Volume 5 (2022) Issue 4 No.3 Pages 107-115
Abstract
Purpose: Increasing attention is being paid to the importance of nutritional management of allogeneic hematopoietic stem cell transplant (allo-HSCT) patients. However, few studies have conducted detailed evaluations of both nutritional intake and quality of life (QOL) in allo-HSCT patients. Therefore, we investigated the nutritional status and quality of life of our allo-HSCT patients.
Methods: The subjects were 26 adults who underwent allo-HSCT at Hamamatsu University Hospital between August 2018 and October 2021. Early nutritional intervention was provided from the time of the decision to perform allo-HSCT to the time of discharge, and it incorporated regular QOL assessments. The analyzed indices were nutritional intake, anthropometric measurements, body mass index (BMI), grip strength, body composition analyzer (InBody S10) measurements, and blood laboratory values including transthyretin levels. QOL was assessed using the QLQ-C30 questionnaire of the European Organization for Research and Treatment of Cancer (EORTC) (version 3.0) and calculated according to the EORTC scoring manual. The indices were compared at pre-transplantation, 30 days post-transplantation, 60 days post-transplantation, and at discharge. The association between pre-transplantation nutritional status and QOL was examined.
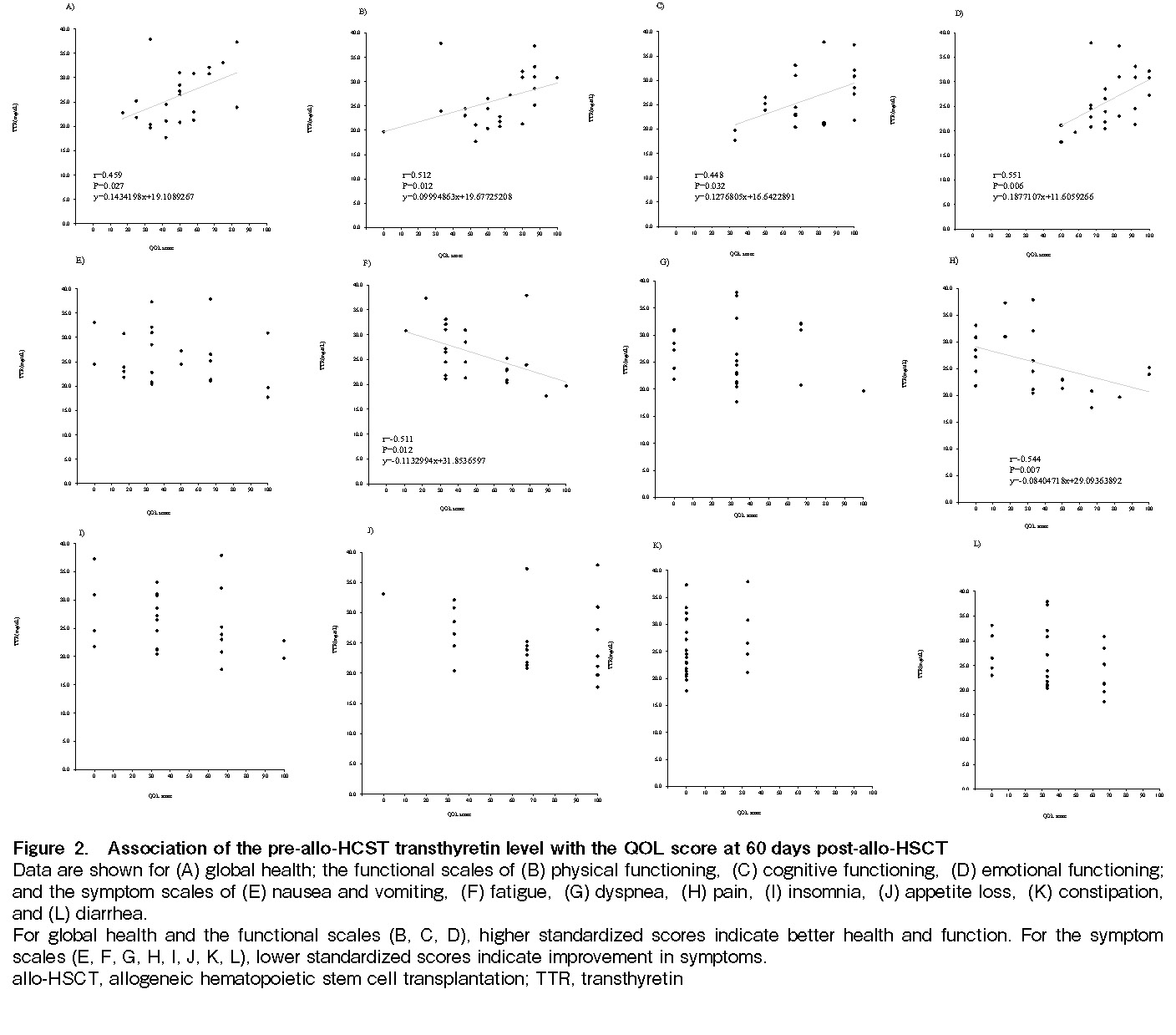
Results: The median hospital stay after transplantation was 97 days (range, 78-123 days). Energy intake was maintained at 31 kcal/day/kg through 30 days post-transplantation, 60 days post-transplantation, and discharge, and protein intake was maintained at 1.0 g/day/kg throughout all time periods. There was a significant positive correlation between the pre-transplantation transthyretin level and the 60-day post-transplantation QOL scores for
Conclusion: Early nutritional management of allo-HSCT patients prior to transplantation allowed maintenance of nutritional intake, and higher pre-transplant transthyretin levels were associated with higher QOL scores at 60 days post-transplantation.
