Volume 6 (2023) Issue 1 No.1 Pages 1-4
Abstract
Background: Pulmonary veno-occlusive disease (PVOD) is a rare but fatal complication of hematopoietic stem cell transplantation (HSCT). Although literature on PVOD post-HSCT is scarce, a recent study has indicated that this condition may be underestimated. Respiratory syncytial virus (RSV) is a common respiratory pathogen that causes common cold in healthy individuals but may lead to severe lower respiratory infection accompanied by respiratory distress in infants and immunocompromised individuals, such as post-HSCT patients. However, little is known about the relationship between PVOD and RSV infections.
Case report: A 4-year-old boy was diagnosed with metastatic neuroblastoma and underwent intensive chemotherapy, autologous HSCT, and allogeneic cord blood transplantation (CBT). He experienced PVOD on day 194 following CBT after displaying upper respiratory symptoms and positive RSV antigen test results approximately one month prior. Pathological examination of a lung biopsy specimen revealed lung injury suspected to be associated with viral infection in addition to PVOD-related findings, suggesting that RSV infection might have contributed to the onset of PVOD.
Conclusions: The patient's clinical history and histological findings indicated that RSV could have triggered the development of PVOD under potential endothelial damage caused by HSCT and other prior treatments. Common respiratory viral infections, such as RSV infection, may evoke the development of PVOD.
Introduction
Pulmonary veno-occlusive disease (PVOD) is a rare but fatal complication following hematopoietic stem cell transplantation (HSCT) presenting as cough, dyspnea, and pulmonary hypertension (PH)1,2. Endothelial damage is considered the main pathophysiology of PVOD which implicates risk factors such as graft-versus-host disease (GVHD), infection, prior use of alkylating agents, and total body irradiation (TBI)1. Only 19 cases of PVOD after HSCT have been reported in the literature (
Respiratory syncytial virus (RSV) is a frequent cause of common cold in healthy individuals; however, it may lead to severe lower respiratory infections accompanied by respiratory distress in infants and immunocompromised individuals, such as post-HSCT patients4,5. However, little is known about the relationship between RSV infection and PVOD after HSCT.
Herein, we describe the clinical outcomes of a pediatric patient who experienced PVOD on day 194 after allogeneic cord blood transplantation (CBT). His clinical course, in conjunction with histological lung biopsy findings, suggested RSV infection as a trigger.
Case Report
A 4-year-old boy with stage IV neuroblastoma received four cycles of chemotherapy, including high-dose chemotherapy including busulfan and melphalan, followed by autologous peripheral blood stem cell transplantation with autologous bone marrow supplementation (
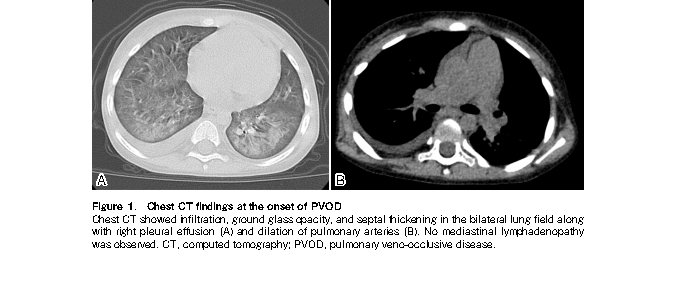
The patient remained healthy with no evidence of GVHD until presentation at our hospital with a productive cough on day 159. As his older brother displayed similar cold symptoms, a rapid antigen test for RSV was performed, which revealed a positive result. His respiratory symptoms gradually worsened, and he revisited our hospital on day 194 with dyspnea and intercostal retractions. Upon admission, he was given 0.7-1.0 mg/kg of prednisolone, which failed to improve his respiratory condition. Chest computed tomography on day 231 revealed infiltration, ground-glass opacity, and septal thickening in the bilateral lung fields along with right pleural effusion (Figure 1,
The patient's respiratory condition transiently improved after the initiation of inhaled NO, ambrisentan, sildenafil, and increased methylprednisolone doses. Tacrolimus was administered throughout the clinical course at target concentrations of 4-6 ng/mL for continuous infusion and 2 ng/mL for oral intake. His respiratory condition improved temporarily after increasing the dose of methylprednisolone, with PH improvement on echocardiography showing disappearance of tricuspid regurgitation and rounding of the interventricular septum shape. However, he continuously required inhaled NO and high-dose prednisolone to maintain his respiratory condition (
Although mechanical ventilation with inhaled NO enabled a relatively stable respiratory status for 55 days, a metastatic relapse was identified on day 336 based on abnormal accumulations in the cranium and liver, and a right mediastinal mass was detected by I123-MIBG scintigraphy. Despite no additional radiation, chemotherapy, or changes in the management of PH, the patient succumbed to respiratory failure on day 349.
Discussion
The present patient had been aggressively treated with HSCT and multiple courses of chemotherapy, including TBI and large amounts of alkylating drugs, which is consistent with other reported cases of PVOD following HSCT1,2. Moreover, such an extensive treatment history may have been associated with potential endothelial damage and the subsequent development of PVOD.
