Volume 6 (2023) Issue 3 No.1 Pages 66-71
Abstract
The preferred choice for hematopoietic cell transplantation (HCT) donors in India is a matched related donor (MRD) followed by a haploidentical (haplo) donor for patients with hematological malignancies. International data in the haplo-HCT setting is mainly using bone marrow as a source. Almost all HCTs in India use peripheral blood stem cells (PBSC), which increases the risk of graft-versus-host disease (GVHD). In this single-center prospective study from 2017 to 2021, we sought to compare these outcomes prospectively in adult patients with hematological malignancies. Patient, disease, donor, and HCT details were prospectively recorded. GVHD prophylaxis included cyclosporine + methotrexate in MRD-HCT and post-transplant cyclophosphamide (PTCy) based in haplo-HCT. The primary endpoint GVHD relapse-free survival (GRFS) was defined as the time post-HCT without any of the following events: grade III-IV acute GVHD, chronic GVHD requiring systemic immunosuppressive treatment, disease relapse, or death from any cause. A total of 41 MRD and 33 haplo-HCT recipients were included in the study. Both cohorts were matched for age, sex, diagnosis, disease risk index, donor age, sex and CMV mismatches, and CD34 counts. A lower proportion of MRD-HCT recipients than haplo-HCT received myeloablative conditioning (39% vs. 76%, p = 0.002). There was no difference in the cumulative incidence of grade III-IV acute GVHD (16% vs. 27%, p = 0.2) or moderate-to-severe chronic GVHD (58% vs. 71%, p = 0.5). The one-year GRFS was not significantly different (53% vs. 38%, p = 0.2), with median GRFS of 420 and 274 days. The relapse incidence (22% vs. 19%, p = 0.6) and non-relapse mortality (25% vs. 35%, p = 0.4) did not differ. There was no difference in overall survival at one year (60% vs. 52%, p = 0.3). Despite a higher proportion of myeloablative conditioning in the haplo-HCT cohort, all outcomes, including GRFS, were comparable to those of the MRD-HCT cohort. This should encourage patients without an MRD to undergo haplo-HCT.
Introduction
Haploidentical (haplo) hematopoietic cell transplantation (HCT) using peripheral blood stem cells (PBSC) and post-transplant cyclophosphamide (PTCy) is the preferred alternative donor transplant in the absence of a fully matched related donor (MRD) HCT for patients with hematological malignancies in India. MRD includes matched sibling donors (MSD) and an ~10% probability of a full match with parents/children1. Haplo-HCT is preferred over matched unrelated donor (MUD)-HCT because of the low likelihood of full matching in local registries and the high costs. In addition, data showing comparable outcomes between MRD-, MUD-, and haplo-HCT have increased confidence in offering haplo-HCT when MRD is not available2, 3. However, most international data use bone marrow as a source, as it has a lower risk of chronic GVHD4. Our preliminary experience with PBSC haplo-HCT using PTCy revealed a high incidence of chronic GVHD5. Therefore, GVHD-relapse-free survival (GRFS), which reflects the true success of HCT as survival without ongoing morbidity/mortality, would be necessary for this setting. As no data are available comparing outcomes after PBSC haplo-HCT versus MRD in India, we sought to compare these outcomes prospectively in this study.
