Volume 5 (2022) Special Edition No.3 Pages S15-S24
Abstract
A variety of cellular therapies including hematopoietic cell transplantation (HCT) hold the promise to treat medical conditions and diseases that currently have limited or no effective therapeutic options. A number of cellular therapies other than HCT, such as CAR T-cell therapy, are currently in preclinical and clinical development and the field is rapidly growing. The current activity of cellular therapies, including HCT, in the clinical setting are summarized in this article. Collaborative efforts from all relevant professionals and organizations will be of great importance to overcome substantial challenges in clinical development and post-launch evidence collection of cellular therapies. Harmonization among decision-makers also plays a critical role in reinforcing consistency and improving efficiencies of the regulatory and health technology assessment process. For the long-term safety follow-up of patients undergoing cellular therapies, registries for HCT are able to manage the complexity of data and in the best position to introduce and monitor future innovative cellular therapies for a variety of hematological disorders.
Introduction
Hematopoietic cell transplantation (HCT) has been performed for the treatment of a variety of hematological malignancies, and is now recognized as the most successful cellular therapy. A number of cell therapies other than HCT such as CAR T-cell therapy are currently in preclinical and clinical development and the field is rapidly growing. This rapid growth in research and development of cellular therapies has spurred regulatory agencies to develop a regulatory framework to facilitating accelerated marketing authorizations for those therapy to satisfy unmet medical need. We herein report the current global activity of HCT and the activity of cellular therapy in Europe, North America, and Asia-pacific regions together with the regulatory flamework in those three regions.
Activity and Regulation of Cellular Therapies in Asia-Pacific (AP) Region
As of April 2022, twenty-two countries/regions are participating in APBMT. Among those countries/regions hematopoietic cell transplantation (HCT) and other cellular therapies are actively performed; however, the activity in each country/region varies significantly. Regarding HCT, the access rate exceeds 75% in one quarter of countries/regions, but the rate is less than 25% in 9 countries, and less than 10% in 2 countries/regions. Our recent survey on the activity of CAR T-cell therapy showed that the therapy is currently available only in 9 out of 19 countries/regions. Among those, the therapy is available as clinical trial/clinical practice in 5 countries/regions, and as clinical trial only in 4 countries/regions.
The major sites performing CAR T-cell therapy are HCT centers in all countries/regions, but in about 20% of countries/regions the therapy is also available in the hematology or medical oncology service. Regarding commercially available CAR-T cells, Kymriah (tisagenlecleu-cel) and Yescarta (axicabtagene ciloleucel) has been approved in the Asia-Pacific countries of Australia and Japan over the past 3 years. Zolgensma (onasemnogene abeparvovec) was approved in Japan in March 2020. As of 2021 CAR T-cells have been approved in South Korea (Kimmriah and Zolgensma), China Hong Kong (Kymriah), Japan (Breyanzi), China (Yescarta), and Singapore (Kymriah). Another survey conducted by the National Cancer Center in Japan revealed that the other cellular immunotherapies, including antigen-specific T-cell therapy, are also performed as clinical trials/clinical practice in our regions. Although the factors that impede the access to CAR T-cell therapy and other cellular therapies remain to be elucidated, it is likely that some of the factors impeding the access to CAR T-cells are similar to those in HCT. Those include financial constrain of patients, inadequate funding from government, inadequate number of sites performing the therapy, inadequate laboratory infrastructure support, and poor public awareness.
Cellular therapy products are an emerging medical product class undergoing rapid scientific and clinical innovation worldwide. These products pose unique regulatory challenges both for countries with existing regulatory frameworks and for countries where regulatory frameworks for cellular therapy products are under development. This rapid growth in research and development of advanced therapies in our region has spurred regulatory agencies to develop a regulatory framework for facilitating accelerated marketing authorizations for advanced therapies to meet unmet medical needs.
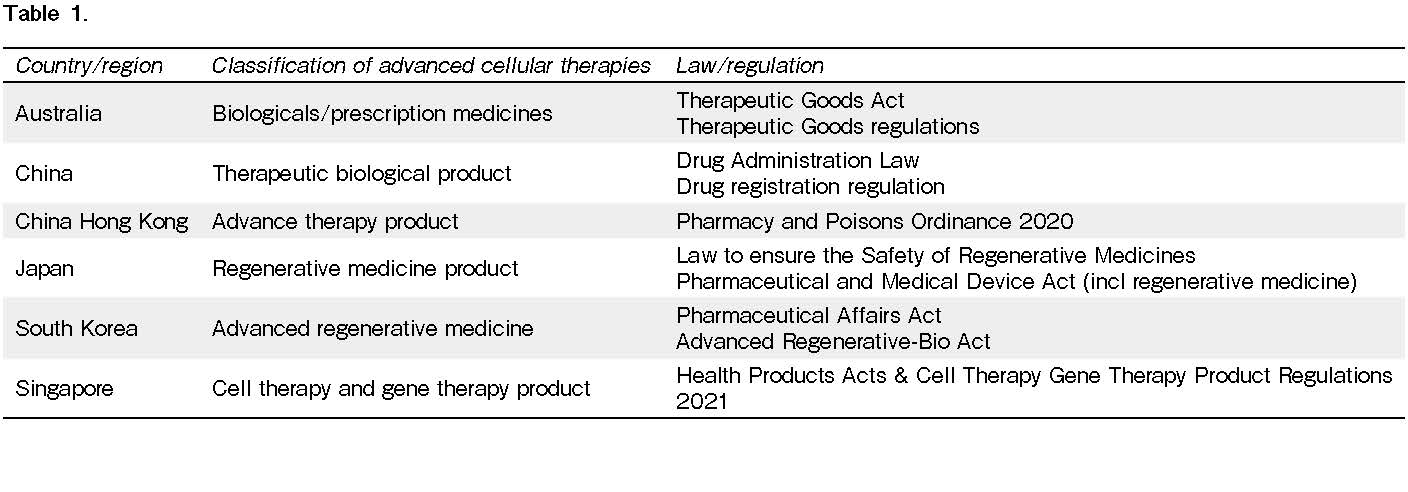
In Asia-Pacific regions, the regulatory environment is also heterogeneous, with varying levels of maturity and capacity across the national healthcare and healthcare products regulatory systems. Not unexpectedly, regulatory oversight of advanced therapies reflects this heterogeneity. Table 1 summarizes the current classification of advanced cellular therapies and its related regulation/law for Advanced therapies in 7 countries in Asia-Pacific region1.
