Volume 5 (2022) Special Edition No.2 Pages S6-S14
Abstract
There is a significant need for alternative donors other than full-matched related or unrelated donors for allogeneic hematopoietic stem cell transplantation, especially in the Asia Pacific, where donor registries are smaller, and ethnicities are far more diverse. Both umbilical cord blood (UCB) and haploidentical transplantation can be carried out despite significant human leukocyte antigen (HLA) mismatches between patients and donors and help to meet this need. There are advantages and disadvantages to UCB and haploidentical transplantation, though enhancements in technology continue to improve outcomes in both. Donor selection for these cell sources is dependent on the presence of donor specific anti-HLA antibodies in the recipient's serum, degree and characteristics of donor-recipient HLA mismatches, ABO compatibility. Specific to haploidentical transplantation, additional factors like donor age, sex, donor-recipient CMV serology as well as NK cell alloreactivity are also important.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-SCT) remains a mainstay for the treatment of many hematological disorders but many patients in Asia are unable to find full matched unrelated donors due to small registry sizes and considerable human leukocyte antigen (HLA) diversity across Asia.
There is a significant need for alternative donors other than full-matched related or unrelated donors for allogeneic hematopoietic stem cell transplantation, especially in the Asia Pacific, where donor registries are smaller and ethnicities are far more diverse1. Both UCB and haploidentical transplantation can be carried out despite significant HLA mismatches between patients and donors and help to meet this need.
Allogeneic Stem Cell Transplantation Using Alternative Donor in Acute Leukemia – Current Status
Allogeneic stem cell transplantation using alternative donor
Allo-SCT is an established curative treatment for acute leukemia. It has been proven to improve survival in patients with intermediate or high-risk acute myeloid leukemia (AML) in first complete remission, or acute leukemia of any risk beyond first remission. However, only about 30% of patients have an available HLA-matched sibling donor2. Therefore searching for alternative donor had been developed in past three decades.
Up to present, there are three options for choosing alternative donor: matched unrelated donor (MUD) (or even mismatched unrelated donor) or UCB or mismatched related donor [including haploidentical (Haplo) related donor]2–4.
Unrelated donor stem cell transplantation
Firstly, the alternative strategy pursued for the remaining 70% of patients was an HLA MUD. Unfortunately, even with the use of large unrelated donor banks, such as the National Marrow Donor Program (NMDP), around 40% of patients are unable to find an HLA-matched donor among all ethnic groups. HLA-mismatched unrelated donors are available for some patients, but outcomes historically have been inferior to HLA-matched donor stem cell transplant (SCT)2, 4.
UCB stem cell transplantation
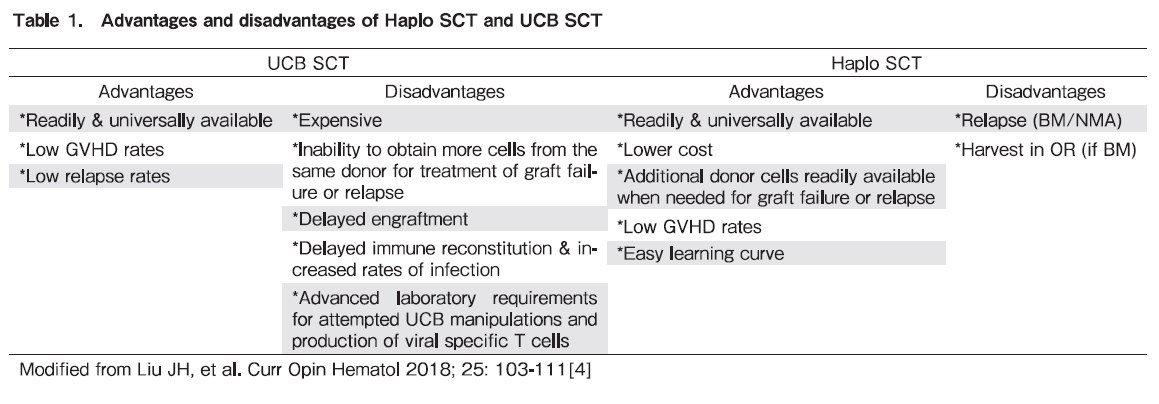
UCB SCT offers several benefits such as immediate graft availability compared with other strategies using HLA mismatched donors; less strict HLA matching requirements; reduced incidence of chronic graft versus-host disease (GVHD), and favorable graft-versus-leukemia (GVL) effects. A reduced HLA matching requirement is especially helpful for finding donors for patients from ethnic minorities, providing an UCB unit of adequate dose for up to 81-91% of adult patients and 95-99% of patients below 20 years old. Experienced UCB SCT centers are currently reporting comparable disease-free and overall survival (OS) rates to MUD SCT, especially in patients with minimal residual disease prior to SCT. The main obstacles for UCB SCT remain the expense, high graft failure rate, delayed engraftment, slow immune reconstitution, high rates of opportunistic infections, and relatively high rate of non relapse mortality. There are additional difficulties with UCB SCT in the treatment of acute leukemia, including the inability to acquire new cells from the donor for use as donor lymphocyte infusions in cases of disease relapse or to perform a second transplant from the same donor in cases of graft failure or poor graft function. Novel strategies of ex vivo expansion of UCB and manufacturing UCB-derived virus-specific T cells to treat post-UCB SCT virus infections may hold promise to improve outcomes of UCB SCT3.