Methods
This single-center, prospective study enrolled all consecutive patients aged ≥ 12 years with hematological malignancies undergoing either MRD or haplo-HCT using PBSC between 2017 and 2021. The intensity of the conditioning regimen was based on the patient's age, comorbidities, and organ function according to the institutional criteria. Myeloablative conditioning regimens included busulfan (12.8 mg/kg)-based regimens for myeloid malignancies, and TBI (12 Gy)-based regimens for lymphoid malignancies. Reduced-intensity regimens (RIC) included fludarabine-melphalan 140 mg/m2 for MRD-HCT and the Johns Hopkins regimen fludarabine-cyclophosphamide-TBI (200 cGy) for haplo-HCT. GVHD prophylaxis included cyclosporine (CSA) + methotrexate for MRD-HCT and PTCy + CSA + mycophenolate for haplo-HCT. Immunosuppressive therapy was tapered and stopped between day +60 and day
Results
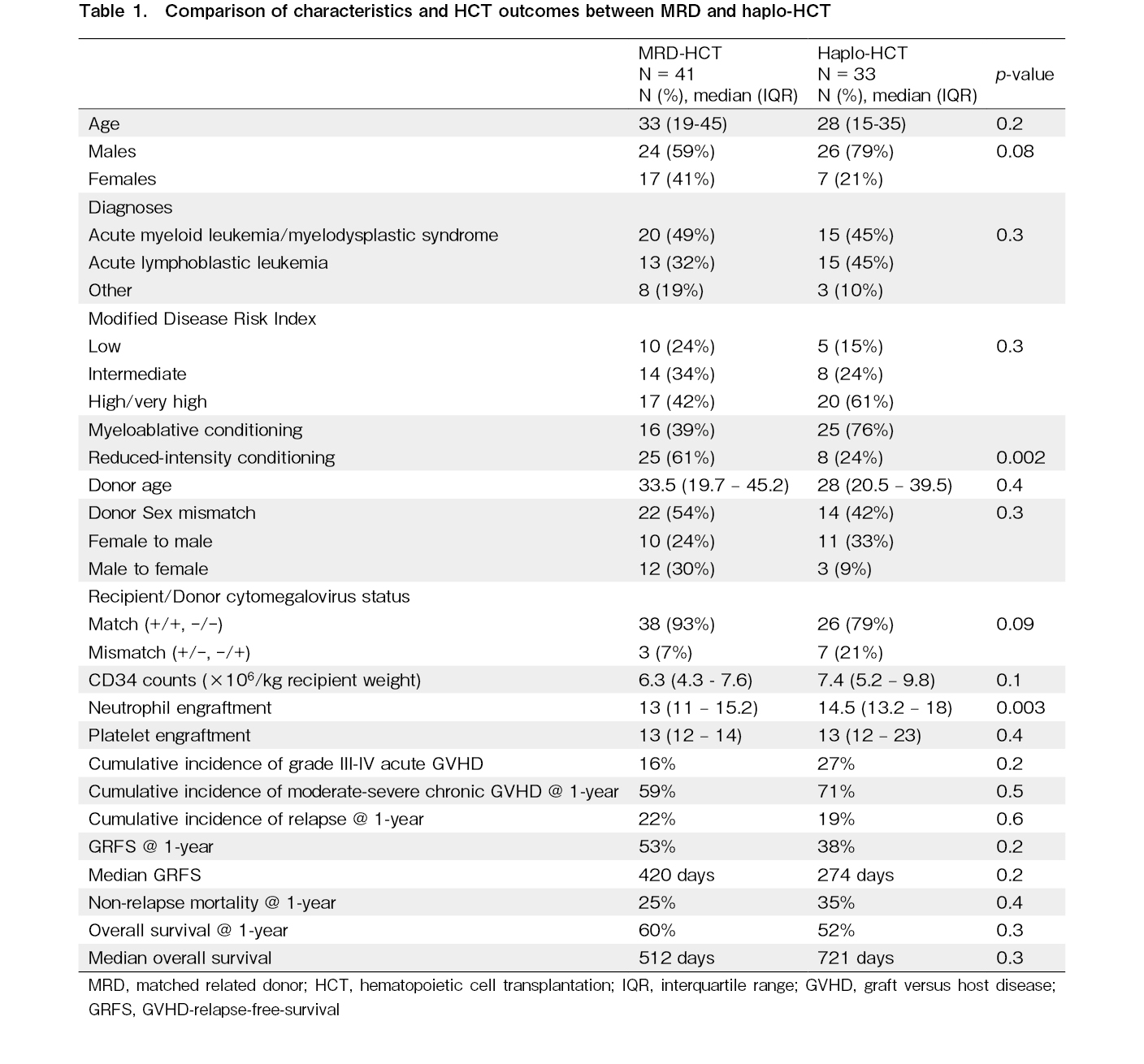
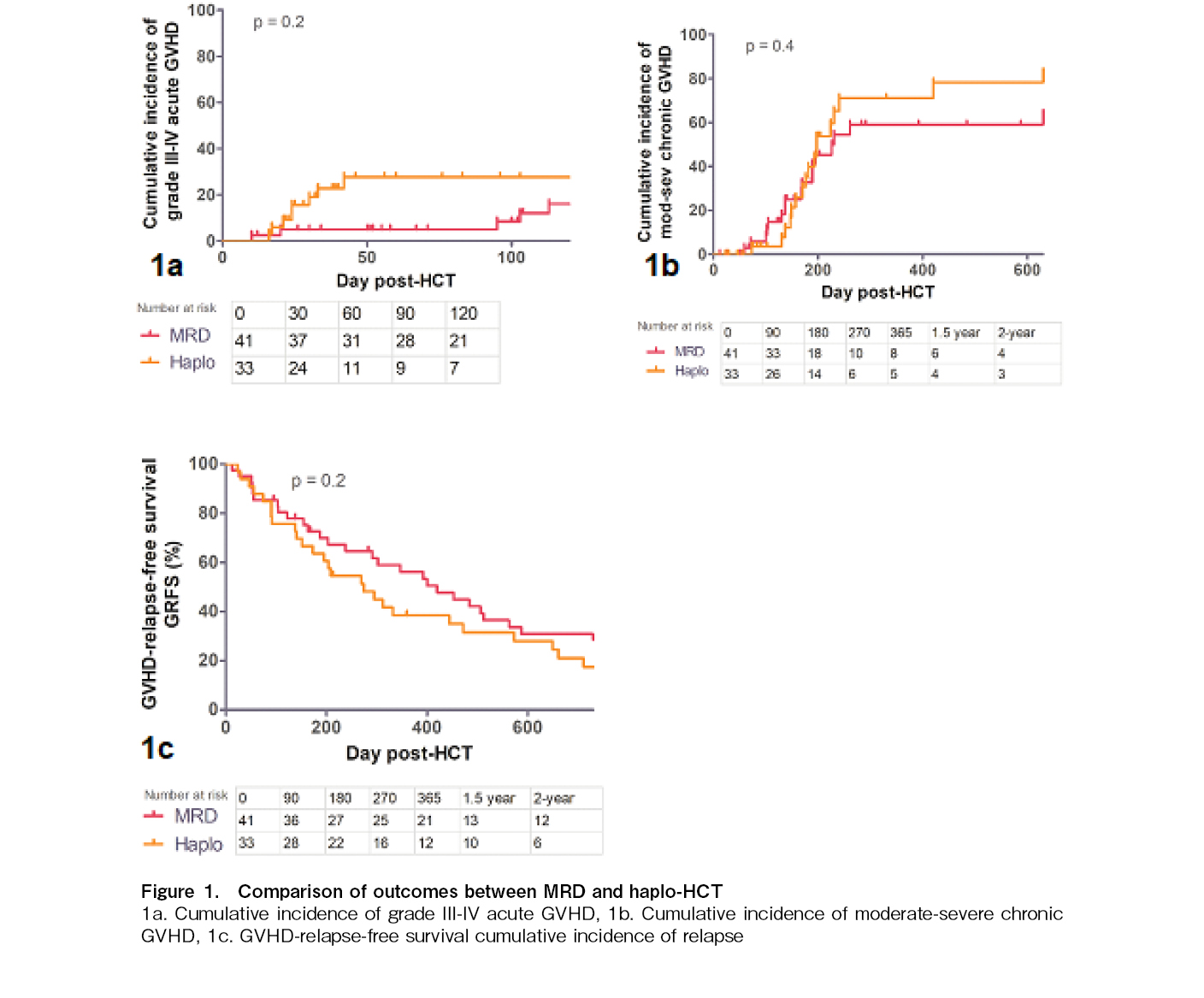
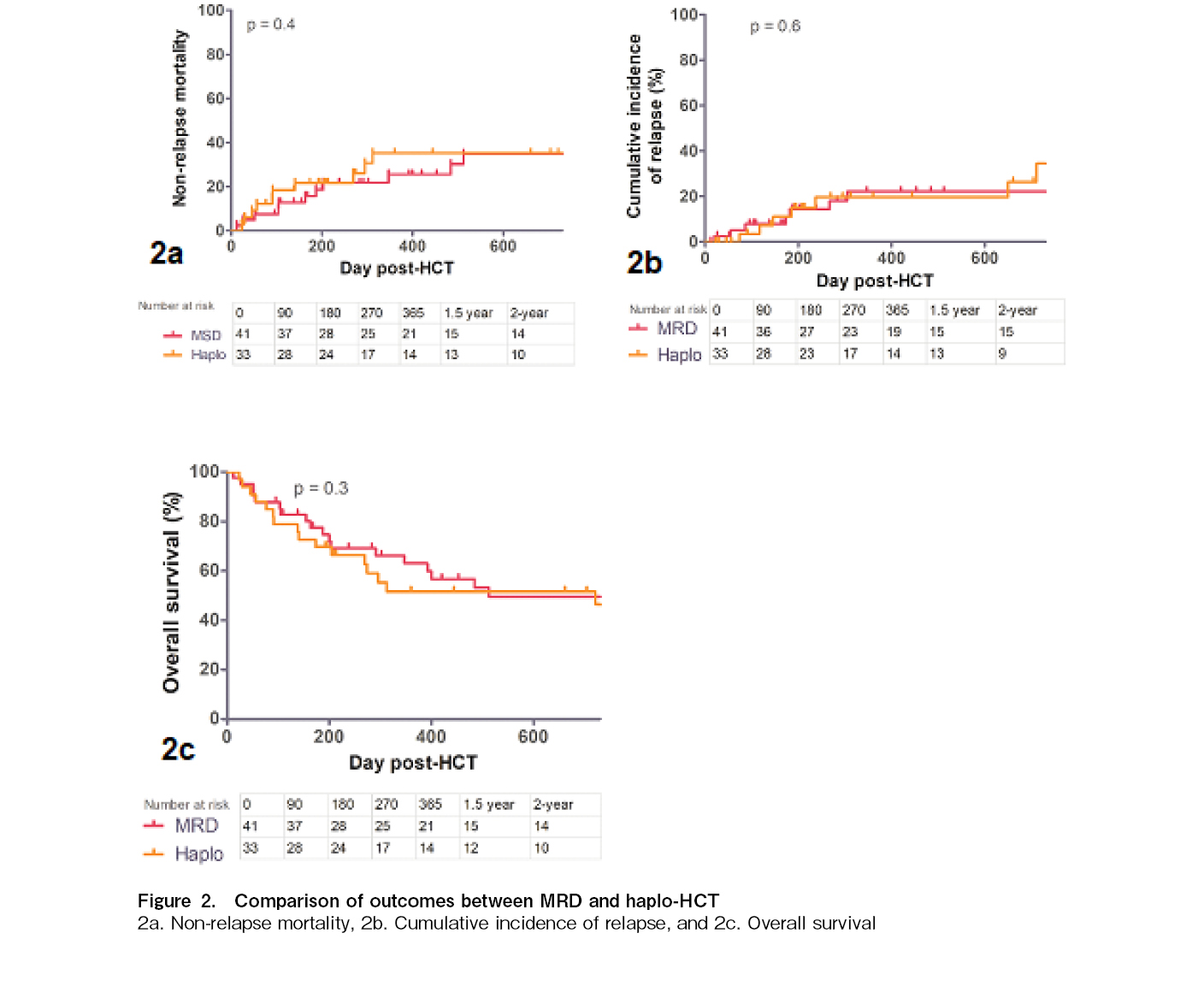