In Australia, human cell and tissue therapy products are classified as biologicals and CAR T-cells are classified as Class 4 biologicals (high-risk products) that are more than minimally manipulated to artificially introduce a function of the cells or tissues that are not intrinsic to the original cells or tissues2.
In China, the National Medical Products Administration (NMPA) is the regulatory agency that provides the regulatory oversight of advanced therapies. In 2020, the NMPA published the New Drug Registration Regulation, which established the four expedited review pathways for drugs used in serious life-threatening diseases to fulfill local unmet medical need. Most advanced therapies would qualify for these expedited pathways, which allow for quicker research and development and eventually faster product approvals3.
In 2020, the South Korea Ministry of Health and Welfare (MoHW) brought the new Advanced Regenerative-Bio Act into effect to improve access to advanced regenerative medicine and the advanced biopharmaceuticals products. In this new framework, MoHW has regulatory oversight of medical practice and clinical research use of regenerative medicine, whereas the Ministry of Food and Drug Safety (MFDS) is overseeing the commercial use and manufacturing of cell and gene therapy4.
In Singapore, advanced therapeutics are known as cell, tissue, and gene therapy products (CTGTPs) and are regulated by the Health Sciences Authority (HSA). The Health Products Act (HPA) and Health Products (Cell, Tissue and Gene Therapy Products) Regulations, which came in effect on 1 March 2021, provide the main regulatory framework for CTGTPs5.
In Japan, there are two main laws regulating regenerative medical technology and products are the Act on the Safety of Regenerative Medicine and the Act on Pharmaceuticals and Medical Devices (PMD). The law on the safety of regenerative medicine provides regulatory oversight on all medical technologies using processed cells in medical care or academic research purpose, whereas the PMD Act regulates the marketing authorization and commercialization of a regenerative medicinal product. Under this framework, regenerative medical products include both cellular and tissue-based products to be used in the reconstruction, repair, or formation of structures or functions of the human body and the treatment or prevention of human diseases, and gene therapy6.
It is crucial to harmonize the activity and data collection between HCT and a variety of non-HCT cellular therapies especially in the field of HCT. New cellular therapies should be considered as a complement and asset rather than as a competitor of HCT. However, some regulations antagonizes this concept. In Japan, the CAR T-cells are considered as regenerative medicine (RM) product and the clinical data of CAR T-cell therapy should be submitted to the registry of regenerative medicine by the act for regenerative medicine. However, for best positioning of CAR T-cells, its clinical data should be in the HCT outcome registry. The Japanese data center for HCT (JDCHCT) also collates and shares the data with the Center for International Blood and Marrow Transplant Research (CIBMTR) to benchmark local outcomes with international data. With this system we can also share the data with the RM registry and pharmaceuticals for their PMS report.
To conclude, multi-stakeholder collaboration is paramount to strengthen the research and development capacity and promote early patients access while ensuring that varying interests and expectations of all relevant stakeholders have been sufficiently balanced. Harmonization among decision-makers on the other hand, plays critical roles in reenforcing consistency and improving efficiencies of the regulatory process. Both elements need to be emphasized to achieve timely patient access and to realize the potential of innovative cellular therapies.
