (KB) model) based on a large amount of data, including clinical information and genomics data8. Using this model, Fenwarth et al. analyzed the prognosis of 545 patients with AML in CR1 who were eligible for allo-SCT. They showed that the KB score had better prognostic power as measured by a higher concordance index than the ELN model and that patients in the good prognosis group (KB score ≥ 40) had a worse prognosis with allo-SCT than with chemotherapy alone9. Although the ELN intermediate group comprised only 30% of this cohort, the KB score-based index can help identify those who can spare allo-SCT in CR1.
Another aspect of personalized medicine is the optimization of SCT preparative regimens. The AML working party of the EBMTR, using data of 2,292 allo-SCT recipients, showed that the group with MRDpos and aged <50 years had better OS, leukemia-free survival (LFS), and lower relapse rate when conditioned with myeloablative conditioning (MAC) regimen compared with reduced-intensity conditioning (RIC)10. However, this was not the case in the over-50 age group or in the under-50 age group with MRDneg. This represents an example of the role of MRD in allo-SCT preparative regimen selection. In this risk group, autologous SCT (auto-SCT) is sometimes considered for patients ineligible for allo-SCT. Although several retrospective studies have suggested the advantage of auto-SCT over chemotherapy11, 12, prospective trials have failed to prove its superiority13.
In ELN adverse risk cases, allo-SCT is usually recommended whenever possible. In ELN 2022, the list of genetic alterations that assign AML cases to the adverse risk group expanded dramatically based on the clinical introduction of NGS-based molecular profiling. Molecular abnormalities included in this risk group are characterized by a substantial overlap with those observed in myelodysplastic syndromes (MDS)14. This finding reflects the poor prognosis of MDS-derived AMLs. However, it is noteworthy that this list includes gene mutations whose prognostic impact is distinct between MDS and AML. For example, SF3B1, which is almost always associated with a favorable prognosis in MDS, confers a poor prognosis in AML. More precisely, SF3B1 is no longer a favorable factor when co-occurring with other drivers or 5q deletions in MDS, and the fact that SF3B1 mutations rarely occur as a single abnormality in AML might account for its position as a poor prognostic factor. In ELN 2022, TP53 mutation is assumed to be an unfavorable factor, irrespective of allelic status (mono- or bi-allelic alteration). This is different from MDS, where mono-allelic TP53 mutations are completely distinct from bi-allelic mutations in terms of the profile of co-existing mutations, genomic stability, and prognosis15. In contrast, the clinical significance of mono-allelic TP53 has not been well characterized in AML, and it remains unclear whether the risk of mono-allelic TP53 mutations deserves allo-SCT in CR1. Patients with the germline variant of DDX41 also constitute a group of AML cases that should be reviewed for prognostic value. DDX41 is among the most common germline variants, accounting for 3-8% of MDS and AML cases. Patients with DDX41 germline variants often present with blast ratios of 10-30% and are diagnosed with either MDS excess blasts (MDS-EB) or AML-MDS-related changes (AML-MRC), both of which are regarded as unfavorable disease entities, and upfront allo-SCT is often considered. Additionally, the predisposing genetic risk is considered a factor by which allo-SCT should be considered. However, recent reports indicate that germline DDX41 variants are favorable prognostic factors and show a specific response to azacitidine treatment. This finding is more obvious in MDS than in AML; however, it should be urgently clarified for both MDS and AML whether this group can dispense with upfront allo-SCT as consolidation.
Allo-SCT in the elderly is often limited by high treatment-related mortality (TRM) and a lower remission rate. Improved remission induction with the introduction of molecularly-targeted drugs, expanded donor sources as represented by cord blood or haploidentical donors, and the prevalence of RIC regimens all paved the way for allo-SCT in the elderly. Although limited by selection bias due to adverse performance status and comorbidities, retrospective observational studies showed longer OS for patients who underwent allo-SCT compared to those without16, supporting allo-SCT for patients aged 60 to 75 years in CR1. This advantage was consistently observed irrespective of disease risk and MRD status; however, a low HCT-comorbidity index (HCT-CI) score remained an adverse factor predicting shorter OS. This underscores the importance of the critical assessment of patient-derived factors.