Haploidentical stem cell transplantation
Haplo SCT has also been investigated in the past few decades and seemed more and more popular. The two most utilized approaches, either using
Potential benefits from HLA-mismatched stem cell transplantation
Interestingly, use of HLA-mismatched SCT provides several advantages for patients with AML in need of transplant. First, it provides rapid access to donors, allowing patients with high-risk leukemias to be transplanted quickly. Second, there is the potential that there may be more potent GVL with HLA-disparity in high-risk cases. For patients with AML in complete remission, UCB SCT had a similar relapse rate, but inferior OS when compared with MUD SCT. However, for acute leukemia patients with minimal residual disease (MRD) before SCT, retrospective analyses have shown that UCB SCT exhibited a lower relapse rate and similar OS in comparison with MUD SCT4–6.
Perspectives
UCB SCT has the advantages of serving as an immediate
Bone Marrow Or PBSC: Does It Really Matter In T-replete Haploidentical Transplantation
PTCy platform for GVHD prevention has become standard of care for T-cell replete haploidentical SCT. Although initial reports using this approach used bone marrow (BM) as the preferred donor source, several studies have since shown the efficacy of peripheral blood stem cells (PBSC) with comparable outcomes8. Traditionally, it is recognized that there are advantages and disadvantages to using BM versus PBSC in human leukocyte antigen (HLA)-identical sibling and matched and mis-matched unrelated donor transplantation. The cellular composition of the two graft sources varies, which leads to differences in engraftment kinetics, immune reconstitution, and risks of acute and chronic GVHD. Hence, one graft source may be preferred over the other in certain clinical scenarios based on patient, disease, and donor related variables. In addition, there are logistical issues that need consideration such as harvest expertise and operating room resources for BM and apheresis facilities for PBSC. Given that it is still relatively new, there is considerable interest in understanding the use of PBSC and BM in recipients of T-cell replete haploidentical transplantation using PTCy for GVHD prevention.
Irrespective of the graft source, there are significant differences in T- and NK-cell reconstitution after T-cell replete haploidentical SCT using PTCy compared to sibling and unrelated donor SCT receiving conventional GVHD prophylaxis regimens, which explains the general lower incidence of GVHD and the higher incidence of infections such as cytomegalovirus (CMV) and BK virus9. Haploidentical SCT is characterized by delayed recovery of naïve T-cells and NK cells along with relative sparing of CD4+ regulatory T-cells, which leads to an immune milieu that promotes tolerance and lower rates of GVHD. Furthermore, B-cells remain phenotypically naïve through as long as 1-year post-transplantation, and with the delayed recovery of de novo T-cells, predisposes the recipients to viral infections. The kinetics of immune recovery after haploidentical SCT are generally complex and are also dependent on other variables such as conditioning regimen intensity, recipient-donor CMV status, and degree of HLA mismatch.