1. Introduction
The number of hematopoietic stem cell transplantations (HSCTs) performed in Japan has increased dramatically since 1990, and more than 5,000 HSCT procedures are now performed annually. The collection and analysis of data regarding complications and the course after HSCT have played an important role in improving HSCT outcomes worldwide1.
Allogeneic hematopoietic stem cell transplantation (allo-HSCT), a conditioning regimen with high-dose chemotherapy and whole-body radiation prior to transplantation, causes oral mucositis, diarrhea, nausea and vomiting, taste abnormalities, and commonly, loss of appetite. In addition, graft-versus-host disease (GVHD) and various complications may occur following transplantation. These post-transplant symptoms reduce oral intake and can in turn impair nutritional status and quality of life (QOL).
Patients with hematopoietic tumors have been shown to have lower QOL and health-related QOL (HRQOL) compared with the general population. Therefore, it is important to assess QOL at the beginning of treatment2. Allo-HSCT also has short- and long-term effects on QOL and physical and psychological health. Many patients experience significant mental distress after HSCT, and the treatment has a negative influence on QOL and systemic function3–8. In the basic guidelines for allo-HSCT at Hamamatsu University Hospital (hereafter
Adverse physical symptoms are a general indicator of the physical domain of HRQOL9. It has been suggested that HSCT is associated with decreased psychological domains of HRQOL10, increased distress, anxiety, and depression11–15, and decreased health outcomes and survival16,17. Numerous studies have focused on the physical function of patients post-HSCT. A certain percentage of patients still suffer from long-term side effects such as chronic GVHD and do not reach pre-transplantation HRQOL levels at 2 years post-transplantation18. Others have reported that full recovery is a three- to five-year process8. We previously reported that nutritional management is necessary from the early stage, because nutritional status will deteriorate after transplantation even with Nutrition Support Team (NST) intervention19. As a result of this finding, we have implemented aggressive early (pre-transplantation) nutritional intervention as a new NST protocol. Patients who received this intervention had better QOL than those described in other reports20. However, few studies have focused on changes in QOL and nutritional care during hospitalization. Therefore, we analyzed the trends in nutritional status and QOL of allo-HSCT patients over time at our hospital. We hypothesized that a nutritional intervention protocol would improve the nutritional status and QOL of patients who had undergone allo-HSCT.
2. Patients and Methods
Patients
Of 30 adults who underwent allo-HSCT at our hospital between August 2018 and October 2021, 26 were enrolled in the study. Four patients who died during the observation period were excluded.
Nutritional management of patients with allo-HSCT
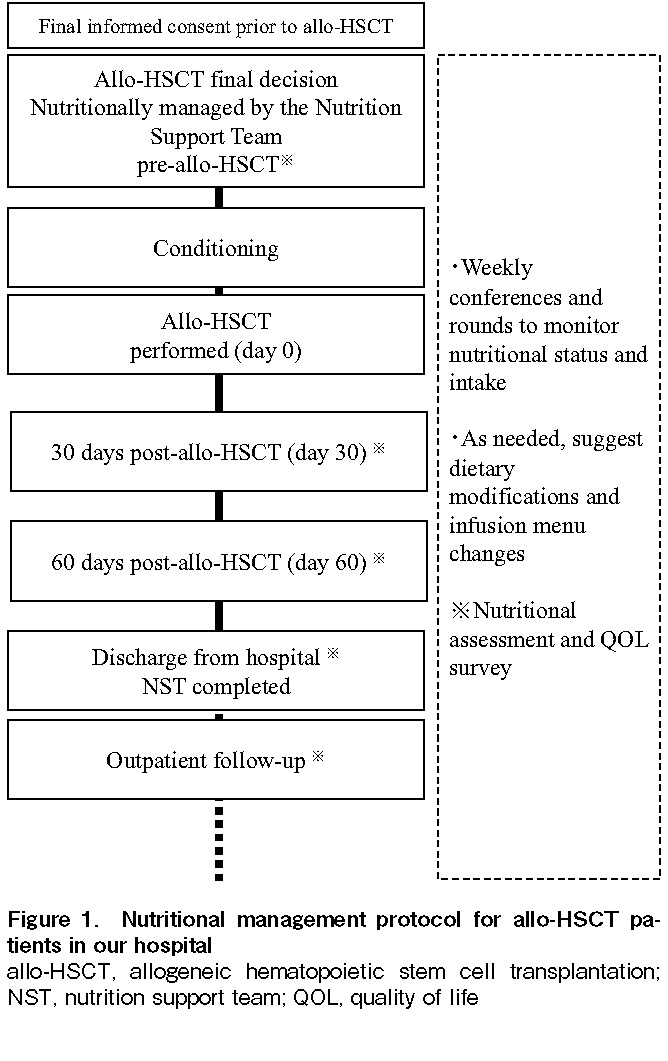
Figure 1 shows the nutritional management protocol implemented at our hospital for allo-HSCT patients. After obtaining the patient's final informed consent before allo-HSCT, NST intervention was initiated as soon as the transplant decision was made, and weekly conferences and rounds were held to monitor nutritional status and intake. As needed, we also suggested dietary modifications and considered changes and additions to the content of nutritional supplements and changes to the parenteral nutrition menu. The intervention was terminated at the time of discharge, after which the patient was followed up at an outpatient clinic. Detailed nutritional assessments were performed at pre-transplantation (pre-conditioning regimen), at 30 days post-transplantation, at 60 days post-transplantation, and at discharge. In principle, oral intake was continued until the time of discharge. In cases where diet alone was insufficient, nutritional supplements were added. At the time of discharge, dietary intake was by oral intake alone in all patients.