In conclusion, genetic profiling and MRD measurements allow for refined risk assessment and are useful for developing personalized medicine in AML CR1 cases.
Prophylactic/Pre-emptive Strategies in AML Post Allo Transplant. What Is Relevant for 2022?
Allo-SCT offers long-term survival or cure in high-risk AML patients. However, relapse remains a major barrier post allo-SCT. Approximately 40% of AML patients relapse post allo-SCT, and the 2-year survival post relapse remains less than 20%. Relapse-related risk factors can be disease-related (cytogenetic risk group, adverse molecular markers, CR status, MRD status both pre- and post-transplant) or transplant-related (use of RIC regimens, intense immunosuppression including T-cell depletion, stem cell source, and absence of chronic graft-versus-host disease (GVHD))17, 18.
Because of the dismal outcome in patients with AML who relapse post allo-SCT, either a prophylactic (without any evidence of disease relapse with 100% donor chimerism) or a pre-emptive (with disease detected at the MRD level or mixed chimerism or molecular relapse) strategy is preferred as a modality of intervention to achieve long-term remission in high-risk AML patients17, 18.
Immune intervention
Withdrawal of immunosuppression (IST) and donor lymphocyte infusion (DLI) are well-studied immune interventions for preventing relapse. Early withdrawal of IST reportedly prevents overt relapse. Both prophylactic and pre-emptive DLI have been shown to maintain long-term remission in post-transplant settings17, 18. Challenges with DLI have been associated with unpredictable severe GVHD in a few patients and the risk of pancytopenia.
Pre-emptive use of DLI based on bone marrow (BM) MRD status has been used by different investigators. Published data from China have shown that the use of pre-emptive DLI is associated with a reduction in relapse rate and improvement in disease-free survival (DFS). Although it was associated with GVHD development, there was no difference in the NRM rate between those who received DLI and those who did not. This study included related, MUD, and haploidentical patients and included patients who had persistent MRD after IL-2 therapy. It is important to use pre-emptive DLI early post allo-SCT if there is no evidence of GVHD and persistent MRD positivity17. There have been recommendations to repeat BM MRD at 3 months' timeline intervals and to intervene if MRD persists. However, this is an open question, as it depends on the molecular markers and the type of assay used to monitor MRD.
Prophylactic DLI has been mainly tested in T-cell-depleted (TCD) allo-SCT. The use of prophylactic DLI prior to D+100 is associated with a very high rate of GVHD. Preferably, prophylactic DLI is recommended for those with high-risk AML after day +120 if there is no evidence of GVHD and IST has been successfully discontinued 30 days prior to DLI. Prophylactic DLI has been shown to reduce the relapse rate and have more favorable long-term outcomes in several prospective studies. The dose of CD3+ cells needs to be tailored according to the type of donor (MRD vs. MUD vs. haploidentical), and dose escalation is recommended at 6-8 weeks intervals if there is no GVHD. Prophylactic DLI in high-risk AML settings has been shown to be associated with improved OS in a retrospective analysis by EBMT17, 18. Recent data have shown the efficacy and feasibility of concurrent use of DLI with low-dose immunosuppression to prevent severe GVHD.