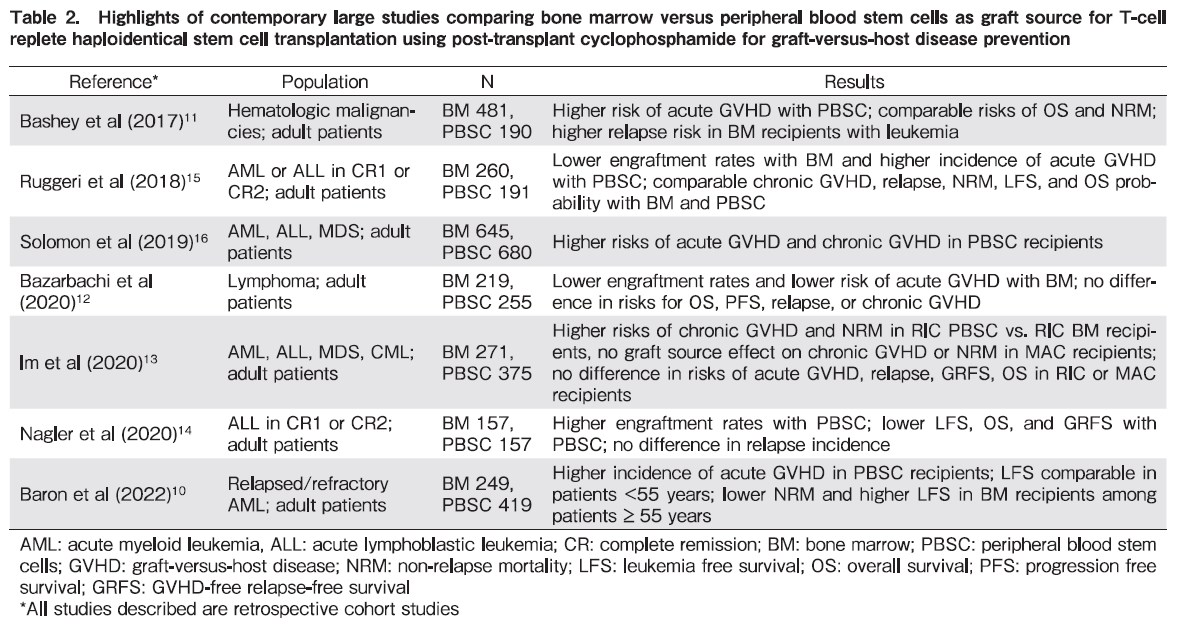
Table 2 summarizes large contemporary studies that have investigated the role of graft source in recipients of T-cell replete haploidentical SCT using PTCy as GVHD prevention strategy10–16. Notwithstanding the limitations typical of registry based retrospective analyses, there are common themes that can be identified with respect to the influence of graft source on outcomes. Similar to other donor sources, there are advantages and disadvantages to the use of PBSC and BM as a graft source in haploidentical SCT17. PBSC recipients have been observed to experience shorter time to neutrophil engraftment and lower rates of graft failure compared to BM recipients. However, patients receiving PBSC grafts have higher risks of acute and chronic GVHD. Arcuri et al, in a meta-analysis that compared the role of graft source and conditioning regimen intensity in haploidentical SCT recipients reported no difference in OS, progression free survival, GVHD-free relapse free survival, and non-relapse mortality with the use of PBSC or BM grafts. However, PBSC recipients had lower risk of relapse but higher rates of grade II-IV acute GVHD, grade III-IV acute GVHD, chronic GVHD, and extensive chronic GVHD8. Their results did not change in analyses that were stratified by conditioning regimen intensity.
With this background, how should clinicians determine which graft source is appropriate for a given patient who is being considered for T-cell replete haploidentical SCT with PTCy based GVHD prophylaxis? Foremost in this decision-making process are the data that OS in most studies has been shown to be comparable between BM and PBSC recipients. Hence, both graft sources are acceptable for proceeding with transplantation. Donor characteristics that are associated with SCT outcomes need to be considered in addition to the graft source (e.g., donor age and sex, presence of donor-specific antibodies, ABO compatibility, and CMV status)18. In our clinical practice, BM is the preferred graft source for haploidentical SCT given its association with lower risks of acute and chronic GVHD. This approach is definitely desirable in non-malignant diseases such as severe aplastic anemia where there is no need for an alloreactive graft-versus-tumor effect. However, we lean towards using PBSC in patients where there is higher risk of delayed engraftment or primary graft failure (e.g., older recipients and in diseases such as myelodysplastic syndromes, myeloproliferative neoplasms, and myelofibrosis) and in patients with high-risk leukemia given the suggestion that PBSC may possibly be associated with lower risks of relapse. An important caveat in graft source selection is logistics, since many centers do not have the experience, expertise, and set up to perform bone marrow harvests while PBSC collection using apheresis is more universally available.
In conclusion, BM and PBSC are acceptable graft sources for T-cell replete haploidentical SCT using PTCy based GVHD prophylaxis. The decision to use a specific graft source needs to be tailored towards patient characteristics and transplant center experience. Randomized clinical trials are needed to further clarify appropriate populations for the use of BM vs. PBSC in this setting.