A total of 41 MRD and 33 haplo-HCT recipients were included in the study. The median follow-up in both cohorts was 347 days (IQR 146-937) and 274 days (IQR 139-1,036), respectively, (p = 0.8). The median ages were 33 years (IQR 19-45 years) and 28 years (IQR 15-35 years) (p = 0.2) (Table 1). Male patients were predominant in both cohorts (59% vs. 79%, p = 0.08). An equal proportion of patients had acute myeloid leukemia/myelodysplastic syndrome (AML/MDS) (49% vs. 45%, p = 0.3) and acute lymphoblastic leukemia (ALL) (32% vs. 45%). The disease risk index was also matched between both cohorts: low-risk (24% vs. 15%), intermediate-risk (34% vs. 24%), and high/very high-risk in 42% and 61% (p = 0.3), respectively. A lower proportion of MRD-HCT recipients received the MAC regimen than did the haplo-HCT (39% vs. 76%, p = 0.002). The median donor ages of 33.5 years (19.7-45.2) vs. 28 years (20.5-39.5) were comparable in both cohorts (p = 0.4). Both cohorts had similar proportions of recipient-donor gender-mismatch transplants (54% vs. 42%, p = 0.3) and CMV-matched transplants (93% vs. 79%, p = 0.09). The median CD34 counts were similar despite capping in the MRD-HCT cohort (6.3 × 106/kg vs. 7.4 × 106/kg, p = 0.1). Neutrophil engraftment was delayed in the haplo-HCT cohort by a median of 1.5 days (13 days vs. 14.5 days, p = 0.003). There