FLT3 inhibitors
Approximately 30% of AML patients have FLT3-ITD mutations. AML patients with FLT3-ITD mutations have been associated with a higher relapse rate and are considered an indication for allo-SCT. Sorafenib is an oral FLT3 kinase inhibitor that has been shown to be efficacious post allo-SCT in patients with AML with FLT3 mutations. The randomized, double-blind, placebo-controlled SORMAIN trial used a dose of up to 400 mg bid sorafenib for FLT3-ITD positive patients prophylactically post allo-SCT. After a median follow-up of 42 months, the median RFS was 31 months in the placebo group and not reached in the sorafenib group. The two-year RFS was 53% vs. 85% (p=0.002)19. A phase III randomized trial using sorafenib at a dose of 400 mg bid for 6 months post allo-SCT in AML showed statistically significant 2-year RFS (85% vs. 53%), LFS (81% vs. 54%) and OS (83% vs. 72%)20. Acute Leukemia Working Party of the EBMT recently published the clinical practice recommendation on allo-SCT in AML patients with FLT3-ITD. The group recommends post-transplant maintenance with sorafenib in all cases except in patients with active acute GVHD. Sorafenib should be started as soon as possible after disease evaluation and MRD assessment at a dose of 400 mg daily in two divided doses, and the dose may be increased to 800 mg daily in case of positive MRD and for a minimum of 2 years, depending on tolerance21. Other FLT3 inhibitors used in this setting include midostaurin and gilteritinib. Randomized phase III trial data on gilteritinib were collected, and the final results are awaited. However, the optimal dose, duration of therapy, and risk-based stratification when using
Hypomethylating agents
Azacytidine (AZA) has been shown to expand circulating T-reg cells and upregulate the expression of tumor antigens in leukemic blasts, leading to an increased GVL effect without increasing GVHD. This makes it an ideal drug for maintenance therapy post allo-SCT in high-risk AML patients. AZA and decitabine have been tested in several prospective and retrospective studies as maintenance therapies to prevent relapse post allo-SCT. In phase 1 dose-finding study, the optimal dose of AZA in this setting was confirmed to be 32 mg/m2 administered for 5 consecutive days every 28 days. It has been tested in both prophylactic and pre-emptive conditions (based on the MRD status and donor chimerism level in CD34-positive cells). This has been found to be well tolerated without a significantly increased rate of GVHD. Recently, a group from MD Anderson Cancer Center reported the results of the first randomized controlled trial of AZA. In this study, 187 patients with high-risk AML or MDS who were in CR after allo-SCT received AZA (n = 93) or placebo (n = 94) at a dose of 32 mg/m2/day for 5 days for 12 months. However, most patients in the AZA arm (74.6%) did not receive the planned 12 cycles of treatment due to relapse, death, toxicity, or upon the patient's request. The investigators closed the study early because of slow accruals. RFS was comparable between both groups; however, stratification by the number of AZA cycles administered showed a trend toward improved RFS in patients receiving more AZA therapy cycles22. In addition to injectable AZA, an oral formulation of AZA (CC-486) has been tested in a phase 1/2 dose-finding study. A phase II randomized controlled trial (RCT) from China demonstrated that minimal-dose decitabine maintenance combined with recombinant human granulocyte colony-stimulating factor after allo-SCT could reduce relapse in high-risk AML patients undergoing allo-SCT, with a 2-year relapse rate of 15.0% and 38.3% in the intervention and non-intervention groups, respectively. Two-year LFS was 81.9% in the intervention group and 60.7% in the non-intervention group.
Combination of AZA and DLI
Epigenetic therapies, such as AZA, with immunotherapy, such as DLI, have been combined to prevent relapse post allo-SCT in high-risk AML patients. This combination is well tolerated in post-transplant settings. AZA is usually administered subcutaneously at a dose of 32 mg/m2/day for 5 days every 28 days with a combination of DLI, which is started after 1-3 cycles of AZA and 4 weeks after discontinuation of IST in prophylactic or pre-emptive treatment settings. Depending on the response and GVHD status, escalated DLI was administered at 8-week intervals. The cell dose was determined based on the type of stem cell transplant (sibling vs. unrelated vs. haplo). Using this algorithm, a French retrospective analysis showed that OS and PFS at 2 years were 70.8% and 68.3%, respectively, and a relapse rate of 22%23, 24.