Donor Selection for Haploidentical Hematopoietic Stem Cell Transplantation
Over the past two decades, significant advancement has been made in alleviating HLA alloreactivity between the donor and recipient, which has permitted an increase in use of haploidentical donors for transplantation, now the fastest growing source of hematopoietic stem cells, with improved transplant outcomes comparable to HLA matched donor transplants. The utility of HLA-haploidentical related donor provides several benefits including increase donor availability for almost all patients in need. The great majority of patients have more than one potential haploidentical donor available for donation and it is clear that not all of these donors can provide equivalent transplant outcomes, making donor considerations become increasingly complex. In an effort to optimize donor selection strategies, multiple studies have been published on the impacts of donor characteristics on outcomes including the presence of donor specific anti-HLA antibodies in the recipient's serum, donor age, sex, degree and characteristics of donor-recipient HLA mismatches, ABO compatibility, donor-recipient CMV serology as well as NK cell alloreactivity19. It is also important to mention that these donor characteristics may have different impact on outcomes when different haploidentical transplant platforms are used, i.e., T-cell depleted (TCD) versus T-cell replete (TCR) haploidentical transplantation.
1. Donor specific anti-HLA antibodies (DSAs)
Approximately 10-20% of recipients of haploidentical transplant have pre-formed anti-HLA antibodies against their donor's HLA, with higher incidences in female and heavily transfused recipients. The presence of DSAs has been shown to be associated with primary graft failure, delayed engraftment, primary poor graft function as well as lower post-transplant survival20–23. The ability of DSAs in causing primary graft failure depends on both antibody levels and activation of the complement system22. It has been now recommended to routinely test for DSAs and their ability to activate complement pathway such as C1q assay before choosing haploidentical donors.
Using hematopoietic stem cells from a donor without the corresponding HLA antigens is an ideal option for a recipient with anti-HLA antibodies. However, if there are no such donors available, recipients with DSAs should undergo desensitization treatment prior to transplantation to prevent graft failure24.
2. Donor age
Using stem cells from a younger donor has been associated faster immune recovery, less severe GVHD, low transplant-related mortality (TRM) and better survival in both TCD and unmanipulated haploidentical transplant with PTCy3. Not only better survival but younger donor can provide other potential benefits such as the ability to better tolerate the collection procedure, providing higher CD34+ cell yield and lower likelihood of clonal hematopoiesis and future risk of developing malignancies.
3. Donor Sex
It has been shown that minor HLA antigens in Y chromosome may increase GVHD as well as graft versus tumor effect in a setting of a female donor to a male recipient transplantation. We have previously shown in an HLA-matched SCT that female donors for male recipients associated with higher incidence of acute GVHD, higher TRM and lower relapse resulted in similar survival compared with other donor-recipient sex combinations25. This is particularly important when the main target of GVL from the graft is minor HLAs. However, in the setting of a major HLA mismatch like haploidentical transplantation, using stem cells from a female donor to a male recipient seems to have more negative impact on outcomes. In the PTCy platform, Kasamon and colleagues found that transplantation using a female donor to a male recipient resulted in lower survival26. It is therefore recommended that a male donor should be a preferred donor choice when selecting a donor for male recipients at least in the TCR haploidentical transplantation using PTCy.
4. Donor-recipient Relationship
In an unmanipulated haploidentical SCT, high risk of graft failure has been reported using a parent donor in comparison with an offspring. This impact is independent to donor age27. While in TCR haploidentical transplantation using the Beijing protocol, a higher NRM, acute GVHD and lower survival with mother than father donors has been reported28. On the contrary, in TCD haplo, it has been shown that a mother donor was associated with less relapse, lower NRM and better EFS compared with a father donor29.
A second degree relatives have also been used as a haploidentical donor which showed similar results to first-degree relative donors30. However, using the Beijing protocol, Wang and colleagues found that second-degree haploidentical donors associated with higher TRM and lower survival in comparison with 1st degree relatives28.
5. Donor-recipient ABO compatibility
ABO mismatch between a donor and a recipient can cause immunologic complications in allo-SCT. Major ABO mismatch can induce anti-donor isoagglutinin causing delayed RBC engratment, pure red cell aplasia as well as hemolytic anemia, while minor ABO mismatch can cause acute hemolysis from donor plasma or donor passenger lymphocyte syndrome. However, the impact of ABO mismatch on major transplant outcomes like NRM or survival remains controversy with conflicting data have been reported to date. Data from the EBMT showed that major ABO mismatch was associated with inferior engraftment rate whereas bi-directional mismatching increased risk of acute GVHD. Interestingly, this study also demonstrated that major ABO mismatch was associated with poor survival in patients receiving BM but not PB graft31.