was no difference in platelet engraftment between the cohorts (13 vs. 13 days, p = 0.4). The cumulative incidence of grade III-IV acute GVHD at day+100 (16% vs. 27%, p = 0.2) (Figure 1a) as well as moderate-to-severe chronic GVHD (58% vs. 71%, p = 0.5) (Figure 1b) at 1-year post-HCT was not significantly different between the cohorts. The primary endpoint GRFS was not significantly different (53% vs. 38%, p = 0.2), with median GRFS of 420 and 274 days, respectively (Figure 1c). The NRMs at one year were 25% and 35%, respectively (p = 0.4) (Figure 2a). The cumulative incidences of relapse at one year were 22% and 19%, respectively (p = 0.6) (Figure 2b). There was no difference in the overall survival at one year (60% vs. 52%, p = 0.3), with median OS of 512 and 721 days, respectively (Figure 2c).
Discussion
Registry studies from both EBMT and CIBMTR have reported similar survival outcomes with MRD and unmanipulated haplo-HCT for AML11, 12 and ALL13, 14. These studies showed a higher risk of acute GVHD balanced by a lower risk of chronic GVHD with PTCy haplo-HCT. Our study had a higher incidence of moderate-to-severe chronic GVHD, mostly related to the use of PBSC, and a higher proportion of patients receiving MAC regimens in the haplo-HCT cohort. While overall survival is the true success measure of HCT, patients often feel deceived when they have to experience chronic issues of GVHD, as it significantly affects their quality of life. Despite this, our cohort's one-year GRFS of 53% with PBSC MRD and 38% with haplo-HCT were not significantly different. The two-year GRFS after MRD and haplo-HCT in the EBMT studies using predominantly bone marrow and RIC regimens were 50% vs. 47%, respectively, in the AML cohort11 and 39% vs. 40%, respectively, in the ALL cohort13. There is a need to delay immunosuppression discontinuation using a risk-model-based clinical application in haplo-HCT to balance the higher risk of chronic GVHD with PBSC. Early conventional tapering of immunosuppressive therapy around day +60 to day
Author Contributions
DPL and PC conceived the study. PC, KSK, and DPL analyzed the data and drafted the manuscript. All authors were involved in patient recruitment, clinical care, and manuscript writing. DPL and KSK confirmed full access to the data in the study and the final responsibility for the manuscript.