Other ongoing trials in post-transplant settings
Other drugs in the post-transplant setting that are being evaluated include Bcl-2 inhibitor (venetoclax), HDAC inhibitor (panobinostat), IDH2 inhibitor (enasidenib), Hedgehog inhibitor (glasdegib), and TP53 (APR-246 with AZA)25, 26.
Conclusion
Relapse remains a major challenge post allo-SCT in high-risk patients with AML. The outcome of patients relapsing after allo-SCT remains poor. The MRD-based treatment algorithm is evolving, although it is not yet widely adopted. Prophylactic and pre-emptive strategies to prevent relapse should be part of the overall treatment algorithm in high-risk AML patients post allo-SCT. The options can be immunotherapy using donor lymphocyte infusion, FLT3 inhibitors in FLT3-mutation-positive AML patients, hypomethylating agents, or a combination of DLI with hypomethylating agents. Multiple promising drugs are being used in pipelines that are undergoing clinical trials. Risk stratification: Personalized therapy is the key to preventing relapse post allo-SCT in high-risk AML settings.
Consolidative Allogeneic SCT after CAR T-cell Therapy in ALL
CD19-targeted chimeric antigen receptor (CAR) T-cell therapy has demonstrated striking responses in B-cell acute lymphoblastic leukemia (B-ALL); however, approximately 40% of recipients experience relapse after CAR-T therapy. The long-term remission or cure rate of B-ALL after allo-SCT could improve if these two modalities are combined27. Evidence supporting the benefit of consolidative allogeneic SCT after CAR T-cell therapy is mounting. These include a study at the Fred Hutchinson Cancer Center and several studies in our institution. In the FRHCC study28, 45 of 53 patients achieved CR after CAR T-cell treatment and achieved MRD-negative status; in contrast, 22 patients relapsed early. Of the 45 MRD-negative responders, 18 underwent transplantations. Of these, 11 (61.1%) achieved CR, three (16.7%) relapsed, and four (22.2%) died because of transplant-related mortality. Of the 27 patients who did not undergo transplantation, 19 (70%) experienced a relapse. These data clearly indicate that consolidation with allotransplantation should be considered in patients achieving CR from CAR T-cell therapy, especially in high-risk patients. The risk factors associated with relapse after CAR-T cell therapy when considering consolidation allo-SCT are listed in Table 2.
In our study at Lu Daopei Center, 110 patients, who were consolidated by allo-SCT after CAR T-cell therapy, showed better LFS and OS than those receiving CAR T-cell therapy only29. Our experience suggested that allo transplant should be considered after the patient achieves MRD-negative CR from CAR T-cell treatment. In contrast, analyses of potential factors associated with poor response to and relapse after CAR T-cell therapy are limited. We also summarized the long-term follow-up results of 254 B-ALL patients treated with CD19 CAR-T cells from five clinical trials30. The analysis showed that TP53 mutations, bone marrow blasts > 20%, prior CAR-T/blinatumomab treatment, and severe cytokine release syndrome (CRS) were associated with a lower CR rate. However, age, extramedullary disease, complex cytogenetics, history of prior transplant, prior courses of chemotherapy, CAR-T cell dose, and the manufacturing source of cellular products did not affect the rate of achieving CR. Patients who underwent consolidative allo-SCT after CAR-T therapy had superior OS and LFS compared to those who did not. This benefit was also observed in pediatric and adult patients and in patients in either the high- or low-risk groups. Further studies to identify the factors associated with CR, LFS, and OS rates are warranted to maximize the clinical benefits of CAR T-cell therapy.
In addition, we compared the long-term outcomes of 33 patients with B-ALL who relapsed after allo-SCT and received CAR-T therapy with 23 patients who chose to undergo a second allo-SCT after achieving CR with CAR T-cell therapy31. Significant differences were observed in patients who received second transplantation following CAR T-cell therapy vs. those who received CAR T-cell therapy alone in OS and LFS (1-year OS 44.1% vs. 68.0%, 2-year OS 30.2% vs. 54.4%, p=0.016; 1-year DFS 32.9% vs. 68.0%, 2-year DFS 17.6% vs. 54.4%, p=0.002). Our study demonstrates that even for R/R B-ALL patients who relapsed after the first allo-SCT, MRD-negative CR status can still be achieved through CAR T-cell therapy without increasing CRS or neurotoxicity. CAR T-cell therapy followed by consolidation second allo-SCT may also be considered for young and fit patients.
