Volume 6 (2023) Issue 1 No.2 Pages 5-10
Abstract
Introduction: Allogeneic hematopoietic stem cell transplantation (HSCT) is currently the only curative treatment option for myelofibrosis (MF). Despite the benefits of long-term relapse-free survival, HSCT can be associated with substantial treatment-related morbidity and mortality.
Methods: This is an observational retrospective study of 15 consecutive patients with MF who underwent allogeneic HSCT at a tertiary care center in Northern India between June 2012 and January 2020. The pre-transplant Dynamic International Prognostic Scoring System (DIPSS) and hematopoietic cell transplantation-specific co-morbidity index (HCT-CI) scores were used. The primary endpoints were overall survival (OS) and disease-free survival (DFS), and the secondary endpoints were post-transplant complications (acute and chronic graft-versus-host-disease [GvHD], graft failure [GF], and cytomegalovirus reactivation [CMV]).
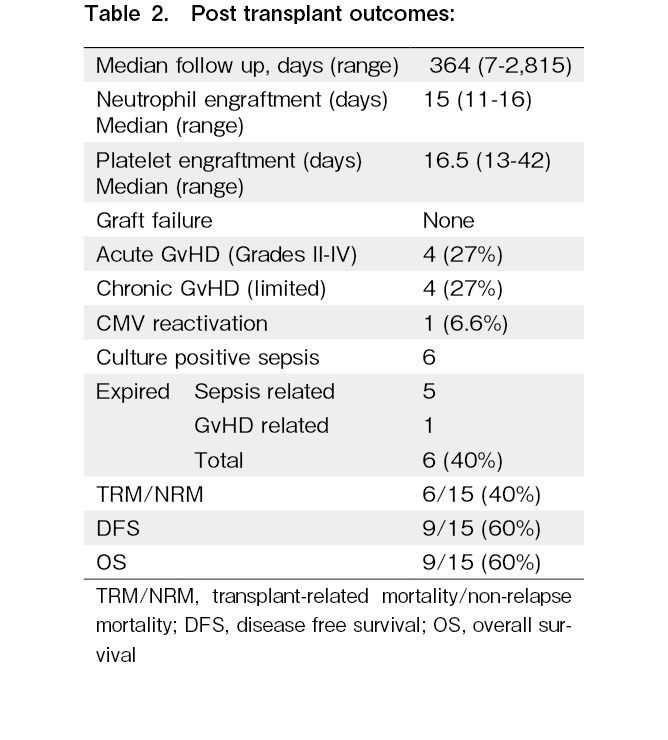
Results: The OS and DFS in our study were 60% with no relapse at a median follow-up of 364 days (range 7-2,815 days). Twenty-seven percent of patients developed acute GvHD and 27% of patients developed chronic (limited) GvHD. The non-relapse mortality (NRM) was 40%, with the main cause of death being sepsis, followed by acute GvHD.
Conclusion: MF remains a challenging condition to treat, with a poor prognosis. Our study showed that reduced toxicity conditioning provided good DFS and OS. Thus, it should be offered to patients with high DIPSS scores. Sepsis was the predominant cause of mortality in this cohort.
Introduction
Myelofibrosis (MF) is a chronic myeloproliferative neoplasm (MPN) with the expansion of clonal stem cells characterized by ineffective hematopoiesis, dysplastic megakaryocytic hyperplasia, osteosclerosis, intramedullary fibrosis, and extramedullary hematopoiesis. Clinical manifestations of MF include progressive cytopenia, constitutional symptoms, hepatosplenomegaly, thrombotic events, bone pain, and cachexia1. MF is subcategorized into de novo MF (primary MF) and secondary MF, which can be post-essential thrombocythemia or post-polycythemia vera2. It is predominantly a disease of the elderly population. Leukemic transformation occurs in approximately 20% of patients, and 98% mortality is observed within 3 months in patients with acute myelocytic leukemia (AML)3.
Patients with intermediate- or high-risk MF are ideal candidates for therapy which includes hydroxyurea, blood transfusions, splenectomy, or JAK inhibitors such as ruxolitinib. These therapies improve symptoms, overall survival (OS), and quality of life1. However, allogeneic hematopoietic stem cell transplantation (HSCT) is the only potentially curative treatment for MF in a select group of patients4,5. The number of transplants performed for MF has increased in the recent past, despite the use of JAK inhibitors5 and the high morbidity and mortality associated with HSCT, particularly in older adults6. Judicious candidate selection for HSCT and the optimal time for transplantation have been the subject of constant debate in MF.