Activity and Regulation of Cellular Therapies in North America
Genetically modified autologous T cells targeting cell surface antigens such as CD19 or B cell maturation antigen (BCMA) have emerged as a promising therapies for patients with advanced hematologic malignancies. These therapies genetically reprogram a patient's T cells by inserting a segment of DNA that encodes a chimeric antigen receptor (CAR), which includes an extracellular or
Since 2017, several CAR T-cell therapies have been approved for patients with advanced hematologic malignances in the U.S. Current approved CAR T-cells target either CD19, present on most B cell malignancies, or BCMA, present on myeloma cells. Tisagenlecleucel (tisa-cel, Novartis) was 1st approved in pediatric and young adult patients (< 26 years) with relapsed/refractory acute lymphoblastic leukemia (ALL)10. In the pivotal trial, the overall remission rate was 81%. Event-free survival (EFS) and overall survival (OS) were 73% (95% CI, 60 to 82) and 90% (95% CI, 81 to 95), respectively, at 6 months and 50% (95% CI, 35 to 64) and 76% (95% CI, 63 to 86) at 12 months. Three CAR T-cell products are approved for relapsed/refractory aggressive large B cell lymphomas after two lines of therapy: axicabtagene ciloleucel (axi-cel, Kite/Gilead), tisa-cel and lisocabtagene maraleucel (liso-cel, Bristol Myers Squibb)11–13. In the ZUMA-1 phase 2 study, the objective response rate with axi-cel, a retroviral-based CD19-CD28 CAR T-cell product, was 82%, with a complete response (CR) rate 54%, and median duration of remission 11.1 months among 101 evaluable patients. In the phase 2 JULIET study, which investigated tisa-cel, a lentiviral-based CD19-4-1BB CAR T-cell product, the best overall response rate (ORR) was 52%, with a CR rate 40%, and median duration of remission not reached. Long-term follow-up of both trials showed sustained durable remission, indicating potential cure after CAR T-cell therapy14, 15. Liso-cel was approved for patients with relapsed/refractory LBCL in early 2021 based on the result of the phase 1b TRANSCEND NHL-001 trial16. In addition, axi-cel and liso-cel were recently approved in second line following the results of large prospective phase 3 randomized clinical trials17–19. Axi-cel is also approved in advanced follicular lymphoma (FL)20. Brexucabtagene autoleucel (brexu-cel, Kite/Gilead) is approved for relapsed/refractory mantle cell lymphoma (MCL) and adult patients with relapsed/refractory B-ALL21, 22. Idecabtagene vicleucel (ide-cel, Bristol Myers Squibb) and ciltacabtagene autoleucel (cilta-cel, Janssen) are both BCMA-targeted CAR T-cell approved for the treatment of patients with advanced multiple myeloma (MM)23, 24. In addition to these commercially available CAR T-cells, several CAR T-cell products with different targets are under investigation in other blood cancers, such as chronic lymphocytic leukemia, Hodgkin lymphoma, and T-cell lymphoma.
CAR T-cells can cause several unique adverse events including cytokine release syndrome (CRS), immune effector cell associated neurotoxicity syndrome (ICANS), hypogammaglobulinemia and prolonged cytopenia25–28. In general, products that include CD28 as the co-stimulatory signal have higher rates of CRS and ICANS than those that contain 4-1BB. For example, in the recent ZUMA-7 phase 3 trial, patients treated with axi-cel had a 6% incidence of grade ≥ 3 CRS and 21% had grade ≥ 3 neurotoxicity18. These rates were higher than those observed in patients treated with liso-cel (1% grade ≥ 3 CRS; 4% grade ≥ 3 neurotoxicity)19 and tisa-cel (5% grade ≥ 3 CRS; 2% grade ≥ 3 neurotoxicity)29, consistent with observations from the original registration studies11–13.
As part of their approval, the U.S. FDA has required that manufacturers collect data on efficacy, safety and long-term follow-up on a large number of patients (1,000 to 1,500) treated post-approval. Most US centers have reported the outcomes of patients treated with CAR T-cells to the CIBMTR Cellular Immunotherapy Data Resource (CIDR). As of the summer of 2021, the total accrual to the CIDR included 6,343 CAR T-cell recipients and 6,624 infusions30. Data is being collected from 195 reporting centers from the U.S., Canada and Israel. The most common indication for CAR T-cells is LBCL (66%), followed by ALL (16%), MM (6%), MCL (5%) and FL (4%). There has been an evolution in patient selection over time from 2017 to 2021. In particular, the proportion of patients who did not receive an HCT prior to CAR T-cell therapy is steadily increasing with 84% and 81% of patients with ALL and NHL without a prior HCT in 2021, respectively. CAR T-cells are being used in earlier lines of treatment with HCT being saved as a backup option. Outcomes of patients treated with CAR T-cells reported by the CIDR as well as other real-world data generally have shown similar results in terms of efficacy of safety as was seen in the pivotal trials, including in patients that did not meet inclusion criteria.