Nutritional assessment index
The survey items included nutritional intake, body measurements (triceps skinfold [TSF] and arm muscle circumference [AMC]), body mass index (BMI), grip strength, body composition analyzer measurements (InBody S10, InBody Japan Inc., Tokyo, Japan; skeletal muscle mass index (SMI), extracellular water/total body water [ECW/TBW] and phase angle [PA]), and blood test values (total protein, albumin, transthyretin, cholinesterase, triglycerides, total cholesterol, zinc, C-reactive protein, white blood cell count, hemoglobin, platelet count, and total lymphocyte count). Since evaluations were performed every 30 days, it was necessary to assess nutritional status using a rapid turnover protein that has a short blood half-life. Therefore, transthyretin, which has a half-life of 1.9 days, was used to evaluate the association between nutritional status and QOL.
QOL score
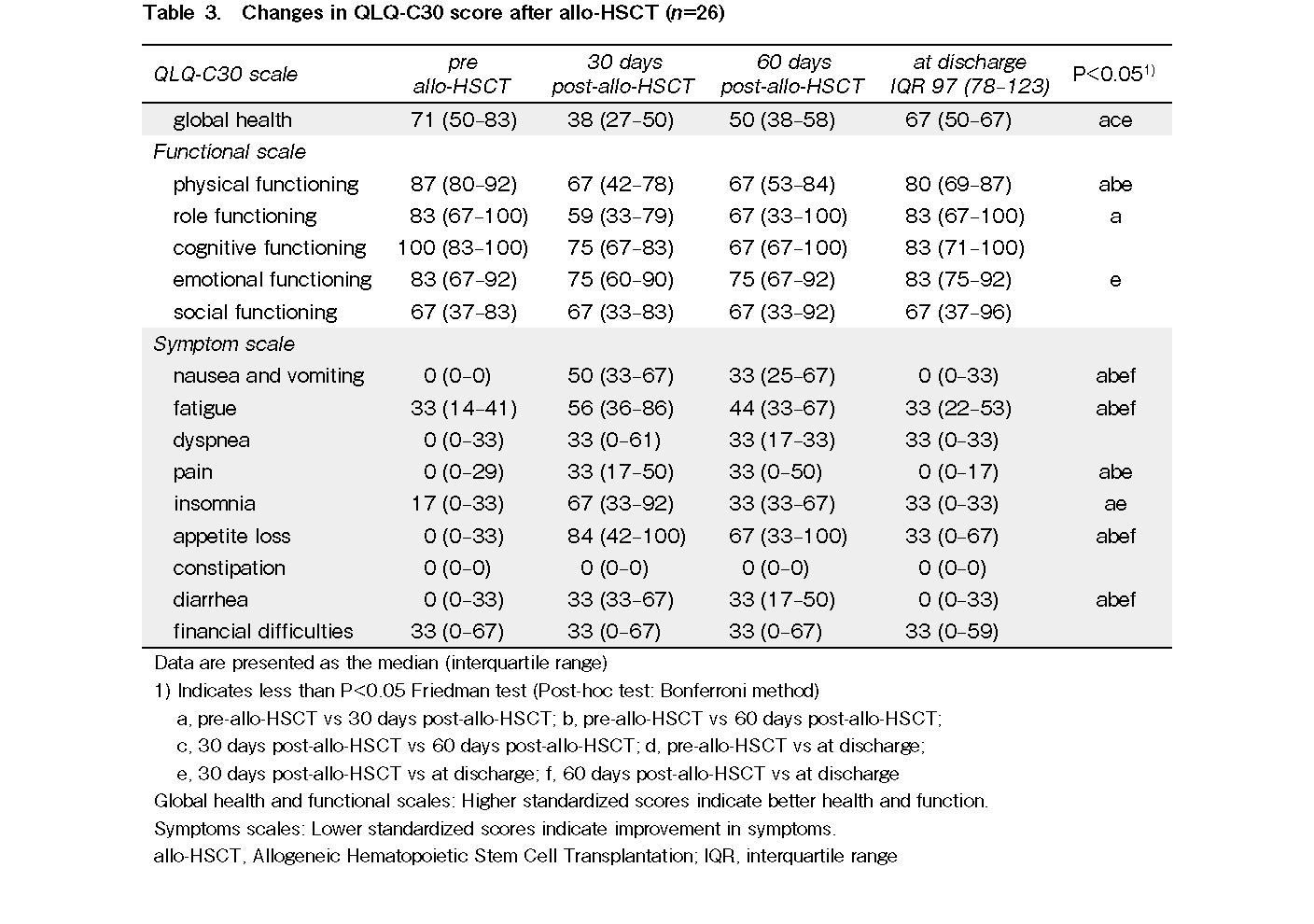
We used the QOL questionnaire of the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C3021 (version 3.0). Specifically, the Japanese version of the EORTC QLQ-C30, which has been shown to be reliable and valid22, was used in the present study. Prior permission to use this questionnaire was obtained from EORTC and the developer of the Japanese version, Kojiro Shimozuma. The participants were asked to answer a total of 30 self-administered questions about their global health; five functional scales (physical functioning, role functioning, cognitive functioning, emotional functioning, and social functioning); and nine symptom scales (nausea and vomiting, fatigue, dyspnea, pain, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). QOL scoring followed the EORTC scoring manual23. Higher standardized scores on the global health and functional scales indicated better status, whereas lower standardized scores on the symptom scale indicated better status.
