Volume 5 (2022) Issue 2 No.3 Pages 54-60
Abstract
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an integral part of the treatment strategy for patients with malignant or non-malignant hematological diseases. Clinical outcomes of patients undergoing allo-HSCT have significantly improved in recent decades. However, transplant-related morbidity and mortality remain major issues for allo-HSCT recipients.
With regard to nutrition, patients undergoing allo-HSCT are at high risk for malnutrition. It is expected that clinical practice concerning nutritional support in allo-HSCT has been improving in recent decades; however, no data directly support this expectation. One major issue in managing nutritional support during allo-HSCT is the lack of large-scale randomized prospective studies, which leads to a lack of well-established strategies. Accordingly, we need to gather data from studies in non-HSCT and allo-HSCT settings. In some Asia-Pacific countries, a physician's lack of knowledge of nutritional support may impede the application of nutritional support practices recommended by existing guidelines. Another barrier may be the lack of access to an adequately qualified or trained registered dietitian (RD) at allo-HSCT units. Adequate training in the nutritional management of allo-HSCT patients should be provided to all RDs working with HSCT. Herein, we summarize the information on nutritional support in allo-HSCT, focusing on an Asian perspective.
1. Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an integral part of the treatment strategy for patients with malignant or non-malignant hematological diseases. The clinical outcomes of patients undergoing allo-HSCT have improved significantly in recent decades1, 2. However, morbidity and mortality undoubtedly remain major issues in allo-HSCT recipients.
Patients undergoing allo-HSCT are at high risk for malnutrition3, 4. Although we expect that clinical practice relating to nutritional support in allo-HSCT has improved in recent decades, there is no evidence directly supporting this expectation. One major issue is the lack of large-scale randomized prospective studies, resulting in a lack of well-established strategies in nutritional support during allo-HSCT. Accordingly, data from studies in non-HSCT and allo-HSCT settings need to be collated. Herein, we summarize information on nutritional support in allo-HSCT, with emphasis on an Asian perspective.