Activity and Regulation of Cellular Therapies in Europe
The approval of CD19-specific chimeric antigen receptor (CAR) T-cell therapies by the US Food and Drug Administration (FDA) in 201731 and by the European Medicines Agency (EMA) in 201832 generated excitement in the field, as it offers a new treatment modality to many patients suffering from refractory acute lymphoblastic leukemia and other B cell malignancies. Among the current generations of CAR T-cells, four autologous CAR19 T-cell products―tisagenlecleucel (KymriahⓇ, Novartis), axicabtagene ciloleucel (
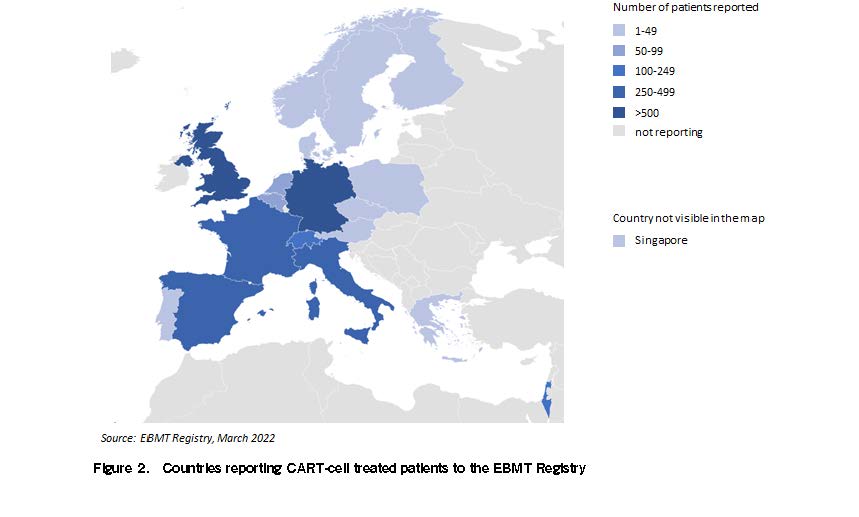
In the mid-1970s, the EBMT created a pan-European Registry which now captures the majority of stem-cell transplantation (HCT) activities across Europe. HCT is the most frequently used cellular therapy intervention, with more than 40,000 HCT infusions per year and with more than 700,000 transplant procedures reported overall34. With the creation of an additional cellular therapy module half a decade ago, the EBMT registry was upgraded to allow data to capture in more detail on other cellular therapy interventions. Patients treated with CAR T- cells have been collected by EBMT centers and reported to the EBMT registry since July 2019; 3,250 CAR T-cell treatments have been reported by March 2022, most of them using a commercial autologous construct (Figure 1, 2).
In late 2016, the EMA reached out to the EBMT to explore solutions for registering toxicities and efficacies associated with CAR T-cell treatments. The EMA wanted to investigate the possibility of using this new cellular therapy module of the EBMT registry for the capture of long-term follow-up data of CAR T-cells, consistent with approval requirements issued by the EMA for CAR T-cell therapies. This strategy was part of the ongoing Patient Registry Initiative by the EMA which aims to make better use of existing registries and to capture real word data to support post-authorization studies (PAS) such as post-authorization safety studies and post-authorization efficacy studies (PAES) and regulatory decision making35. These PAS studies are based on secondary use of EBMT Registry data. To test suitability, in October 2017, EBMT submitted a request to EMA to qualify the cellular therapy module. This process ended with a positive opinion on the cellular therapy module of the EBMT registry by the EMA in early 201936. The additional potential benefits of registering commercial CAR T-cells in such a global registry are manifold, and this was also evident to many stakeholders. This global CAR T-cells registry would avoid data sets in private registries of market authorization holders (MAHs) or disease-focused groups, allowing for comparison of safety and efficacy data between different advanced therapy medicinal products (ATMPs), assessment of the cost effectiveness of this rather expensive intervention between different CAR T-cell products, and comparisons with alternative interventions such as bispecific molecules and stem-cell transplantation. This knowledge would improve the quality and affordability of patient care. Such a registry could also facilitate shorter and less expensive approval procedures, which is important considering that many commercial GTMPs with small variations will enter the market in the near future at a high price33, with many novel targets still to be discovered36. The global registry would thereby support that patients get earlier access to CAR T-cells. Although the EMA had explored the use of registries with the EBMT beginning in 2016 and had recommended approval for the first two CAR T-cell products on the market on June 29, 201832, it wasn't until early 2020, when more than 300 patients had already been treated with commercial products in Europe, that the first contracts were signed between the EBMT and MAH37. PAS are already ongoing under the EBMT umbrella and the PAS portfolio will be expanded with the approval of new CAR T-cell constructs.
A major challenge when querying data is how a specific item is defined and collected. To harmonize this process across major registries, data definition groups have been established during the past decades between the EBMT and the CIBMTR, initially installed to increase quality and harmonize HCT data sets. Most recently the two groups started harmonizing cellular therapy registry forms across the globe. Given the fact that both organizations now capture CAR T-cells from the very same MAHs, with EBMT in the EU, and CIBMTR in the United States, this harmonization process is even more important, as the ability to easily fuse such data sets for global regulatory oversight is becoming essential.
To better structure the process of data collection and data access, in 2020 the EBMT launched multi-stakeholder group for immune effector cells such as Chimeric Antigen Receptor T cells (GoCART), a CAR T-cell community platform. The primary aim of GoCART is to create a public private/multistakeholder collaboration to maximize the potential of CAR T, minimize duplication of efforts, and create a strong community for pan-EU leadership in this area. The community aims to use the EBMT cellular therapy module as a central EU data registry, working in partnership with national cooperative and registry groups, as well as national organizations. In addition, it will be key to create transparent data access policies and harmonized data collection of CAR T-cell therapies in the EBMT registry in line with the mandatory
In summary, the introduction of CAR T-cells has not only changed the long-term outcome of a significant number of patients with relapsed/refractory hematological malignancies but has also been the starting point of the creation of a comprehensive structure and legal framework that did not exist before and EBMT has been a contribute factor to it.