The data of patients with MF who underwent allogeneic SCT at our transplant center were analyzed retrospectively.
Patients and Methods
Fifteen consecutive patients who underwent allogeneic HSCT for MF at our center from June 2012 to January 2020 were evaluated retrospectively using the hospital information system and by analyzing the medical records. Informed consent was obtained from all patients, and the study was approved by Hospital Ethical Committee and Institutional Review Board (in accordance with the Declaration of Helsinki: EC/AARCE/APPROVALLETTER/JANUARY/2022/22a; dated 18/01/2022). All transplants were conducted in HEPA-filtered rooms.
Stem cells were obtained from human leukocyte antigen (HLA)-matched related, haploidentical, and unrelated donors. Peripheral blood stem cells were used for all patients who underwent allogeneic HSCT. Donor peripheral blood stem cells were mobilized using granulocyte colony-stimulating factor (G-CSF). All patients received antimicrobial prophylaxis, including fluconazole, acyclovir, and Co-trimoxazole, and febrile neutropenia was treated according to hospital policy. Engraftment was defined as an absolute neutrophil count > 500/μL for three consecutive days and a platelet count > 20,000/μL for 7 days after the last platelet transfusion. Patients were regularly monitored in the outpatient clinic after discharge.
A reduced-intensity conditioning regime was used for all 12 matched sibling (MSD) transplants. Fludarabine (30 mg/m2/dose intravenously for 6 days), melphalan (140 mg/m2/dose intravenously for 1 day). One patient who underwent a matched unrelated donor (MUD) transplant received busulfan (3.2 mg/kg/dose intravenously for 2 days), fludarabine (30 mg/m2/dose intravenously for 5 days), and anti-thymocyte globulin (Thymoglobulin-1.5 mg/kg intravenously for 3 days). Two patients who underwent haploidentical HSCT received fludarabine (30 mg/m2/dose intravenously for 5 days), cyclophosphamide (14.5 mg/kg intravenous for 2 days), and total body irradiation (TBI) 400cGy (divided into two fractions).
In all HLA-matched transplants, standard cyclosporine A (CsA) and methotrexate therapy were implemented as prophylaxis for graft-versus-host disease (GvHD). Post-transplant, the GvHD prophylaxis used for haploidentical HSCT was cyclophosphamide (PTCy) 50 mg/kg/day intravenously on days +3 and +4 along with oral tacrolimus (0.06 mg/kg twice daily) and oral mycophenolate sodium (10 mg/kg/dose three times daily) from day +5. Tapering of immune suppression was initiated 3 months after allogeneic HSCT in the absence of acute or chronic GvHD, with the aim of ceasing it approximately 6 months post-HSCT.
The primary endpoints of the study were OS and DFS, and the secondary endpoints were post-transplant complications (acute and chronic GvHD, graft failure [GF], and CMV reactivation [CMV]). OS was defined as the time from transplantation to death from any cause or the last follow-up. DFS was defined as the time between transplantation and relapse. The surviving patients were censored at the last follow-up visit. Acute and chronic GvHD were graded according to the related consensus criteria7,8. Relapse was defined as falling chimerism on serial monitoring; if it was persistently falling, bone marrow aspirations and biopsies were performed.