2. Pre-transplant Nutritional Support
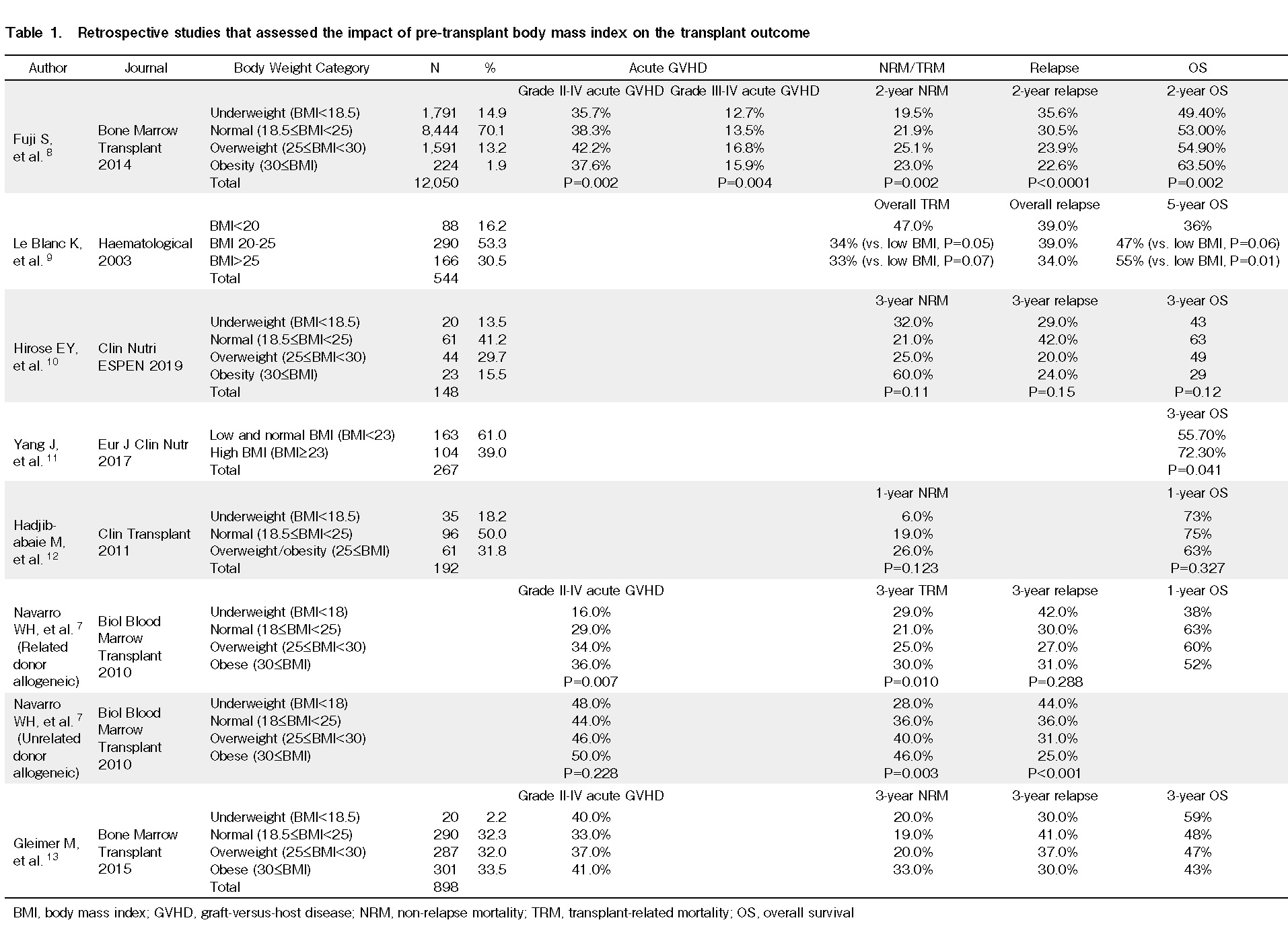
After the diagnosis of hematological malignancy, patients typically have a lead time up to allo-HSCT, and this interval varies among hematological diseases. For instance, patients with acute myeloid leukemia receive induction chemotherapy and a few courses of consolidation therapy before allo-HSCT, which takes approximately 3 months. Thus, there is sufficient time to intervene from the viewpoint of nutritional support during this period. Importantly, malnutrition should be avoided before allo-HSCT3. Patients who undergo intensive chemotherapy are at a high risk of malnutrition4, 5. Various retrospective studies have reported the impact of pre-transplant malnutrition on clinical outcomes after allo-HSCT (Table 1)6–13. Furthermore, poor performance status during allo-HSCT is a well-established adverse prognostic factor14, 15. Accordingly, in such cases, intensive nutritional support incorporating rehabilitation should be undertaken to maintain good performance status4, 16. Malnutrition status, so-called
At the time of diagnosis of hematological malignancy, patients can begin a treatment pathway leading to allo-HSCT. Therefore, it is recommended that the nutritional status be assessed early at initial diagnosis, as stated in the European Society for Clinical Nutrition and Metabolism (ESPEN) and the American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines4, 16. Screening is usually performed by a registered dietitian (RD). Weight loss, low body mass index, reduced muscle mass, reduced food intake, and the presence of inflammation are key parameters often employed to diagnose malnutrition22, 23. Screening tools incorporating these parameters are commonly used to assess the nutritional status. It is important to regularly repeat assessments in patients who are planning to undergo HSCT to detect and identify any signs of malnutrition.