Global Trends in Cellular Therapy and its integration with Hematopoietic Stem Cell Transplantation (HSCT) – Worldwide Network for Blood & Marrow Transplantation (WBMT) Perspective
In the year 1957, engraftment of allogeneic hematopoietic cells was demonstrated in humans opening new exciting treatment opportunities38. After progress in understanding immune alloreactivity, in drug development and in supportive care, outcome of hematopoietic stem cell transplantation (HSCT) continuously improved and worldwide activity progressively increased. Today, HSCT is the only curative treatment option for several hematologic disorders and, what was learned from HSCT, paved the way for cellular therapy with advanced therapy medicinal products (ATMP). WBMT, a worldwide network of scientific societies and NGO in working relations with the World Health Organization, plays an important role in this process by promoting excellence and access to cellular therapy. To reach his mission, WBMT publishes a survey collating information from European Blood and Marrow Transplantation Group (EBMT), Center for International Blood and Marrow Transplant Research (CIBMTR), Asia Pacific Blood and Marrow Transplantation Group (APBMT), Eastern Mediterranean Blood and Marrow Transplantation (EMBMT), Australasian Bone Marrow Transplant Recipient Registry (ABMTRR), Cell Therapy Transplant Canada (CTTC), Latin America Blood and Marrow Transplantation (LABMT), African Blood and Marrow Transplantation (AFBMT) and transplantation centers.
In 2016, 1662 centers reported more than 82,000 HSCT procedures corresponding to an increase of 77.6% from 200639. Preliminary analyses revealed increases to 92,812 HSCT in 2018 and estimates of 100.000/year after 2020. HSCT/10 million inhabitants showed regional differences ranging from 561 and 439 in North America and Europe to 77, 54, 36 and 9 in Latin America, South-East Asian Region/ Western Pacific Region (SEAR/WPR), Eastern Mediterranean Region and Africa regions (EMRO), respectively. The relative increase in HSCT was higher in low as compared to high/intermediate income countries.
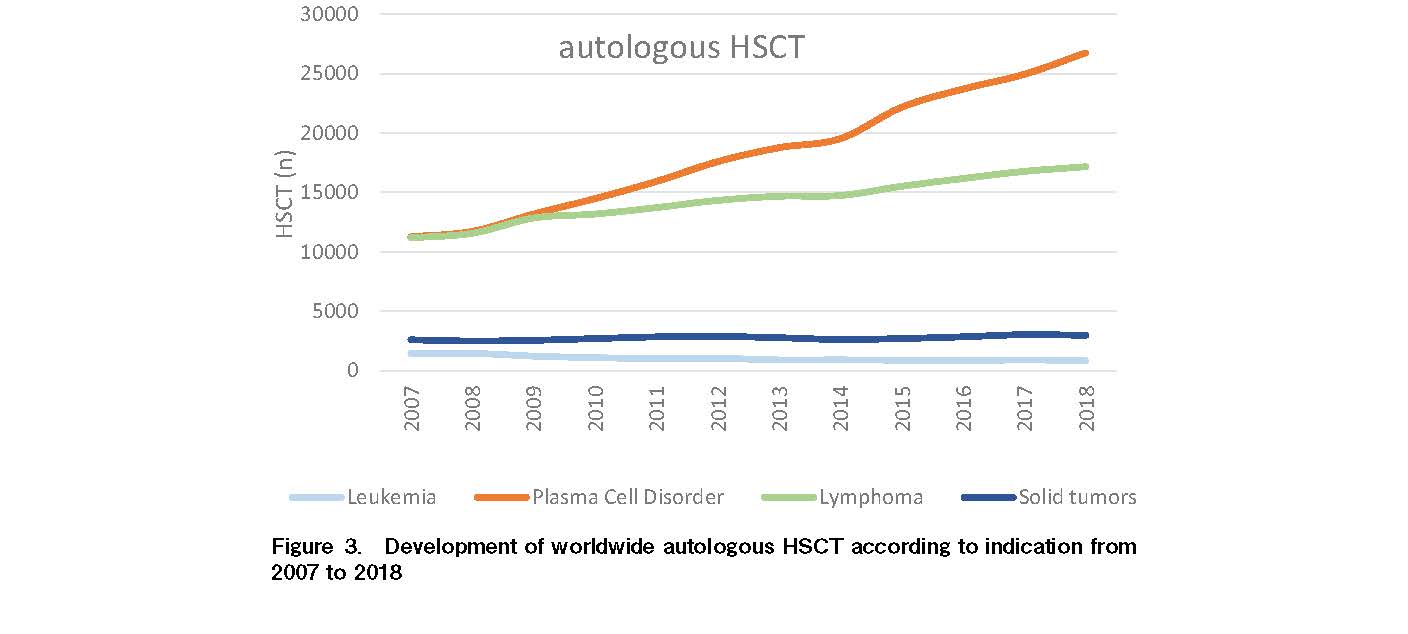
The leading indications for autologous HSCT, which showed an increase of 68.9% since 2006 and amounted to 53.5% of all HSCT, were lymphoproliferative diseases (+45.7% from 2006). The main indication of this group was plasma cell disorders (+122.0%) and, to a lesser extent, lymphomas (+84.2%; Figure 3). Solid tumors as indication for autologous HSCT showed only a slight increase (+11.4%) and leukemia as indication for autologous HSCT a decrease (−51.1%) in almost all world regions except in SEAR/WPR (+39%).