6. NK Cell Alloreactivity
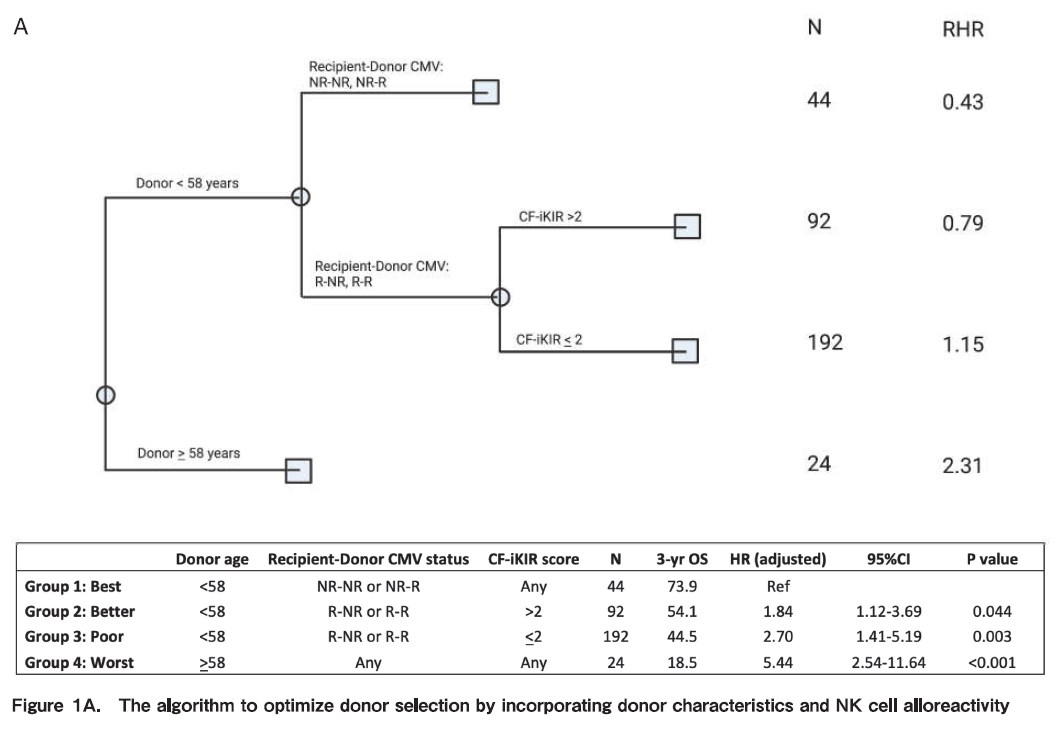
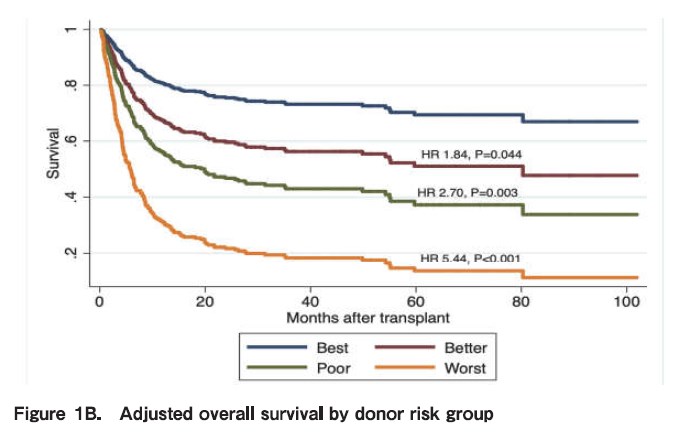
NK cell alloreactivity has been shown to have different impact on outcomes of haploidentical SCT when different platforms and different KIR hypotheses are used. For instance, in the TCD and unmanipulated haploidentical SCT with PTCy, NK cell alloreactivity seems to have positive impact on outcomes, such as reduce risk of relapse and increase survival. On the other hand, in the Beijing protocol, higher incidence of GVHD, NRM and worse survival were reported when having KIR ligand mismatch between the donor and recipient. We recently studied the impact of NK alloreactivity using several models on outcomes of haploidentical SCT and found that a donor with NK cell alloreactivity predicted by count functional inhibitory KIR score is associated with improved PFS and OS of patients. Based on this result, we developed an algorithm to optimize donor selection by incorporating donor characteristics and NK cell alloreactivity (Figure 1A) (Kongtim P et al, Submitted manuscript).
7. Donor-recipient CMV Serostatus
Conflicting data have been reported on the impact of donor-recipient CMV serostatus on clinical outcomes of haploidentical SCT. Our group has demonstrated that recipient- but not donor CMV serostatus influenced OS and PFS (Figure 1B) (Kongtim P et al, Submitted manuscript).