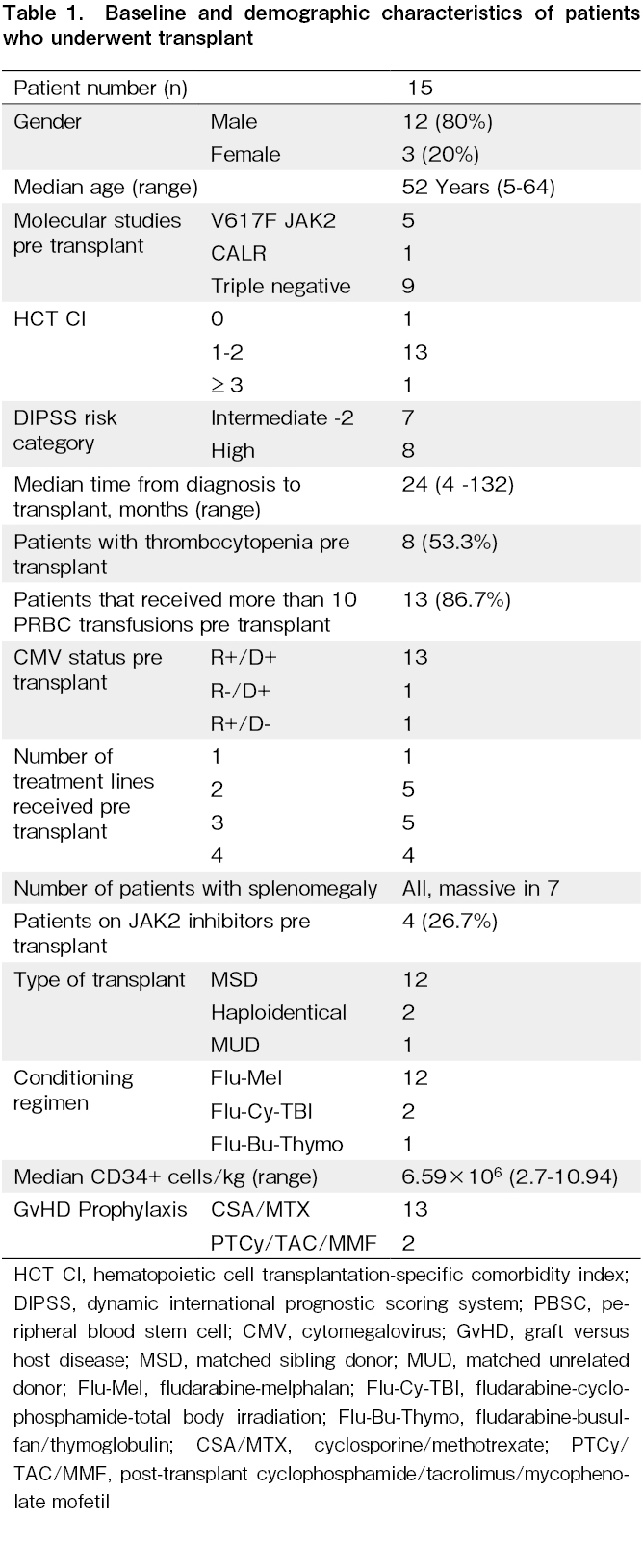
Human leukocyte antigen (HLA) typing was performed using high-resolution typing (HLA A, B, C, DRB1, and DQB1). Donor-specific antibodies (DSA) were screened by the Single Bead assay on Luminex for all haploidentical transplants, and if borderline or high (median fluorescence intensity [MFI] >2000), the patients were administered a dose of rituximab (375 mg/m2) pre- transplant or plasmapheresis was performed. The DSA was repeated pre-transplant to re-evaluate the MFI (Table 1).
Statistical Analysis
Statistical Package for Social Science (SPSS) 25 (IBM Corp., USA) was used to perform the statistical analyses. Survival analyses were performed using Kaplan-Meier tests. Univariate analyses of risk factors for HSCT were performed using the Cox regression test, and a P value <0.05 was considered statistically significant.