Ethical considerations
Approval to conduct this study was obtained from the Ethics Committee of Hamamatsu University School of Medicine (Approval No. 18-077). Consent was obtained on an opt-out basis.
Statistical analyses
The following assessments of nutritional indices and QOL scores were performed. The Friedman test (post hoc test: Bonferroni method) was used to compare the results at pre-transplantation, 30 days post-transplantation, and 60 days post-transplantation. Spearman's rank correlation coefficient was used to evaluate the association between the pre-transplantation transthyretin level and the QOL score at 60 days post-transplantation, with a significance level of less than 5%. EZR24 version 1.54 was used as the statistical software.
3. Results
Patient characteristics
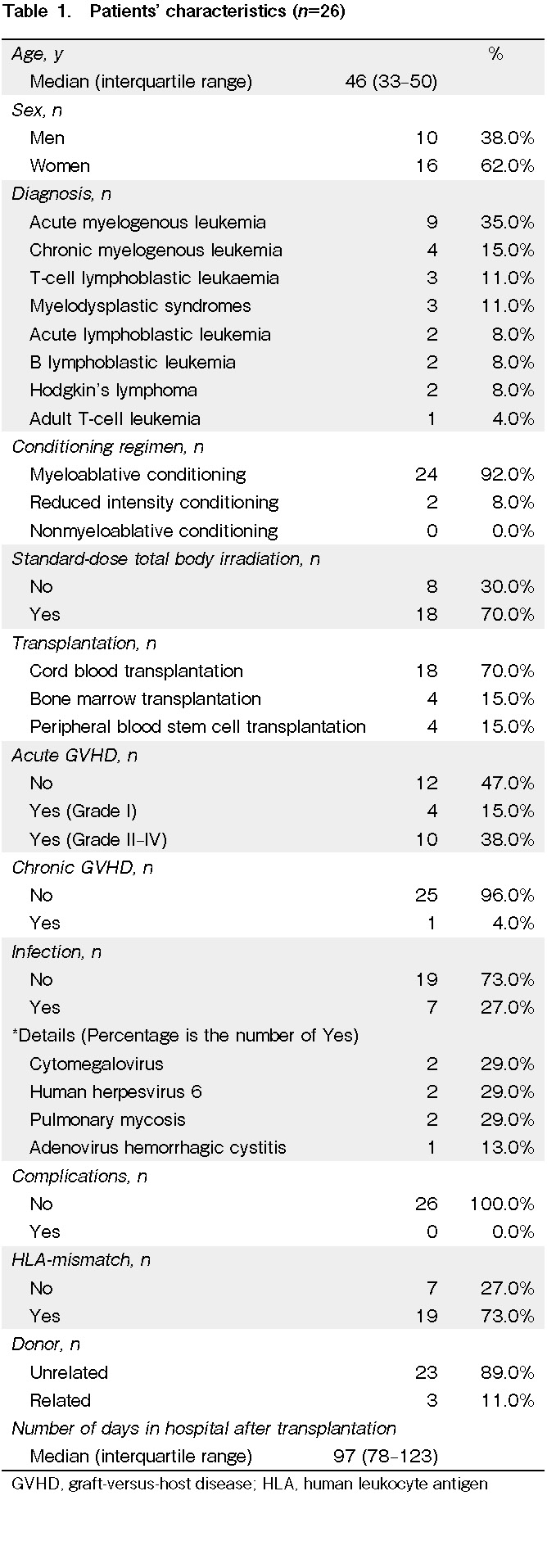
The median age of the 26 patients was 46 years (range, 33-50 years; male, 38.0% and female, 62.0%). The underlying diseases were acute myeloid leukemia (35.0%), chronic myeloid leukemia (15.0%), T-cell acute lymphocytic leukemia (11.0%), myelodysplastic syndrome (11.0%), acute lymphoblastic leukemia (8.0%), B lymphoblastic leukemia (8.0%), Hodgkin's lymphoma (8.0%), and adult T-cell leukemia (4.0%). The types of allo-HSCT were cord blood transplantation (70.0%), bone marrow transplantation (15.0%), and peripheral blood stem cell transplantation (15.0%). The type of conditioning regimen was myeloablative conditioning (92.0%) and reduced intensity conditioning (8.0%). Total body irradiation was standard dose in 70.0% and not standard dose in 30.0%. Grade II-IV acute GVHD was seen in 38.0% of all patients. No post-transplantation complications occurred, but post-transplantation infections occurred in 27.0%. There was human leukocyte antigen mismatch in 73.0%. The donor was unrelated in 89.0%. The median hospital stay after transplantation was 97 days (range, 78-123 days) (Table 1).