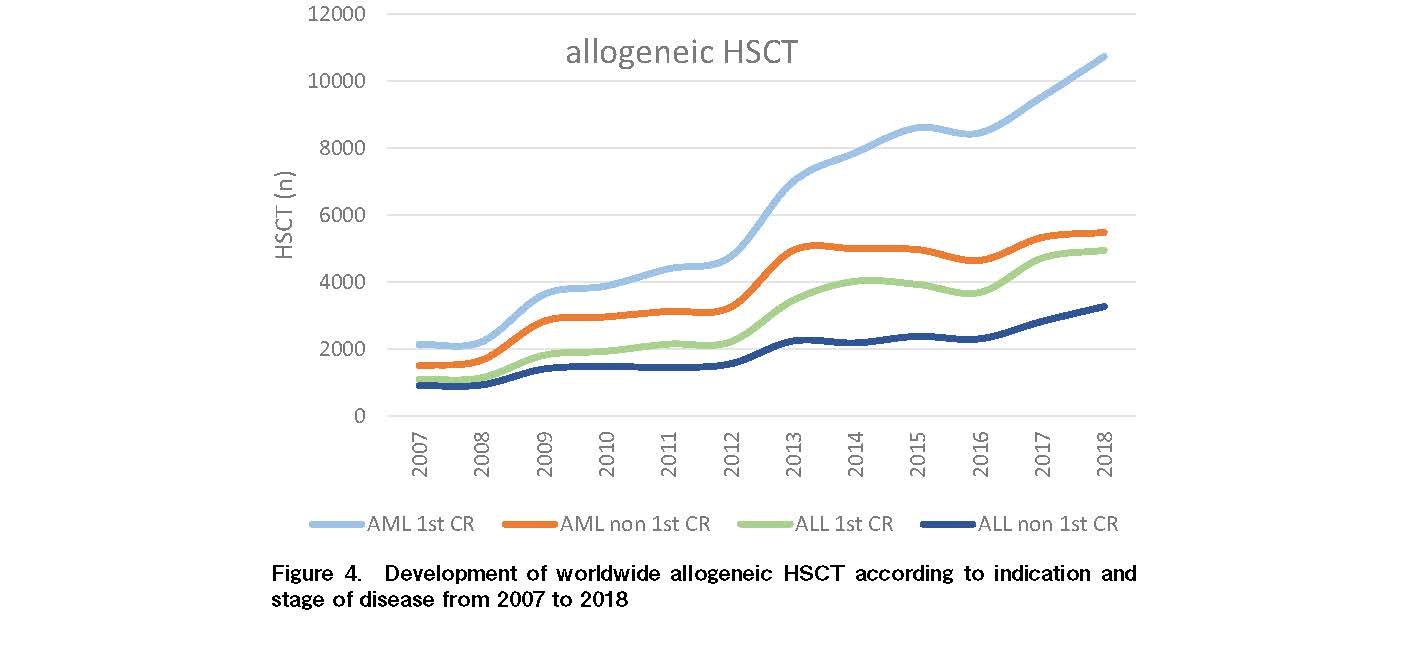
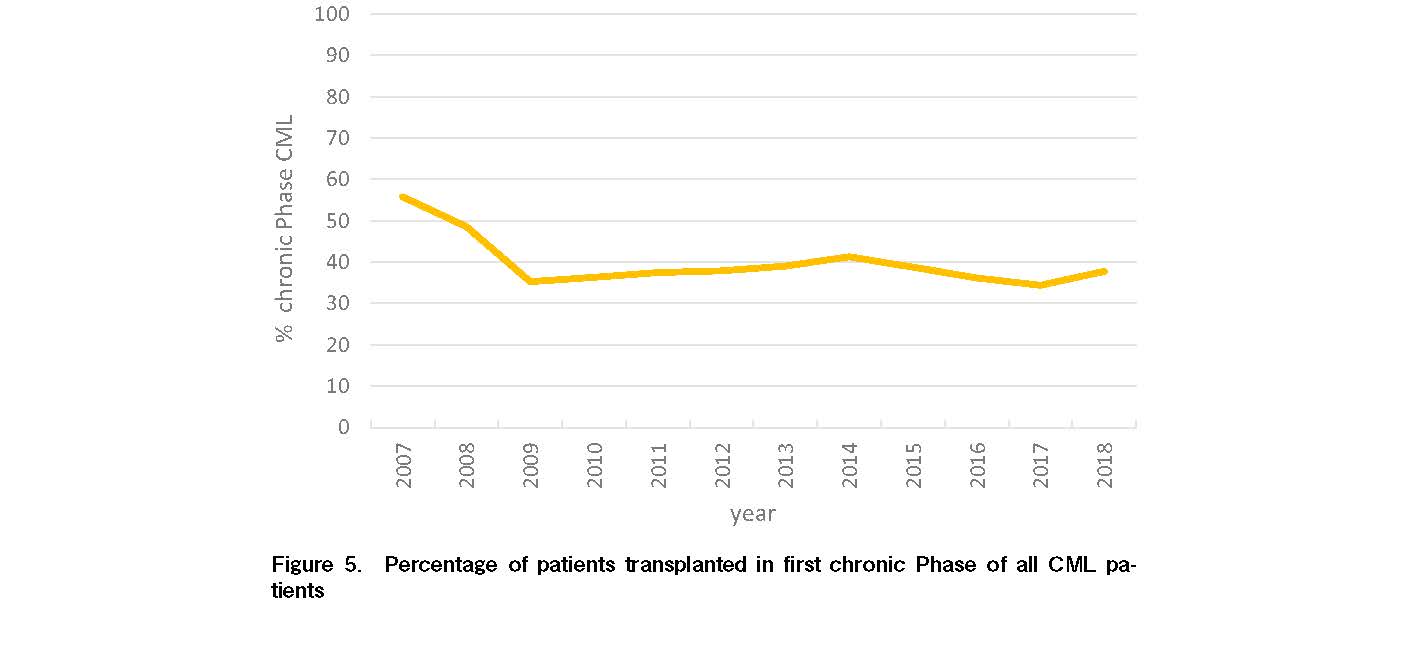
The increase of allogeneic HSCT from 2006 amounted to +77.6% (n=38.425) as result of increased activity in acute leukemias (+116.8% for AML and +97.7% for ALL), MDS/MPS (+147.6%) and non-malignant diseases. It is reassuring that mainly HSCT in AML and ALL in CR1 and not non-CR1 prevailed (Figure 4). Negative developments were observed in chronic myeloid (CML; −16.9%) and chronic lymphoblastic leukemias (CML; −17.3%). Interestingly, the percentage of patients transplanted in non-chronic Phase CML remains higher than chronic Phase CML despite the availability of multiple generations of TKI (Figure 5). Even higher was the increase in non-malignant disorders (+139.4%) from 2006 especially Hemoglobinopathies (+222.2%), Immune Deficiencies (+118.4%) and Bone Marrow Failures (+107.7%).