In addition, it is critical to improve preexisting metabolic syndrome before allo-HSCT, including diabetes mellitus and obesity. Diabetes mellitus (DM) is a well-known poor prognostic factor for allo-HSCT. Pre-transplant DM, which requires treatment, is a factor for the hematopoietic cell transplantation comorbidity index (HCT-CI)24, 25. Other retrospective studies have also found that pre-transplant hyperglycemia was associated with poor clinical outcomes post-allo-HSCT26–28. The most common cause of DM is type 2 DM, which can be improved by nutritional intervention, including lifestyle modifications such as dietary interventions and physical activity29, 30. Additionally, pre-transplant obesity is a well-known poor prognostic factor in allo-HSCT. Pre-transplant obesity is also a factor for HCT-CI24, 25. According to a few retrospective studies, pre-transplant obesity was associated with an increased risk of non-relapse mortality, although pre-transplant obesity was not associated with a poor clinical outcome in these studies7, 8.
In summary, assessment of nutritional status by an RD and consultation with a physiotherapist for rehabilitation are recommended during the diagnosis of hematological disease in transplant candidates. Interventions to improve pre-transplant nutritional status and performance status are crucial for improving clinical outcomes following allo-HSCT31.
3. Early Period after Allo-HSCT
Patients who undergo allo-HSCT are at a high risk of morbidities and mortalities after allo-HSCT, particularly during the early period post-allo-HSCT. Nutritional support during this period is crucial for maintaining an adequately healthy nutritional status after allo-HSCT3, 32. First, total caloric intake should be closely monitored, and when total caloric intake becomes insufficient, nutritional support should be implemented to maintain adequate total caloric intake to meet estimated needs31, 33. The target total caloric intake can be determined using the Harris-Benedict formula or other formulas. If the oral caloric intake decreases, additional snacks, protein/caloric enrichment of food, or energy/protein-dense drinks can be supplemented. If the total caloric intake continues to be insufficient, artificial nutritional support such as enteral nutrition (EN) or parenteral nutrition (PN) can be initiated. In medical inpatients, the use of individualized nutritional support can improve important clinical outcomes, including overall survival, compared with the use of standard hospital food meals alone34. Thus, in patients undergoing allo-HSCT, nutritional support options should also be applied31.
Recently, various studies have reported the possible beneficial effect of EN after allo-HSCT35–37. No published study has demonstrated the favorable effect of EN when compared with PN in prospective randomized controlled trials. However, retrospective studies have suggested that EN affords clinical benefits post-allo-HSCT35, 36. In detail, a French group reported that patients who received EN demonstrated a significantly lower incidence of severe acute graft-versus-host disease (GVHD) and death due to infectious diseases than those who received PN after allo-HSCT35, 36. Additionally, recent studies reporting the clinical relevance of microbiota supported the importance of EN in maintaining the microbiota status after allo-HSCT38–40. Although meeting full caloric needs with EN alone might be challenging, a degree of EN infusion, also known as trophic feeding, could be beneficial41–44. Furthermore, a recent prospective study incorporating prebiotics suggested the beneficial impact of prebiotics using resistant starch and a commercially available prebiotic mixture in patients who underwent allo-HSCT, which shortened the duration of oral mucositis and diarrhea and reduced the incidence and severity of acute GVHD45. The authors performed a detailed analysis of the microbiota, revealing that the microbial diversity, the population of butyrate producers, and butyrate concentration were maintained in patients who consumed prebiotics. A similar beneficial effect of prebiotics has been previously reported46. These results suggest a beneficial effect of prebiotics after allo-HSCT by maintaining the microbiota status, which should be assessed in large-scale studies in the future.