Clinical outcomes and safety profiles were similar between patients who underwent CAR T-cell therapy and those who received chemotherapy before transplantation. In our study32, patients treated with CAR T-cell therapy developed more acute GVHD; however, the incidence of severe acute GVHD was the same between the two groups (approximately 12%). Similarly, the incidence of chronic GVHD was higher after CAR T-cell therapy than after chemotherapy; however, the incidence of severe chronic GVHD was 12%, which was not different between the two groups. Both approaches yielded similar 4-year leukemia-free and OS rates exceeding 70%, and achieving MRD-negative status was the most important factor associated with the best outcomes. However, a recent study that enrolled 74 patients33 showed that humanized CD19 CAR T-cells had a safety profile similar to other CD19 CARs but higher response and durable remission rates without further therapy in children and young adults with relapsed or refractory B-ALL. Importantly, durable remission was achieved in patients whose previous CAR T-cell therapy failed. A larger randomized trial is needed to further understand whether consolidative allo-SCT should be conducted after CAR T cell therapy.
We recently published the first-in-human study on a novel CD7 CAR T-cell therapy for T-cell malignancies34. Derivation of CD7-targeted CAR (7CAR) T cells often requires additional genetic manipulations to ablate the CD7 gene or block CD7 cell surface expression. We report a novel approach to derive naturally selected
In summary, consolidative allo-SCT after CAR T-cell therapy for patients with high-risk features, either pediatric or adult, is recommended. When bridging to allo-SCT, achieving MRD-negative CR or MRD-positive CR after CAR T-cell therapy is essential. Therefore, allo-SCT within 3 months of CAR-T therapy should be considered. Myeloablative or non-myeloablative conditioning regimens can be administered during allo-SCT. The role of consolidation allo-SCT should be continuously redefined with the development of novel CAR-T and combination therapies.
Discussion
This article focuses on three recent advances in allo-SCT for acute leukemia. Genomics-guided prognostication, advances in molecular-targeted therapy, and immuno-cell therapy are the mainstays of this literature, and these technologies are constantly changing the indications for allo-SCT. For example, improved outcomes with molecularly-targeted therapies and CAR-T therapy may spare non-high-risk cases from allo-SCTs. In contrast, these therapies might expand the indications for allo-SCT for very high-risk cases by allowing patients to receive allo-SCT under reasonable control of the disease who previously would not have lived long enough with allo-SCT. Therefore, we must continue to assess treatment strategies, including transplantation, together with the progress of novel diagnostic methods and nontransplant treatment modalities.
Author Contributions
YN wrote the abstract and introduction as well as the section on
Funding Statement
This work was supported by the Japan Agency for Medical Research and Development (AMED) (JP19ck0106353h0003 to Y.N.) and KAKENHI (JP18H02836 to Y.N.).
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website. AV is one of the Editor of Blood Cell Therapy. He was not involved in the editorial evaluation or accept this article for publication.
References
1.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009; 301: 2349-61.
2.Kanda J, Saji H, Fukuda T, Kobayashi T, Miyamura K, Eto T, et al. Related transplantation with HLA-1 Ag mismatch in the GVH direction and HLA-8/8 allele-matched unrelated transplantation: a nationwide retrospective study. Blood. 2012; 119: 2409-16.
3.Zhu HH, Zhang XH, Qin YZ, Liu DH, Jiang H, Chen H, et al. MRD-directed risk stratification treatment may improve outcomes of t(8;21) AML in the first complete remission: results from the AML05 multicenter trial. Blood. 2013; 121: 4056-62.