8. Degree and characteristics of HLA mismatch
In an HLA-matched SCT, higher degree of HLA mismatch is associated with poor outcomes. However, studies have demonstrated that in unmanipulated haploidentical SCT using either PTCy and the Beijing protocol, degree of HLA mismatching did not influence NRM, relapse, PFS or OS.
The impact of HLA mismatches at molecular level has also been studied and recently reported by our group which showed that HLA-A mismatch eplets in HVG direction is associated with a reduced risk of relapse and improved survival. Based on the result from this study, ME analysis of individual HLA loci might assist donor selection and risk stratification in haploidentical SCT32.
In conclusion, data on the impact of different donor characteristics on outcomes of haploidentical SCT have emerged over the recent years. Carefully select donor who can provide the best outcomes for the recipient is one of the most important elements for successful haploidentical SCT.
Is It The End of the Road for Cord Transplant?
Studies of UCB transplantation showed that 1 to 2 antigen mismatched cord blood transplantation could have equivalent results compared to fully matched unrelated bone marrow donors. Studies of transplants carried out with half-matched (haploidentical) donors have also shown equivalent outcomes to full matched donor transplantation with either extensive cell selection methods (e.g. with TCR α/β and CD19 depletion) or novel peri-transplant conditioning and prophylaxis protocols including the widely used PTCy regimen33.
A prospective multicenter comparison of double-unit UCB and haploidentical transplantation with reduced-intensity conditioning did not show a statistically significant difference in 2-year PFS between the donor sources, albeit higher transplant-related mortality (TRM) with UCB transplantation34. Specifically, 2-year OS after UCB was 46% compared with 57% after haploidentical transplantation (p = .04). Studies using a uniform myeloablative regimen (comprising thiotepa, busulfan, and fludarabine with anti-thymocyte globulin) revealed similar results, with no significant differences in relapse, disease-free, or OS35. These results show that haploidentical transplant is at least equivalent to UCB transplantation in outcomes.
However, UCB has certain advantages including immediate availability. UCB in cord blood banks are fully tested and can be thawed from liquid nitrogen for transplantation upon request. Furthermore, despite the lack of donor lymphocyte infusion, UCB transplantation is associated with lower post-transplant relapse rates. A study of 582 patients comparing UCB and fully matched or mismatched adult donor transplantation revealed that mismatched UCB grafts had reduced relapse rates (hazard ratio 2.92, p=0.007 with HLA matched adult donors vs UCB) and superior leukemia-free survival versus fully-matched adult donor stem cells, suggesting superior leukemia control with UCB grafts despite more manageable GVHD36. This phenomenon could be related to the robust immunological potential albeit allogeneic pliability of UCB immune cells.
Ongoing studies show continued progress in UCB transplantation with improving outcomes with modifications in conditioning regimens as well as hematopoietic stem cell expansion. A prospective phase 3 multicenter study of ex-vivo expanded hematopoietic stem cell versus conventional UCB transplantation, patients who received expanded grafts experienced accelerated neutrophil engraftment (12 days vs 22 days; p < 0.001), faster platelet recovery, lower incidence of first grade 2 to 3 bacterial or invasive fungal infection, and spent more time out of hospital during the first 100 days after transplant (median, 61 vs 48 days; P = .005) than controls37.
Haploidentical transplantation has largely replaced UCB due to similar outcomes and reduced cost. However, UCB is more rapidly available as a source of cells for transplantation, and continued improvements in UCB technology38 could result in a resurgence in usage if costs could be controlled.
Conclusions
Current results with both UCB and haploidentical transplantation are excellent. In the absence of a readily available full matched related or unrelated donor, no patient should have the lack of an immediately available stem cell donor for transplantation.
Author Contributions
WYKH wrote the abstract, introduction, and conclusions as well as the section on
Conflicts of Interest
The authors declare that they have no relevant conflicts of interest. The disclosure forms provided by the authors are available online. WHYK is a member of the Editorial Board of Blood Cell Therapy and of the Board of Directors of the Singapore Cord Blood Bank. He is not involved in the editorial evaluation or the decision to accept this article for publication.
References
1.Yoshimi A, Suzuki R, Atsuta Y, Iida M, Lu DP, Tong W, et al. Hematopoietic SCT activity in Asia: A report from the Asia-Pacific Blood and Marrow Transplantation Group. Bone Marrow Transplant. 2010; 45: 1682-91.
2.Kanakry CG, de Lima MJ, Luznik L. Alternative donor allogeneic hematopoietic cell transplantation for acute myeloid leukemia. Semin Hematol. 2015; 52: 232-42.