Result
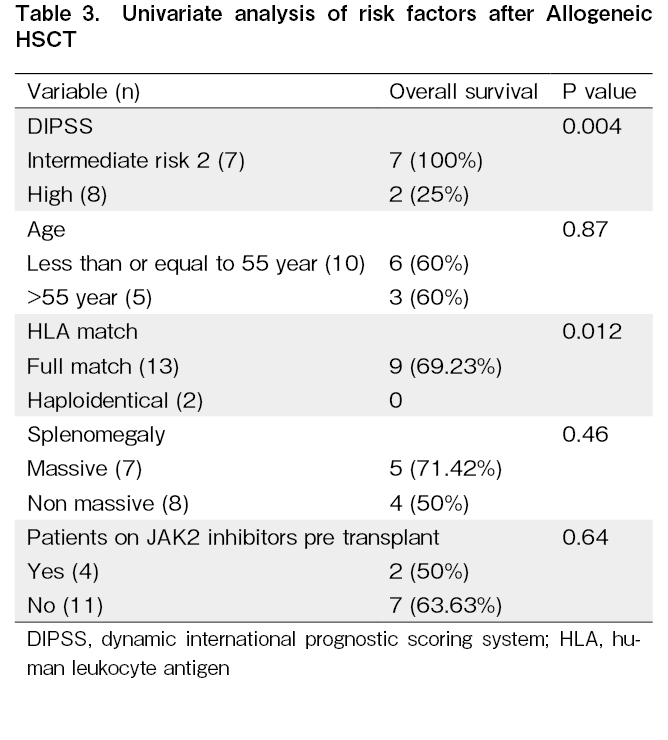
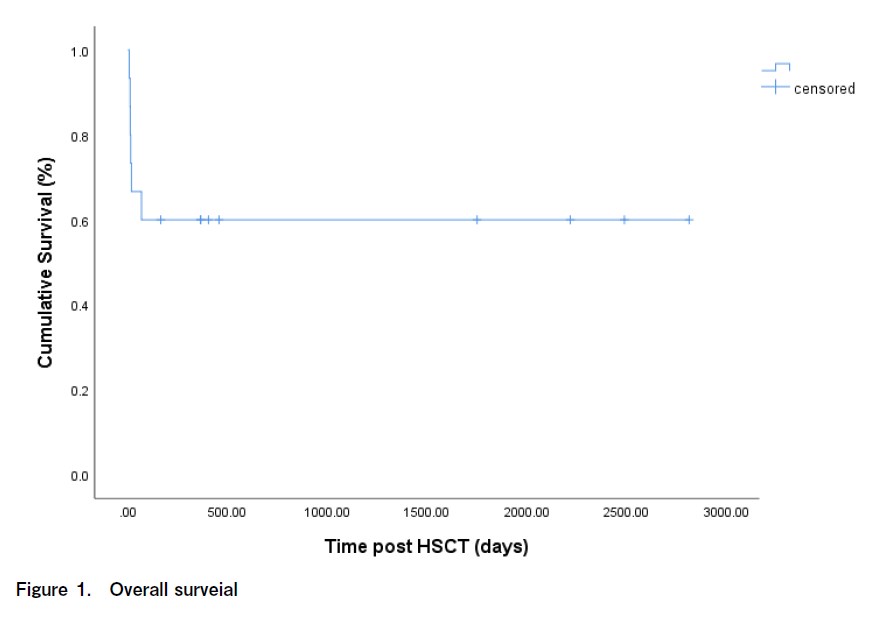
Fifteen transplantation patients were evaluated, 12 of whom were male, and three were female, with a median age of 52 years (range 5-64 years old). Eight patients were in the high-risk DIPSS category, and seven were in the intermediate-risk category (Table 2 and 3). Univariate analysis of the risk factors for HSCT in MF showed that the DIPSS intermediate risk-2 group had a higher OS than the DIPSS high-risk group (P = 0.004). The outcome of HLA-matched patients was also better than that of haploidentical patients (P = 0.012). Mismatched transplantation is a major risk factor for therapy-related mortality; therefore, careful donor selection is required. In our study, 27% of the patients developed acute GvHD, and 27% of the patients developed chronic (limited) GvHD. One patient developed acute gut GvHD (stage 4) and was demised. Two patients developed acute liver GvHD (stage 2), and one patient developed acute skin GvHD (stage 2). Three patients developed chronic liver GvHD, and one patient developed limited chronic oral GvHD. At a median follow-up of 364 days (range, 7-2815 days), the OS of the patients in this study was 60% (Figure 1). The median post-engraftment chimerism on day +21 of all engrafted patients were 97.85% (range 89.56-100%). Of the 40% of deaths reported, 34% were due to sepsis, and 6% were due to GvHD. Five of the six deaths that occurred before engraftment were due to sepsis. The blood cultures of three of the patients were positive for Klebsiella pneumonia, Pseudomonas aeruginosa, and Candida tropicalis.
None of the patients who were demised received pre-transplant ruxolitinib. At the last follow-up, no disease relapse was noted in any patient.