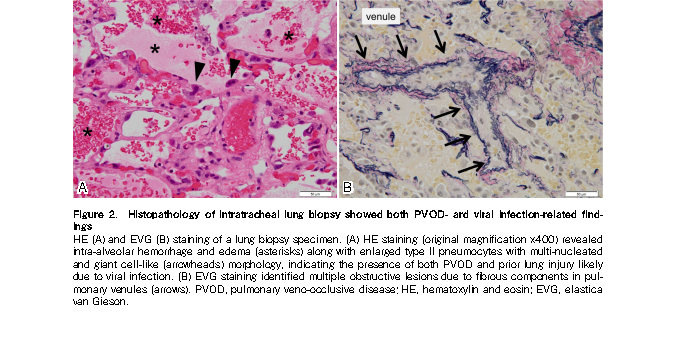
The patient presented with upper respiratory symptoms along with positive RSV rapid antigen test results approximately one month before the onset of PVOD. Moreover, histological examination of a lung biopsy specimen revealed lung injury findings indicative of prior viral infection, in addition to PVOD-related damage. Of note, the pathological findings of loose fibrocellular intimal thickening with swollen endothelial cells in the pulmonary veins/venules suggested subacute changes rather than long-standing alterations characterized by dense fibrous occlusions. Considering the timing of onset, these findings support the contribution of RSV in PVOD development in this case.
RSV is known to cause bronchiolitis, pneumonia, and other serious respiratory diseases in addition to long-term complications in post-HSCT patients4,6. However, information regarding the relationship between RSV and PVOD is scarce. Zinter et al.7 described a 20-year-old man who experienced PVOD 77 days after HSCT (
To our knowledge, only 20 cases of PVOD post-HSCT have been reported, including the present case (
A limitation of this report is that we did not evaluate the genetic predisposition to PVOD11; however, no family history of PVOD or PH was noted in the patient or relatives.
In conclusion, we encountered a unique case of PVOD in a post-HSCT patient following an RSV infection. Based on the patient's clinical history and histological findings, RSV was considered to have triggered the onset of PVOD. Clinicians should be aware of the possibility of late-onset complications of HSCT.
Acknowledgments
The authors thank Mr. Trevor Ralph for his English proofreading.
Author Contributions
T.W. and S.S. drafted the original manuscript. S.S. contributed to the conception and design of the report. T.W., K.K., S.S., E.U., T.K., M.K., H.M., K.T., Y.N., and K.S. contributed to patient care. T.W., S.S., Y.O., and K. O-O. contributed to data interpretation. All authors drafted and critically revised the manuscript, agreed to be fully accountable for ensuring the integrity and accuracy of the work, and read and approved the final manuscript.
Acknowledgments
The authors thank Mr. Trevor Ralph for his English proofreading.
Consent for Publication
Written informed consent for publication was obtained from the parents.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Bunte MC, Patnaik MM, Pritzker MR, Burns LJ. Pulmonary veno-occlusive disease following hematopoietic stem cell transplantation: a rare model of endothelial dysfunction. Bone Marrow Transplant. 2008; 41: 677-86.
2.Holcomb BW Jr, Loyd JE, Ely EW, Johnson J, Robbins IM. Pulmonary veno-occlusive disease: a case series and new observations. Chest. 2000; 118: 1671-9.
3.Gazourian L, Spring L, Meserve E, Hwang D, Diaz AA, Ash SY, et al. Pulmonary Clinicopathological Correlation after Allogeneic Hematopoietic Stem Cell Transplantation: An Autopsy Series. Biol Blood Marrow Transplant. 2017; 23: 1767-72.
4.Khawaja F, Chemaly RF. Respiratory syncytial virus in hematopoietic cell transplant recipients and patients with hematologic malignancies. Haematologica. 2019; 104: 1322-31.
5.Houist AL, Bondeelle L, Salmona M, LeGoff J, de Latour RP, Rivière F, et al. Evaluation of prognostic scores for respiratory syncytial virus infection in a French multicentre cohort of allogeneic haematopoietic stem cell transplantation recipients. Bone Marrow Transplant. 2021; 56: 3032-41.
6.Seo S, Campbell AP, Xie H, Chien JW, Leisenring WM, Englund JA, et al. Outcome of respiratory syncytial virus lower respiratory tract disease in hematopoietic cell transplant recipients receiving aerosolized ribavirin: significance of stem cell source and oxygen requirement. Biol Blood Marrow Transplant. 2013; 19: 589-96.
7.Zinter MS, Melton A, Sabnis AJ, Dvorak CC, Elicker BM, Nawaytou HM, et al. Pulmonary veno-occlusive disease in a pediatric hematopoietic stem cell transplant patient: a cautionary tale. Leuk Lymphoma. 2018; 59: 1494-7.
8.Kimura D, McNamara IF, Wang J, Fowke JH, West AN, Philip R. Pulmonary hypertension during respiratory syncytial virus bronchiolitis: a risk factor for severity of illness. Cardiol Young. 2019; 29: 615-9.
9.Kimura D, Saravia J, Jaligama S, McNamara I, Vu LD, Sullivan RD, et al. New mouse model of pulmonary hypertension induced by respiratory syncytial virus bronchiolitis. Am J Physiol Heart Circ Physiol. 2018; 315: H581-9.
10.Groskreutz DJ, Babor EC, Monick MM, Varga SM, Hunninghake GW. Respiratory syncytial virus limits alpha subunit of eukaryotic translation initiation factor 2 (eIF2alpha) phosphorylation to maintain translation and viral replication. J Biol Chem. 2010; 285: 24023-31.
11.Eyries M, Montani D, Girerd B, Perret C, Leroy A, Lonjou C, et al. EIF2AK4 mutations cause pulmonary veno-occlusive disease, a recessive form of pulmonary hypertension. Nat Genet. 2014; 46: 65-9.
12.Kawashima N, Fukasawa Y, Nishikawa E, Ohta-Ogo K, Ishibashi-Ueda H, Hamada M, et al. Echocardiography Monitoring of Pulmonary Hypertension after Pediatric Hematopoietic Stem Cell Transplantation: Pediatric Pulmonary Arterial Hypertension and Pulmonary Veno-Occlusive Disease after Hematopoietic Stem Cell Transplantation. Transplant Cell Ther. 2021; 27: 786.e1-e8.
Search
News