Major changes were observed in donor type since 2007. A clear increase in HSCT from unrelated donors was noted (+73.2%), while the increase in HLA-identical related was +24%. Remarkable was the increase of 440% in related non-identical HSCT. Proportion of HSCT from related non-identical donors of all allogeneic HSCT were 34% in SEAR/WPR, 26% in Latin America, but only 16.7% in North America and 14% in Europe.
Non-HSCT cellular therapies included donor lymphocyte infusion, CAR T-cells, selected/expanded T-cells, T-REGS, NK cells, dendritic cells, mesenchymal cells and genetically modified CD34 cells.
In conclusion, a progressive increase in HSCT activity is observed worldwide with variability in indications and variability in regional performance. Of importance is the notable increase in haploidentical HSCT and performance of other cellular therapies outside HSCT. The survey shows additional important messages like the need to improve timing of HSCT for CML. The increased use of HSCT in AML CR1 confirms the improved utilization of HSCT in early treatment of the disease. Furthermore, an increased use of HSCT for non-malignant indications is noted.
Acknowledgments
SO thanks Minako Iida (APBMT, Aichi Medical University School of Medicine, Nagakute, Japan) and Yoshiko Atsuta (Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT), Japan) for their dedicated support in writing this manuscript. DN acknowledges Helen Baldomero (WBMT, Transplant Activity Survey Office, University Hospital, Basel, Switzerland), Wael Saber (CIBMTR, Medical College of Wisconsin, Milwaukee, WI, USA), Minako Iida (APBMT, Aichi Medical University School of Medicine, Nagakute, Japan) , Daniel Neumann (IMISE, University of Leipzig, Germany), Mickey B. C. Koh (Infection and Immunity Clinical Academic Group St George's Hospital and Medical School, London, UK and Academic Cell Therapy Facility and Programme Health Sciences Authority Singapore, Singapore), Sebastian Galeano (Hospital Británico, Montevideo, Uruguay), Yoshiko Atsuta (Japanese Data Center for Hematopoietic Cell Transplantation (JDCHCT), Nagakute, Japan) and Mahmoud Aljurf (King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia) for their help in making this evaluation possible.
Author Contributions
SO wrote the abstract and introduction as well as the section on
Conflicts of Interest
The authors declare no conflicts of interest associated with this article. Disclosure forms provided by the authors are available on the website.
SO is a member of the Editor of Blood Cell Therapy. He was not involved in the editorial evaluation or the decision to accept this article for publication.
References
1.Ellen Sem, BS (Pharmacy), RAC, Vicky Han, BPharm, MBA, Annetta C. Beauregard, MS, MBA. The regulatory landscape of advanced therapies in Asia-Pacific. https://www.raps.org/news-and-articles/news-articles/2021/10/the-regulatory-landscape-of-advanced-therapies-in [Accessed: 30 August 2022]
2.Therapeutic Goods Administration. What is regulated as a biological? https://www.tga.gov.au/what-regulated-biological [Accessed: 30 August 2022]
3.National Medical Products Administration. Provisions for drug registration. http://english.nmpa.gov.cn/2019-07/25/c_390595.htm [Accessed: 30 August 2022]
4.KoBIA Newsletter. National assembly pass a bill, ‘Advanced Biopharmaceuticals.’ https://www.kobia.kr/bbs/board.php?tbl=e_newsletter&mode=VIEW&num=72& [Accessed: 30 August 2022]
5.Health Sciences Authority. Regulatory overview of cell, tissue or gene therapy products. https://www.hsa.gov.sg/ctgtp/regulatory-overview [Accessed: 30 August 2022]
6.Maruyama Y. Regulation of regenerative medicine in Japan. Health Products Regulatory Conference 2017. https://www.pmda.go.jp/files/000219466.pdf [Accessed: 30 August 2022]
7.Jain T, Bar M, Kansagra AJ, Chong EA, Hashmi SK, Neelapu SS, et al. Use of chimeric antigen receptor T cell therapy in clinical practice for relapsed/refractory aggressive B cell non-hodgkin-lymphoma: An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Biol Blood Marrow Transplant. 2019; 25: 2305-21.