Comparison of nutritional indices and QOL scores
Nutritional assessment index
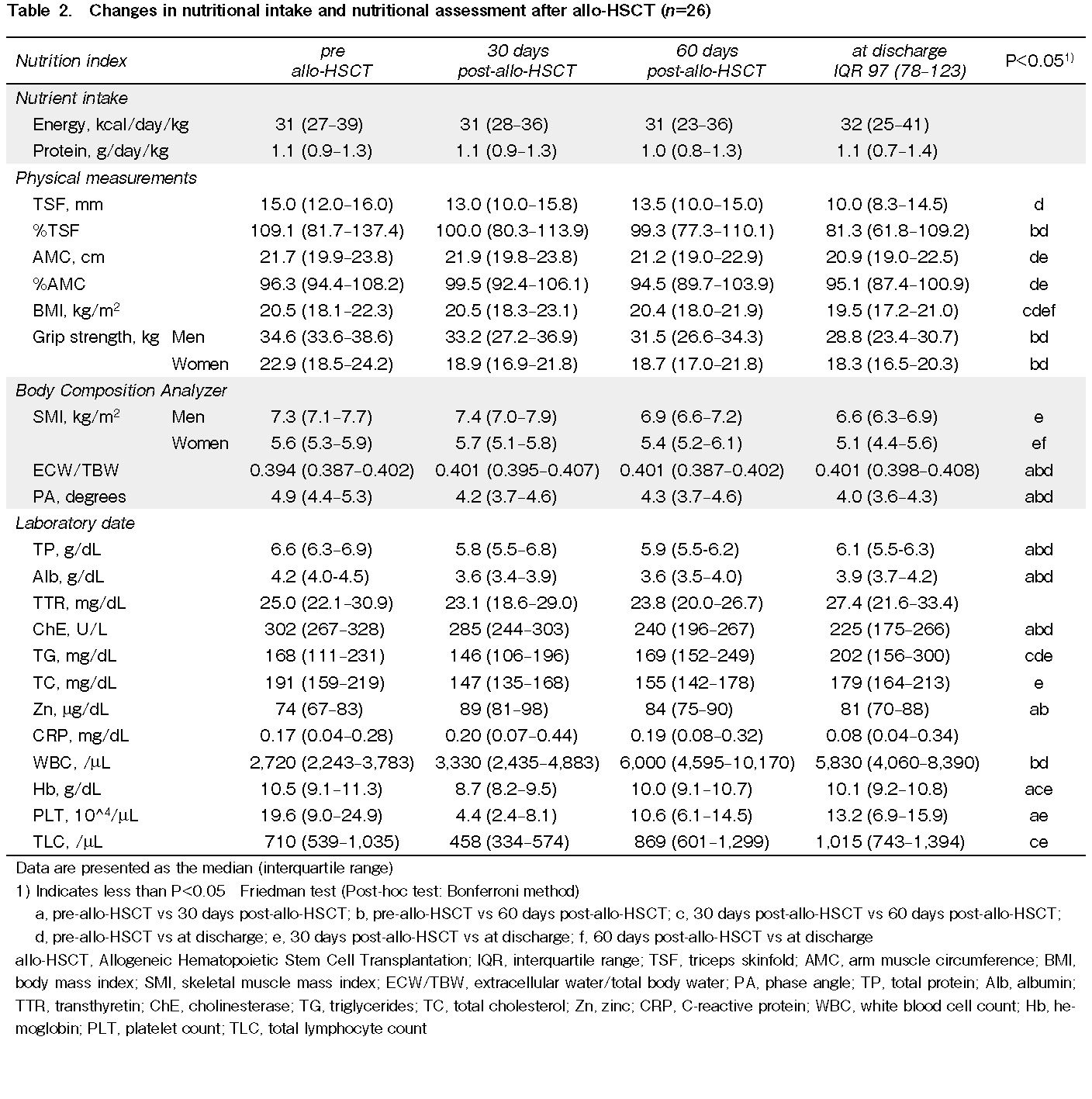
Energy intake was maintained at 31 kcal/day/kg through the 30 days post-transplantation, 60 days post-transplantation, and at discharge. Protein intake was maintained at 1.0 g/day/kg throughout all periods. There was no difference in energy or protein intake by time point.