Financial Support
This work was performed as a part of the American Society of Hematology Global Research Award to DPL.
Ethics Approval
This study was approved by the Postgraduate Institute of Medical Education and Research Institutional Ethics Committee (IEC) letter no. INT/IEC/2021/SPL-982.
Informed Consent
Informed consent was obtained from all participants.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Balcı YI, Tavil B, Tan CS, Ozgur TT, Bulum B, Cetin M, et al. Increased availability of family donors for hematopoietic stem cell transplantation in a population with increased incidence of consanguinity. Clin Transplant. 2011; 25: 475-80.
2.Meybodi MA, Cao W, Luznik L, Bashey A, Zhang X, Romee R, et al. HLA-haploidentical vs matched-sibling hematopoietic cell transplantation: a systematic review and meta-analysis. Blood Adv. 2019; 3: 2581-5.
3.Gagelmann N, Bacigalupo A, Rambaldi A, Hoelzer D, Halter J, Sanz J, et al. Haploidentical stem cell transplantation with posttransplant cyclophosphamide therapy vs other donor transplantations in adults with hematologic cancers: a systematic review and meta-analysis. JAMA Oncol. 2019; 5: 1739-48.
4.Im A, Rashidi A, Wang T, Hemmer M, MacMillan ML, Pidala J, et al. Risk factors for graft-versus-host disease in haploidentical hematopoietic cell transplantation using post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2020; 26:1459-68.
5.Kasudhan KS, Patil AN, Jandial A, Khadwal A, Prakash G, Jain A, et al. Post-transplant cyclophosphamide pharmacokinetics and haploidentical hematopoietic cell transplantation outcomes: an exploratory study. Leuk Lymphoma. 2022; 63: 2679-85.
6.Maffini E, Labopin M, Blaise D, Ciceri F, Gülbas Z, Deconinck E, et al. CD34+ cell dose effects on clinical outcomes after T-cell replete haploidentical allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia using peripheral blood stem cells. A study from the acute leukemia working Party of the European Society for blood and marrow transplantation (EBMT). Am J Hematol. 2020; 95: 892-9.
7.Harris AC, Young R, Devine S, Hogan WJ, Ayuk F, Bunworasate U, et al. International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the mount sinai acute gvhd international consortium. Biol Blood Marrow Transplant. 2016; 22: 4-10.
8.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21: 389-401.e1.
9.Schoemans H, Goris K, Durm RV, Vanhoof J, Wolff D, Greinix H, et al. Development, preliminary usability and accuracy testing of the EBMT ‘eGVHD App' to support GvHD assessment according to NIH criteria-a proof of concept. Bone Marrow Transplant. 2016; 51: 1062-5.
10.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015; 125: 1333-8.
11.Salvatore D, Labopin M, Ruggeri A, Battipaglia G, Ghavamzadeh A, Ciceri F, et al. Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2018; 103: 1317-28.
12.Rashidi A, Hamadani M, Zhang MJ, Wang HL, Abdel-Azim H, Aljurf M, et al. Outcomes of haploidentical vs matched sibling transplantation for acute myeloid leukemia in first complete remission. Blood Adv. 2019; 3: 1826-36.
13.Nagler A, Labopin M, Houhou M, Aljurf M, Mousavi A, Hamladji RM, et al. Outcome of haploidentical versus matched sibling donors in hematopoietic stem cell transplantation for adult patients with acute lymphoblastic leukemia: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. J Hematol Oncol. 2021; 14: 53.
14.Wieduwilt MJ, Metheny L, Zhang MJ, Wang HL, Estrada-Merly N, Marks DI, et al. Haploidentical vs sibling, unrelated, or cord blood hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood Adv. 2022; 6: 339-57.
15.Pidala J, Martens M, Anasetti C, Carreras J, Horowitz M, Lee SJ, et al. Factors associated with successful discontinuation of immune suppression after allogeneic hematopoietic cell transplantation. JAMA Oncol. 2020; 6: e192974.
Search
News