3.Ciurea SO, Shah MV, Saliba RM, Gaballa S, Kongtim P, Rondon G, et al. Haploidentical transplantation for older patients with acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant. 2018; 24: 1232-6.
4.Liu JH, Kanakry CG, Luznik L. Have haploidentical transplants replaced umbilical cord transplants for acute leukemias?. Curr Opin Hematol. 2018; 25: 103-11.
5.Battipaglia G, Galimard JE, Labopin M, Raiola AM, Blaise D, Ruggeri A, et al. Post-transplant cyclophosphamide in one-antigen mismatched unrelated donor transplantation versus haploidentical transplantation in acute myeloid leukemia: a study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022; 57: 562-71.
6.Wieduwilt MJ, Metheny L, Zhang MJ, Wang HL, Estrada-Merly N, Marks DI, et al. Haploidentical vs sibling, unrelated, or cord blood hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood Adv. 2022; 6: 339-57.
7.Passweg JR, Baldomero H, Chabannon C, Corbacioglu S, de la Cámara R, Dolstra H, et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022; 57: 742-52.
8.Arcuri LJ, Hamerschlak N, Rocha V, Bonfim C, Kerbauy MN. Outcomes after haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide: a systematic review and meta-analysis comparing myeloablative with reduced-intensity conditioning regimens and bone marrow with peripheral blood stem cell grafts. Transplant Cell Ther. 2021; 27: 782.e1-7.
9.Rambaldi B, Kim HT, Reynolds C, Chamling Rai S, Arihara Y, Kubo T, et al. Impaired T- and NK-cell reconstitution after haploidentical HCT with posttransplant cyclophosphamide. Blood Adv. 2021; 5: 352-64.
10.Baron F, Labopin M, Tischer J, Ciceri F, Raiola AM, Blaise D, et al. Human leukocyte antigen-haploidentical transplantation for relapsed/refractory acute myeloid leukemia: Better leukemia-free survival with bone marrow than with peripheral blood stem cells in patients >/=55 years of age. Am J Hematol. 2022; 97: 1065-74.
11.Bashey A, Zhang MJ, McCurdy SR, St Martin A, Argall T, Anasetti C, et al. Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol. 2017; 35: 3002-9.
12.Bazarbachi A, Boumendil A, Finel H, Castagna L, Dominietto A, Blaise D, et al. Influence of donor type, stem cell source and conditioning on outcomes after haploidentical transplant for lymphoma – a LWP-EBMT study. Br J Haematol. 2020; 188: 745-56.
13.Im A, Rashidi A, Wang T, Hemmer M, MacMillan ML, Pidala J, et al. Risk factors for graft-versus-host disease in haploidentical hematopoietic cell transplantation using post-transplant cyclophosphamide. Biol Blood Marrow Transplant. 2020; 26:1459-1468.
14.Nagler A, dholaria B, Labopin M, Savani BN, Angelucci E, Koc Y, et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia. 2020; 34: 2766-75.
15.Ruggeri A, Labopin M, Bacigalupo A, Gülbas Z, Koc Y, Blaise D, et al. Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer. 2018; 124: 1428-37.
16.Solomon SR, St Martin A, Shah NN, Fatobene G, Al Malki MM, Ballen KK, et al. Myeloablative vs reduced intensity T-cell-replete haploidentical transplantation for hematologic malignancy. Blood Adv. 2019; 3: 2836-44.
17.Byrne M, Savani BN, Mohty M, Nagler A. Peripheral blood stem cell versus bone marrow transplantation: A perspective from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Exp Hematol. 2016; 44: 567-73.
18.Ciurea SO, Al Malki MM, Kongtim P, Fuchs EJ, Luznik L, Huang XJ, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. 2020; 55: 12-24.
19.Kongtim P, Ciurea SO. Who is the best donor for haploidentical stem cell transplantation?. Semin Hematol. 2019; 56: 194-200.
20.Ciurea SO, de Lima M, Cano P, Korbling M, Giralt S, Shpall EJ, et al. High risk of graft failure in patients with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009; 88: 1019-1024.
21.Chang YJ, Zhao XY, Xu LP, Zhang XH, Wang Y, Han W, et al. Donor-specific anti-human leukocyte antigen antibodies were associated with primary graft failure after unmanipulated haploidentical blood and marrow transplantation: a prospective study with randomly assigned training and validation sets. J Hematol Oncol. 2015; 8: 84.
22.Ciurea SO, Thall PF, Milton DR, Barnes TH, Kongtim P, Carmazzi Y, et al. Complement-binding donor-specific anti-HLA antibodies and risk of primary graft failure in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015; 21: 1392-8.