Regarding the physical measurements, there was a significant decrease in %TSF at 60 days post-transplantation and at discharge (P=0.002) and a significant decrease in %AMC at discharge (P=0.007) compared with pre-transplantation. BMI decreased significantly between 30 and 60 days post-transplantation and at discharge (P<0.001). Grip strength decreased between pre-transplantation and 60 days post-transplantation and at discharge for both men (P=0.001) and women (P=0.012).
SMI calculated by the body composition analyzer decreased significantly between 30 days post-transplant and discharge for both men (P=0.001) and women (P=0.007), whereas ECW/TBW and PA increased significantly at 30 days post-transplantation, 60 days post-transplantation, and at discharge (P<0.001) compared with pre-transplantation.
Serum total protein and serum albumin levels were significantly lower at 30 days post-transplant and at discharge compared to pre-transplantation (P<0.001), but they tended to recover at discharge. Transthyretin levels remained within the reference range. Zinc levels were significantly lower at 30 days and 60 days post-transplantation (P=0.006). White blood cell counts were significantly higher at 60 days post-transplant and at discharge compared to pre-transplantation (P<0.001). Hemoglobin levels, platelet levels, and total lymphocyte counts all increased significantly between 30 days post-transplantation and discharge (P<0.001) (Table 2).
QOL Score
Associations of the pre-transplant transthyretin level with the QOL scores at 60 days post-allo-HSCT
There were significant positive correlations between the pre-transplantation transthyretin level and the 60 days post-transplant QOL scores for
4. Discussion
This study investigated trends in nutritional status and QOL in allo-HSCT patients before and after transplantation. The results showed that energy and protein intakes were generally maintained at 31 kcal/day/kg and 1.0 g/day/kg, respectively, through pre-transplantation, 30 days post-transplantation, 60 days post-transplantation, and at discharge. In addition, there were associations between the pre-transplantation transthyretin level and QOL scores at 60 days post-transplantation for
According to the Japanese parenteral and enteral nutrition guidelines25, nutritional management in HSCT is absolutely necessary because all patients undergoing HSCT are at nutritional risk. It is recommended that nutritional therapy should be started early, even if there are no nutritional disorders before treatment begins. The American Society for Parenteral and Enteral Nutrition guidelines26 also state that all patients undergoing HSCT are considered to be at nutritional risk and recommend that they undergo nutritional screening to develop a nutrition care plan and identify patients who require nutritional assessment. Furthermore, the European Society for Clinical Nutrition and Metabolism guidelines27 on nutrition for cancer patients recommend maintaining physical activity and ensuring adequate nutritional intake during pre-transplantation high-dose chemotherapy and post-HSCT. Therefore, nutritional management at pre-transplantation is important. Allo-HSCT involves a conditioning regimen with a combination of higher-dose and more intense chemotherapy and whole-body radiation than is commonly used. The risk of malnutrition after transplantation is high because adequate oral intake cannot be maintained due to the development of oral mucositis, nausea, vomiting, and diarrhea28. Reports on diet and its impact on post-HSCT complications are scarce; moreover, the focus has been primarily on nutrition immediately after transplantation29. In contrast, our NST provided early nutritional intervention to improve pre-transplantation nutritional status.
In this study, QOL was evaluated along with nutritional status. HRQOL assesses physical, psychological, social, and emotional functioning from the patients' experience and perspective, and it can identify individuals who may benefit from clinical interventions. An association has been shown between nutritional status and HRQOL outcomes in other cancer types, with malnourished patients reporting lower QOL than well-nourished patients30. The QOL scores of patients in our hospital were higher than those of patients before treatment for other cancers that were reported in a previous study20. QOL is an important metric that reflects the health status of cancer patients, and it is significantly impacted by nutritional factors31. However, there are no reports of the association of nutritional status and QOL in HSCT. In particular, QOL after allo-HSCT may be affected by acute and chronic GVHD, infections, and complications. In the present study, there was no bias in post-transplant QOL scores based on the presence or absence of acute GVHD or infection, but further study is needed to examine more cases. In addition, complications and chronic GVHD are factors that are inherently affected, and it is necessary to look at associations with these factors.