4.Zheng C, Zhu X, Tang B, Zhang L, Geng L, Liu H, et al. The impact of pre-transplant minimal residual disease on outcome of intensified myeloablative cord blood transplant for acute myeloid leukemia in first or second complete remission. Leuk Lymphoma. 2016; 57: 1398-405.
5.Ishikawa Y, Kawashima N, Atsuta Y, Sugiura I, Sawa M, Dobashi N, et al. Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11. Blood Adv. 2020; 4: 66-75.
6.Ahn JS, Kim T, Jung SH, Ahn SY, Jung SY, Song GY, et al. Allogeneic transplant can abrogate the risk of relapse in the patients of first remission acute myeloid leukemia with detectable measurable residual disease by next-generation sequencing. Bone Marrow Transplant. 2021; 56: 1159-70.
7.Schlenk RF, Pasquini MC, Pérez WS, Zhang MJ, Krauter J, Antin JH, et al. HLA-identical sibling allogeneic transplants versus chemotherapy in acute myelogenous leukemia with t(8;21) in first complete remission: collaborative study between the German AML Intergroup and CIBMTR. Biol Blood Marrow Transplant. 2008; 14: 187-96.
8.Gerstung M, Papaemmanuil E, Martincorena I, Bullinger L, Gaidzik VI, Paschka P, et al. Precision oncology for acute myeloid leukemia using a knowledge bank approach. Nat Genet. 2017; 49: 332-40.
9.Fenwarth L, Thomas X, de Botton S, Duployez N, Bourhis JH, Lesieur A, et al. A personalized approach to guide allogeneic stem cell transplantation in younger adults with acute myeloid leukemia. Blood. 2021; 137: 524-32.
10.Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socié G, Ljungman P, et al. Measurable residual disease, conditioning regimen intensity, and age predict outcome of allogeneic hematopoietic cell transplantation for acute myeloid leukemia in first remission: A registry analysis of 2292 patients by the Acute Leukemia Working Party European Society of Blood and Marrow Transplantation. Am J Hematol. 2018; 93: 1142-52.
11.Schlenk RF, Taskesen E, van Norden Y, Krauter J, Ganser A, Bullinger L, et al. The value of allogeneic and autologous hematopoietic stem cell transplantation in prognostically favorable acute myeloid leukemia with double mutant CEBPA. Blood. 2013; 122: 1576-82.
12.Pfirrmann M, Ehninger G, Thiede C, Bornhäuser M, Kramer M, Röllig C, et al. Prediction of post-remission survival in acute myeloid leukaemia: a post-hoc analysis of the AML96 trial. Lancet Oncol. 2012; 13: 207-14.
13.Vellenga E, van Putten W, Ossenkoppele GJ, Verdonck LF, Theobald M, Cornelissen JJ, et al. Autologous peripheral blood stem cell transplantation for acute myeloid leukemia. Blood. 2011; 118: 6037-42.
14.Bernard E, Tuechler H, Greenberg PL, Hasserjian RP, Ossa JEA, Nannya Y, et al. Molecular international prognostic scoring system for myelodysplastic syndromes. NEJM Evid. 2022; 1:.
15.Bernard E, Nannya Y, Hasserjian RP, Devlin SM, Tuechler H, Medina-Martinez JS, et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat Med. 2020; 26: 1549-56.
16.Russell NH, Hills RK, Thomas A, Thomas I, Kjeldsen L, Dennis M, et al. Outcomes of older patients aged 60 to 70 years undergoing reduced intensity transplant for acute myeloblastic leukemia: results of the NCRI acute myeloid leukemia 16 trial. Haematologica. 2022; 107: 1518-27.
17.Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood. 2012; 119: 3256-62.
18.Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML after hematopoietic stem cell transplantation: methods of monitoring and preventive strategies. A review from the ALWP of the EBMT. Bone Marrow Transplant. 2016; 51: 1431-8.
19.Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib maintenance after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with FLT3-internal tandem duplication mutation (SORMAIN). J Clin Oncol. 2020; 38: 2993-3002.