23.Yoshihara S, Maruya E, Taniguchi K, Kaida K, Kato R, Inoue T, et al. Risk and prevention of graft failure in patients with preexisting donor-specific HLA antibodies undergoing unmanipulated haploidentical SCT. Bone Marrow Transplant. 2012; 47: 508-15.
24.Ciurea SO, Al Malki MM, Kongtim P, Zou J, Aung FM, Rondon G, et al. Treatment of allosensitized patients receiving allogeneic transplantation. Blood Adv. 2021; 5: 4031-43.
25.Kongtim P, Di Stasi A, Rondon G, Chen J, Adekola K, Popat U, et al. Can a female donor for a male recipient decrease the relapse rate for patients with acute myeloid leukemia treated with allogeneic hematopoietic stem cell transplantation? Biol Blood Marrow Transplant. 2015; 21: 713-9.
26.Kasamon YL, Luznik L, Leffell MS, Kowalski J, Tsai HL, Bolaños-Meade J, et al. Nonmyeloablative HLA-haploidentical bone marrow transplantation with high-dose posttransplantation cyclophosphamide: effect of HLA disparity on outcome. Biol Blood Marrow Transplant. 2010; 16: 482-9.
27.Solomon SR, Aubrey MT, Zhang X, Piluso A, Freed BM, Brown S, et al. Selecting the best donor for haploidentical transplant: impact of HLA, killer cell immunoglobulin-like receptor genotyping, and other clinical variables. Biol Blood Marrow Transplant. 2018; 24: 789-98. doi: 10.1016/j.bbmt.2018.01.013.
28.Wang Y, Chang YJ, Xu LP, Liu KY, Liu DH, Zhang XH, et al. Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 2014; 124: 843-50. doi: 10.1182/blood-2014-03-563130.
29.Stern M, Brand R, de Witte T, Sureda A, Rocha V, Passweg J, et al. Female-versus-male alloreactivity as a model for minor histocompatibility antigens in hematopoietic stem cell transplantation. Am J Transplant. 2008; 8: 2149-57. doi: 10.1111/j.1600-6143.2008.02374.x.
30.Elmariah H, Kasamon YL, Zahurak M, Macfarlane KW, Tucker N, Rosner GL, et al. Haploidentical bone marrow transplantation with post-transplant cyclophosphamide using non-first-degree related donors. Biol Blood Marrow Transplant. 2018; 24: 1099-102.
31.Canaani J, Savani BN, Labopin M, Huang XJ, Ciceri F, Arcese W, et al. Impact of ABO incompatibility on patients' outcome after haploidentical hematopoietic stem cell transplantation for acute myeloid leukemia – a report from the Acute Leukemia Working Party of the EBMT. Haematologica. 2017; 102: 1066-74.
32.Zou J, Ciurea SO, Kongtim P, Yi M, Carmazzi Y, Rondon G, et al. Molecular disparity in human leukocyte antigens is associated with outcomes in haploidentical stem cell transplantation. Blood Adv. 2020; 4: 3474-85.
33.Chang YJ, Ding Q, Hwang WYK, Sahoo RK. Editorial: recent developments in haploidentical hematopoietic cell transplantation: therapy and complications. Front Immunol. 2021; 12: 746221.
34.Fuchs EJ, O'Donnell PV, Eapen M, Logan B, Antin JH, Dawson P, et al. Double unrelated umbilical cord blood vs HLA-haploidentical bone marrow transplantation: The BMT CTN 1101 trial. Blood. 2021; 137: 420-8.
35.Sanz J, Montoro J, Solano C, Valcárcel D, Sampol A, Ferrá C, et al. Prospective Randomized Study Comparing Myeloablative Unrelated Umbilical Cord Blood Transplantation versus HLA-Haploidentical Related Stem Cell Transplantation for Adults with Hematologic Malignancies. Biol Blood Marrow Transplant. 2020; 26: 358-66.
36.Milano F, Gooley T, Wood B, Woolfrey A, Flowers ME, Doney K, et al. Cord-blood transplantation in patients with minimal residual disease. N Engl J Med. 2016; 375: 944-53.
37.Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, Maziarz RT, et al. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood. 2021; 138: 1429-40.
38.Bari S, Zhong Q, Fan X, Poon Z, Lim AST, Lim TH, et al. Ex vivo expansion of CD34+CD90+CD49f+ hematopoietic stem and progenitor cells from non-enriched umbilical cord blood with azole compounds. Stem Cells Transl Med. 2018; 7: 376-93.
Search
News