8.Perales MA, Kebriaei P, Kean LS, Sadelain M. Building a safer and faster CAR: seatbelts, airbags, and CRISPR. Biol Blood Marrow Transplant. 2018; 24: 27-31.
9.Kansagra AJ, Frey NV, Bar M, Laetsch TW, Carpenter PA, Savani BN, et al. Clinical utilization of Chimeric Antigen Receptor T-cells (CAR-T) in B-cell acute lymphoblastic leukemia (ALL)-an expert opinion from the European Society for Blood and Marrow Transplantation (EBMT) and the American Society for Blood and Marrow Transplantation (ASBMT). Bone Marrow Transplant. 2019; 54: 1868-80.
10.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018; 378: 439-48.
11.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017; 377: 2531-44.
12.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019; 380: 45-56.
13.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020; 396: 839-52.
14.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019; 20: 31-42.
15.Chong EA, Ruella M, Schuster SJ; Lymphoma Program Investigators at the University of Pennsylvania. Five-year outcomes for refractory B-cell lymphomas with CAR T-cell therapy. N Engl J Med. 2021; 384: 673-4.
16.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang ML, Arnason JE, et al. Pivotal safety and efficacy results from transcend NHL 001, a Multicenter Phase 1 Study of Lisocabtagene Maraleucel (liso-cel) in Relapsed/Refractory (R/R) Large B Cell Lymphomas. Blood. 2019; 134 (Suppl-1): 241.
17.Perales MA, Anderson LD, Jr., Jain T, et al. Role of CD19 CAR T cells in second line large B cell lymphoma: lessons from phase 3 trials – An Expert Panel Opinion from the American Society for Transplantation and Cellular Therapy. Transplant Cell Ther. 2022; S2666-6367(22)01411-7.
18.Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022; 386: 640-54.
19.Kamdar M, Solomon SR, Arnason J, Johnston PB, Glass B, Bachanova V, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. The Lancet. 2022; 399: 2294-308.
20.Jacobson CA, Chavez JC, Sehgal AR, William BM, Munoz J, Salles G, et al. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022; 23: 91-103.
21.Shah BD, Ghobadi A, Oluwole OO, Logan AC, Boissel N, Cassaday RD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. Lancet. 2021; 398: 491-502.
22.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020; 382: 1331-42.
23.Munshi NC, Anderson LD Jr, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med. 2021; 384: 705-16.
24.Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet. 2021; 398: 314-24.
25.Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020; 4: 676-86.
26.Wudhikarn K, Palomba ML, Pennisi M, Garcia-Recio M, Flynn JR, Devlin SM, et al. Infection during the first year in patients treated with CD19 CAR T cells for diffuse large B cell lymphoma. Blood cancer journal. 2020; 10: 79.
27.Wudhikarn K, Pennisi M, Garcia-Recio M, Flynn JR, Afuye A, Silverberg ML, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 2020; 4: 3024-33.
28.Jain T, Knezevic A, Pennisi M, Chen Y, Ruiz JD, Purdon TJ, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020; 4: 3776-87.
29.Bishop MR, Dickinson M, Purtill D, Barba P, Santoro A, Hamad N, et al. Second-line tisagenlecleucel or standard care in aggressive B-cell lymphoma. N Engl J Med. 2022; 386: 629-39.
30.Moskop A, Jacobs B, Pasquini MC. Current uses of CAR T cell Therapies in the US: CIDR summary slides, 2021. Available at: https://www.cibmtr.org/About/WhatWeDo/CIDR/Pages/index.aspx [Accessed: 17 July 2022]
31.FDA. FDA approves CAR-T cell therapy to treat adults with certain types oflarge B-cell lymphoma, 2017. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma [Accessed: 18 August 2022]
32.EMA. First two CAR-T cell medicines recommended for approval in the European Union, 2018. https://www.ema.europa.eu/en/news/first-two-car-t-cell-medicines-recommended-approval-european-union [Accessed: 18 August 2022]
33.Chabannon C, Kuball J, Mcgrath E, Bader P, Dufour C, Lankester A, et al. CAR-T cells: the narrow path between hope and bankruptcy? Bone Marrow Transplant. 2017; 52: 1588-9.
34.Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic stem cell transplantation in Europe 2014: more than 40 000 transplants annually. Bone Marrow Transplant. 2016; 51: 786-92.
35.EMA. Patient registries. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/patient-registries [Accessed: 30 August 2022]
36.Sebestyen Z, Prinz I, Déchanet-Merville J, Silva-Santos B, Kuball J. Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov. 2020; 19: 169-84.
37.EBMT. Report all CAR-T cell patients into the EBMT Registry, EBMT centre members and national registries are requested to document CAR T cell therapies, 2019. Available from: https://www.ebmt.org/data-collection-car-t-cells-related-news [Accessed: 18 August 2022]
38.Thomas ED, Lochte HL, Lu WC, Ferrebee JW. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. New Engl J Med. 1957; 257: 491-6.
39.Niederwieser D, Baldomero H, Bazuaye N, Bupp C, Chaudhri N, Corbacioglu S, et al. One and a half million hematopoietic stem cell transplants: continuous and differential improvement in worldwide access with the use of non-identical family donors. Haematologica. 2022; 107: 1045-53.
Search
News