20.Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol. 2020; 21: 1201-12.
21.Bazarbachi A, Bug G, Baron F, Brissot E, Ciceri F, Dalle IA, et al. Clinical practice recommendation on hematopoietic stem cell transplantation for acute myeloid leukemia patients with FLT3-internal tandem duplication: a position statement from the Acute Leukemia Working Party of the European Society for Blood and Marrow. Haematologica. 2020; 105: 1507-16.
22.Oran B, de Lima M, Garcia-Manero G, Thall PF, Lin R, Alousi AM, et al. Maintenance with 5-azacytidine for acute myeloid leukemia and myelodysplastic syn-drome patients. Blood. 2018; 132(Suppl 1): 971.
23.Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective phase II study of prophylactic low dose azacitidine and donor lymphocyte infusions following allogeneic hematopoietic stem cell trans- plantation for high-risk acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2019; 54: 1815-26.
24.Assi R, Masri N, Abou Dalle I, El-Cheikh J, Bazarbachi A. Post-transplant maintenance therapy for patients with acute myeloid leukemia: Current Approaches and the Need for More Trials. J Blood Med. 2021; 12: 21-32.
25.Xuan L, Liu Q. Maintenance therapy in acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. J Hematol Oncol. 2021; 14: 4.
26.Antar AI, Otrock ZK, Abou Dalle I, El-Cheikh J, Bazarbachi A. Pharmacologic therapies to prevent relapse of acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Front Oncol. 2020; 10: 596134.
27.Jiang H, Hu Y, Mei H. Consolidative allogeneic hematopoietic stem cell transplantation after chimeric antigen receptor T-cell therapy for relapsed/refractory B-cell acute lymphoblastic leukemia: who? When? Why?. Biomark Res. 2020; 8: 66.
28.Hay KA, Gauthier J, Hirayama AV, Voutsinas JM, Wu Q, Li D, et al. Factors associated with durable EFS in adult B-cell ALL patients achieving MRD-negative CR after CD19 CAR T-cell therapy. Blood. 2019; 133: 1652-63.
29.Zhang X, Lu XA, Yang J, Zhang G, Li J, Song L, et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020; 4: 2325-38.
30.Zhang X, Yang J, Li J, Li W, Song D, Lu XA, et al. Factors associated with treatment response to CD19 CAR-T therapy among a large cohort of B cell acute lymphoblastic leukemia. Cancer Immunol Immunother. 2022; 71: 689-703.
31.Zhang JP, Yang JF, Zhang X, Li JJ, Cao XY, Zhao YL, et al. Significant long-term benefits of CAR T-Cell therapy followed by a second Allo-HSCT for relapsed/refractory (R/R) B-cell acute lymphoblastic leukemia (B-ALL) patients who relapsed after an initial transplant. Blood. 2020; 136 (Suppl 1): 40-1.
32.Zhao YL, Liu DY, Sun RJ, Zhang JP, Zhou JR, Wei ZJ, et al. Integrating CAR T-cell therapy and transplantation: comparisons of safety and long-term efficacy of allogeneic hematopoietic stem cell transplantation after CAR T-cell or chemotherapy-based complete remission in B-cell acute lymphoblastic leukemia. Front Immunol. 2021; 12: 605766.
33.Myers RM, Li Y, Barz Leahy A, Barrett DM, Teachey DT, Callahan C, et al. Humanized CD19-targeted chimeric antigen receptor (CAR) T cells in CAR-naive and CAR-exposed children and young adults with relapsed or refractory acute lymphoblastic leukemia. J Clin Oncol. 2021; 39: 3044-55.
34.Lu P, Liu Y, Yang J, Zhang X, Yang X, Wang H, et al. Naturally selected CD7 CAR-T therapy without genetic manipulations for T-ALL/LBL: First-in-human Phase I Clinical Trial. Blood. 2022; blood.2021014498.
Search
News