Although the data are not shown, QOL at 30 days was lower at post-transplantation than pre-transplantation, but recovered at discharge, and there was no correlation between pre-transplantation transthyretin levels and QOL at either 30 days post-transplantation or at discharge, because there was little difference in the level in most patients. Therefore, we investigated the relationship between pre-transplantation transthyretin levels and QOL at 60 days post-transplantation, where the distribution of the level varied. There were significant positive correlations between pre-transplantation transthyretin levels and the 60-day post-transplantation QOL scores for
It is debatable whether a statistically significant difference in QOL scores is clinically significant. Previous reports have stated that a difference in score of 10-15 should be considered clinically significant18. In addition, a more detailed report categorized clinical significance according to change in the average patient score, with a change of 5-10 points indicating
A limitation of this study is the small number of cases. Long-term observation after transplantation in a larger number of cases is required in further studies and may yield different results from those obtained in the present study. Since the present study was limited to inpatients, we are currently conducting long-term follow-up on an outpatient basis to evaluate the impact of nutritional management methods that combine early intervention and long-term follow-up on improving QOL.
It is necessary to continue to validate early nutritional management for allo-HSCT patients, such as the nutritional management protocol implemented at our hospital. Enhancements to early nutritional management are expected to improve the quality of patient care.
The present findings indicate the importance of early nutritional management prior to transplantation, and they showed a trend toward better recovery of QOL scores after transplantation in patients who had received this intervention. Therefore, it is necessary to perform early intervention such as monitoring of nutrition and QOL scores pre-transplantation, as well as regular bilateral assessments long after discharge from hospital.
This study examined the association between pre- and post-transplantation nutritional indices and QOL in allo-HSCT patients. Energy and protein intakes were maintained throughout pre-transplantation, 30 days post-transplantation, 60 days post-transplantation, and discharge, all with adequate nutritional intakes. The results showed that maintaining good nutritional status before allo-HSCT may improve QOL after transplantation. To demonstrate the effectiveness of early nutritional management for the QOL of patients undergoing allo-HSCT, comparative studies of patients' QOL with and without nutritional intervention are needed.
In conclusion, early nutritional management of allo-HSCT patients prior to transplantation allowed maintenance of nutritional intake, and higher pre-transplant transthyretin levels were associated with higher QOL scores at 60 days post-transplantation.
Author Contributions
A.I. designed the study; developed the protocol; collected, analyzed, and interpreted the data; generated the figures and tables; and wrote the manuscript. T.T., E.T., Y.N., T.O., and A.K. participated in the design of the study and edited the final manuscript. The final manuscript was reviewed and approved by all authors.
Ethics approval
Approval was obtained from the Ethics Committee of Hamamatsu University School of Medicine to conduct this study (Approval No. 18-077).
Consent for publication
Consent was obtained on an opt-out basis.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.JDCHCT, The Japanese Data Center for Hematopoietic Cell Transplantation Hematopoietic Cell Transplantation. Annual Report of Nationwide Survey in Japan 2021 Summary Slide., 2021.
2.Allart-Vorelli P, Porro B, Baguet F, Michel A, Cousson-Gélie F. Haematological cancer and quality of life: a systematic literature review. Blood Cancer J. 2015; 5: e305.
3.Braamse AM, Gerrits MM, van Meijel B, Visser O, van Oppen P, Boenink AD, et al. Predictors of health-related quality of life in patients treated with auto- and allo-SCT for hematological malignancies. Bone Marrow Transplant. 2012; 47: 757-69.
4.Lee SJ, Joffe S, Kim HT, Socie G, Gilman AL, Wingard JR, et al. Physicians' attitudes about quality-of-life issues in hematopoietic stem cell transplantation. Blood. 2004; 104: 2194-200.
5.Kiss TL, Abdolell M, Jamal N, Minden MD, Lipton JH, Messner HA. Long-term medical outcomes and quality-of-life assessment of patients with chronic myeloid leukemia followed at least 10 years after allogeneic bone marrow transplantation. J Clin Oncol. 2002; 20: 2334-43.
6.Pidala J, Anasetti C, Jim H. Quality of life after allogeneic hematopoietic cell transplantation. Blood. 2009; 114: 7-19.
7.Mosher CE, Redd WH, Rini CM, Burkhalter JE, DuHamel KN. Physical, psychological, and social sequelae following hematopoietic stem cell transplantation: a review of the literature. Psychooncology. 2009; 18: 113-27.
8.Syrjala KL, Langer SL, Abrams JR, Storer B, Sanders JE, Flowers MED, et al. Recovery and long-term function after hematopoietic cell transplantation for leukemia or lymphoma. JAMA. 2004; 291: 2335-43.
9.Kroemeke A, Sobczyk-Kruszelnicka M, Kwissa-Gajewska Z. Everyday life following hematopoietic stem cell transplantation: decline in physical symptoms within the first month and change-related predictors. Qual Life Res. 2018; 27: 125-35.
10.Kenzik K, Huang IC, Rizzo JD, Shenkman E, Wingard J. Relationships among symptoms, psychosocial factors, and health-related quality of life in hematopoietic stem cell transplant survivors. Support Care Cancer. 2015; 23: 797-807.
11.Nelson AM, Coe CL, Juckett MB, Rumble ME, Rathouz PJ, Hematti P, et al. Sleep quality following hematopoietic stem cell transplantation: longitudinal trajectories and biobehavioral correlates. Bone Marrow Transplant. 2014; 49: 1405-11.