Discussion
Allogeneic HSCT remains the only curative approach for transplant-eligible patients despite advances in the availability of novel therapeutic agents for MF. The pre-transplantation use of ruxolitinib to reduce spleen size and facilitate early engraftment has been reported. In a resource-constrained country, the availability and accessibility to non-transplant options are limited and cost-prohibitive for patients with MF.
Multidrug-resistant bacteria, such as New Delhi metallo-b-lactamase (NDM), are a growing concern in tropical countries, especially India9. These bacteria contribute to the high morbidity and mortality rates observed in patients undergoing HSCT. In our study, five patients were demised from multidrug-resistant sepsis. Therefore, stringent monitoring and aggressive treatment of all infections are required.
Massive splenomegaly is a common presenting feature of MF and reflects the chronicity and disease burden of patients. Several studies have highlighted that massive splenomegaly may be associated with a higher risk of graft failure, with a significant delay in neutrophil and platelet engraftment and an increased incidence of poor graft function10. Thus, many physicians prefer to perform splenectomy prior to transplantation. All the patients in this study presented with enlarged spleens, with 46.6% having a massive spleen prior to transplantation. However, none of these were splenectomized pre-transplants. Therefore, massive splenomegaly may have contributed to the high mortality rate in this study population. These patients may represent a more aggressive disease phenotype, and pre-transplant splenectomy or ruxolitinib should be considered to reduce spleen size.
In a prospective study, Gupta et al. reported a trial of ruxolitinib treatment followed by HSCT using a reduced-intensity conditioning (RIC) regimen11. The OS at 2 years was 61% and 70% for MSD and MUD HSCT, respectively. Incidences of graft failure, NRM, acute GvHD, and chronic GvHD at 2 years were 16%, 28%, 64%, and 76%, respectively. There was no difference in any of the outcome variables between those who responded to ruxolitinib and those who failed or lost response to the drug11. Rondelli et al. prospectively analyzed 66 patients with MF who underwent RIC HSCT. The OS was 75% in MSD HSCT cases and 32% in MUD HSCT cases. Grade II-IV acute GvHD occurred in 38% of MSD HSCT and 41% of MUD HSCT12. Kroger et al. demonstrated that after a median follow-up of 33 months (range, 12-76 months), the 5-year DFS and OS were 51% and 67%, respectively. The incidence of acute GvHD grades II-IV was 27%, and that of severe GvHD grades III-IV was 11%. There was no difference in acute GvHD between transplantation from MSD or MUD, and the incidence of NRM at 1 year was 16%. The rate of chronic GVHD was 49%, which was limited to 24% and extensive to 24%11. Risk factors for OS were age, DIPSS score, HLA match status, massive splenomegaly, and pre-transplant treatment with JAK2 inhibitors11,13,14. In a multicenter study by McLornan et al. involving 2,224 patients, of which 1,443 underwent RIC HSCT, the OS and relapse rates were 51% and 19.3%, respectively. The acute grade II-IV GvHD rate was 31%15. Another multicenter study evaluating 1,928 MF patients post-HSCT suggested considering HSCT in DIPSS intermediate-1 risk MF patients and supported the recommendation of HSCT in intermediate-2 to high-risk MF. The adjusted OS in the entire cohort, not stratified by DIPSS, was 47%. Forty-one percent of the patients in this study received an RIC regime16. In our study, only four patients (26.7%) received ruxolitinib pre-transplant. Of the six patients who died, none had received ruxolitinib pre-transplant. Thus, it may be assumed that pre-transplant exposure to ruxolitinib may have reduced the mortality rate.
In our study, univariate analysis of risk factors for HSCT in MF, the DIPSS intermediate risk-2 group had a higher OS than the high-risk group (P = 0.004). The outcome of HLA-matched versus haploidentical patients was also better (P = 0.012). Mismatched transplantation remains a major risk factor for transplant-related mortality and thus careful donor selection is required. Therefore, in high-risk patients, HLA-matched transplants are preferable because of the high transplant-related mortality with mismatched donors. Multivariate analysis showed no significant risk factors in this study. Furthermore, in our study, 27% of the patients developed acute GvHD, and 27% of the patients developed chronic (limited) GvHD. The GvHD rates in our study were comparable to those reported in other studies, and mortality due to GvHD was low. Therefore, conditioning and GvHD prophylaxis were acceptable. The OS in our study was 60%, which is encouraging. Out of 40% of the deaths, 6% deaths were due to GvHD, and 34% were due to sepsis. None of the patients developed relapse, which is possibly due to the short follow-up period (median, 364 days) and is a limitation of the study. Therefore, limitations of this study include its limited size, decreasing potential power of statistical analysis, and retrospective collection of data.