12.Schulz-Kindermann F, Hennings U, Ramm G, Zander AR, Hasenbring M. The role of biomedical and psychosocial factors for the prediction of pain and distress in patients undergoing high-dose therapy and BMT/PBSCT. Bone Marrow Transplant. 2002; 29: 341-51.
13.Bevans MF, Mitchell SA, Marden S. The symptom experience in the first 100 days following allogeneic hematopoietic stem cell transplantation (HSCT). Support Care Cancer. 2008; 16: 1243-54.
14.Wingard JR, Huang IC, Sobocinski KA, Andrykowski MA, Cella D, Rizzo JD, et al. Factors associated with self-reported physical and mental health after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010; 16: 1682-92.
15.Lynch Kelly D, Lyon DE, Ameringer SA, Elswick RK. Symptoms, cytokines, and quality of life in patients diagnosed with chronic graft-versus-host disease following allogeneic hematopoietic stem cell transplantation. Oncol Nurs Forum. 2015; 42: 265-75.
16.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors predicting survival after allogeneic stem cell transplant. Support Care Cancer. 2014; 22: 2547-55.
17.Hamilton BK, Law AD, Rybicki L, Abounader D, Dabney J, Dean R, et al. Prognostic significance of pre-transplant quality of life in allogeneic hematopoietic cell transplantation recipients. Bone Marrow Transplant. 2015; 50: 1235-40.
18.Grulke N, Albani C, Bailer H. Quality of life in patients before and after haematopoietic stem cell transplantation measured with the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Core Questionnaire QLQ-C30. Bone Marrow Transplant. 2012; 47: 473-82.
19.Fukaya A, Tsukahara T, Tachibana E, Ono T, Kato A. Nutrition status of allogeneic hematopoietic stem cell transplants and consultation with the nutritional support team at Hamamatsu University Hospital. ―survey for 6 years―. Nagoya Journal of Nutritional Sciences. 2018; 4: 17-24. In Japanese.
20.Inden A, Tsukahara T, Tachibana E, Ono T, Kato A. Evaluation of quality of life using the EORTC QLQ-C30 in allogeneic hematopoietic stem cell transplantation patients at Hamamatsu University Hospital. Nagoya J Nutr Sci. 2021; 7: 39-52. In Japanese.
21.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993; 85: 365-76.
22.Shimozuma K, Katsumata N, Ohashi Y, Makino H, Takashima S, Sonoo H, et al. Impact of surgical adjuvant chemotherapy on quality of life (QOL) of patients with breast cancer (BC) for the second year of treatment-A phase III randomized trial comparing UFT (Uracil/Tegafur) with CMF in highrisk node-negative patients (NSAS-BC 01). ASCO Pro. 2001; 20: 40 1a.
23.Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 Scoring Manual, 3rd ed. Brussels, EORTC Quality of Life Group, 2001
24.Kanda Y. Investigation of the freely available easy-to-use software
25.Japanese Society for Parenteral and Enteral Nutrition. Transplant recipient. In: Japanese Society for Parenteral and Enteral Nutrition, editors. Japanese guidelines for parenteral and enteral nutrition, 3rd ed. Tokyo, Shorinsha Inc, 2013; p. 362-3. In Japanese
26.August DA, Huhmann MB; American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition support therapy during adult anticancer treatment and in hematopoietic cell transplantation. JPEN J Parenter Enteral Nutr. 2009; 33: 472-500.
27.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017; 36: 11-48.
28.Walrath M, Bacon C, Foley S, Fung HC. Gastrointestinal side effects and adequacy of enteral intake in hematopoietic stem cell transplant patients. Nutr Clin Pract. 2015; 30: 305-10.
29.Farhadfar N, Kelly DL, Mead L, Nair S, Colee J, Irizarry Gatell V, et al. Dietary intake and diet quality of hematopoietic stem cell transplantation survivors. Biol Blood Marrow Transplant. 2020; 26: 1154-59.
30.Mulasi U, Vock DM, Jager-Wittenaar H, Teigen L, Kuchnia AJ, Jha G, et al. Nutrition status and health-related quality of life among outpatients with advanced head and neck cancer. Nutr Clin Pract. 2020; 35: 1129-37.
31.Nguyen LT, Dang AK, Duong PT, Phan HBT, Pham CTT, Nguyen ATL, et al. Nutrition intervention is beneficial to the quality of life of patients with gastrointestinal cancer undergoing chemotherapy in Vietnam. Cancer Med. 2021; 10: 1668-80.
32.Charalambous A, Giannakopoulou M, Bozas E, Paikousis L. Parallel and serial mediation analysis between pain, anxiety, depression, fatigue and nausea, vomiting and retching within a randomised controlled trial in patients with breast and prostate cancer. BMJ Open. 2019; 9: e026809.
33.Monti JM, Baym CL, Cohen NJ. Identifying and characterizing the effects of nutrition on hippocampal memory. Adv Nutr. 2014; 5: 337S-43S.
34.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998; 16: 139-44.
Search
News