Acknowledgments
The authors thank Dr. Amee Patel for statistical analysis and Ms Pallavi Sharma for data compilation.
Author Contributions
DC, DD, and VK performed transplants, DC, DD, RS, and AH designed research, and DC and DD wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Jain T, Mesa RA, Palmer JM. Allogeneic stem cell transplantation in myelofibrosis. Biol Blood MarrowTranspl. 2017; 23: 1429-36.
2.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016; 127: 2391-405.
3.Mesa RA, Li CY, Ketterling RP, Schroeder GS, Knudson RA, Tefferi A. Leukemic transformation in myelofibrosis with myeloid metaplasia: A single-institution experience with 91 cases. Blood. 2005; 105: 973-7.
4.Deeg HJ, Gooley TA, Flowers ME, Sale GE, Slattery JT, Anasetti C, et al. Allogeneic hematopoietic stem cell transplantation for myelofibrosis. Blood. 2003; 102: 3912-8.
5.Lavi N, Rowe JM, Zuckerman T. Allogeneic stem-cell transplantation for myelofibrosis. Curr Opin Hematol. 2017; 24: 475-80.
6.Kröger N. Outcome improvement after allogeneic stem-cell transplantation in myelofibrosis. J Oncol Pract. 2016; 12: 629-31.
7.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974; 18: 295-304.
8.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015; 21: 389-401.e1.
9.Johnson AP, Woodford N. Global spread of antibiotic resistance: the example of New Delhi metallo-b-lactamase (NDM)-mediated carbapenem resistance. J Med Microbiol. 2013; 62: 499-513.
10.Polverelli N, Mauff K, Kröger N, Robin M, Beelen D, Beauvais D, et al. Impact of spleen size and splenectomy on outcomes of allogeneic hematopoietic cell transplantation for myelofibrosis: A retrospective analysis by the chronic malignancies working party on behalf of European society for blood and marrow transplantation (EBMT). Am J Hematol. 2021; 96: 69-79.
11.Gupta V, Kosiorek HE, Mead A, Klisovic RB, Galvin JP, Berenzon D, et al. Ruxolitinib Therapy Followed by Reduced-Intensity Conditioning for Hematopoietic Cell Transplantation for Myelofibrosis: Myeloproliferative Disorders Research Consortium 114 Study. Biol Blood Marrow Transplant. 2019; 25: 256-64.
12.Rondelli D, Goldberg JD, Isola L, Price LS, Shore TB, Boyer M, et al. MPD-RC 101 prospective study of reduced-intensity allogeneic hematopoietic stem cell transplantation in patients with myelofibrosis. Blood. 2014; 124: 1183-91.
13.Kröger N, Holler E, Kobbe G, Bornhäuser M, Schwerdtfeger R, Baurmann H, et al. Allogeneic stem cell transplantation after reduced-intensity conditioning in patients with myelofibrosis: a prospective, multicenter study of the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2009; 114: 5264-70.
14.Shahnaz Syed Abd Kadir S, Christopeit M, Wulf G, Wagner E, Bornhauser M, Schroeder T, et al. Impact of ruxolitinib pretreatment on outcomes after allogeneic stem cell transplantation in patients with myelofibrosis. Eur J Haematol. 2018; 101: 305-17.
15.McLornan D, Szydlo R, Koster L, Chalandon Y, Robin M, Wolschke C, et al. Myeloablative and Reduced-Intensity Conditioned Allogeneic Hematopoietic Stem Cell Transplantation in Myelofibrosis: A Retrospective Study by the Chronic Malignancies Working Party of the European Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2019; 25: 2167-71.
16.Gowin K, Ballen K, Ahn KW, Hu ZH, Ali H, Arcasoy MO, et al. Survival following allogeneic transplant in patients with myelofibrosis. Blood Adv. 2020; 4: 1965-73.
Search
News