Hyperglycemia is another issue during the early phase after allo-HSCT. Several factors, including immunosuppressive drugs, PN, and inflammation, lead to elevated glucose levels post-allo-HSCT. Retrospective studies have shown that the presence of hyperglycemia or malglycemia during the early phase after allo-HSCT is associated with an inferior clinical outcome28, 47–51. Such adverse effects of post-transplant DM have also been observed in organ transplantation52, 53. No large-scale study has prospectively reported the benefits of intensive glucose control after allo-HSCT. One small study incorporated intensive glucose control post-allo-HSCT54. The authors demonstrated the feasibility of intensive glucose control after allo-HSCT, suggesting the possible beneficial effects of intensive glucose control. The incidence of infectious disease was significantly lower in the intervention group than in the historical control group54. Subsequently, a prospective multi-center study incorporating intensive glucose control post-allo-HSCT demonstrated a similar promising clinical outcome: low infectious disease and non-relapse mortality rates, considering that almost all of the patients in this study received a classical myeloablative conditioning regimen55. Intriguingly, a recent study that prospectively assessed the impact of dipeptidyl peptidase 4 (DPP4) inhibitors on the incidence of acute GVHD demonstrated a promising, low incidence of severe acute GVHD56. The authors reported no data on glucose control, but it is expected that glucose levels would decrease when using an anti-hyperglycemic drug, such as a DPP-4 inhibitor. The benefits of incorporating DPP-4 inhibitors after allo-HSCT should be reassessed in future trials.
4. Long-term Follow-up Post-allo-HSCT
Patients who undergo allo-HSCT are at a high risk of long-term malnutrition long-term post-allo-HSCT3, 57, 58. In addition, they are at risk for malnutrition secondary to chronic GVHD, particularly gastrointestinal chronic GVHD. Allo-HSCT recipients are also at risk for metabolic syndrome associated with drugs used for GVHD prophylaxis or treatment, such as systemic corticosteroids, calcineurin inhibitors, and mTOR inhibitors.
In outpatient clinics, access to routine monitoring of nutritional status in allo-HSCT recipients might be limited. However, as discussed above, patients are also at a high risk of malnutrition post-allo-HSCT, especially in the setting of chronic GVHD. Thus, it is recommended to incorporate routine nutritional status assessments by RDs as part of the patient's long-term follow-up regimen.
Moreover, it is important to educate patients and family members regarding nutrition before discharge or during the post-transplant period59. Nutrition education should include topics such as food safety, food hygiene, and food allergies. It is particularly crucial to educate recipients of cord blood transplants, as transplant-acquired food allergy could be a serious complication, and guidance regarding this issue is critical to mitigating the risk of such complications60, 61.
5. Barriers to Improving Clinical Practice in Relation to Nutritional Support post-allo-HSCT
As discussed above, various interventions can be employed to maintain the nutritional status of patients undergoing allo-HSCT. However, in real-world scenarios, there are various barriers to improving clinical practice following allo-HSCT. In some countries, a physician's lack of knowledge regarding nutritional support may hinder the application of nutritional support practices recommended by guidelines, such as ESPEN or ASPEN, in patients who will receive allo-HSCT. In Asia-Pacific countries, another barrier may be the lack of access to adequately qualified or trained RDs at some allo-HSCT units. It is also highly recommended that adequate training in the nutritional management of allo-HSCT recipients be provided to all RDs working within institutions performing allo-HSCT. This is especially pertinent for those institutions or transplant centers that frequently perform allo-HSCT.
6. Conclusion
Undoubtedly, nutritional support in HSCT is crucial for optimizing the nutritional status of patients undergoing allo-HSCT. This is believed to contribute to improving clinical outcomes after allo-HSCT. However, clinical studies assessing the importance of each nutritional intervention are limited. Further studies focusing on nutritional support for HSCT are required.
Author Contributions
S.F. and J.C. planned and reviewed published papers, and all the authors wrote and reviewed the manuscript. All the authors approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010; 363: 2091-101.
2.McDonald GB, Sandmaier BM, Mielcarek M, Sorror M, Pergam SA, Cheng GS, et al. Survival, Nonrelapse Mortality, and Relapse-Related Mortality After Allogeneic Hematopoietic Cell Transplantation: Comparing 2003-2007 Versus 2013-2017 Cohorts. Ann Intern Med. 2020; 172: 229-39.
3.Fuji S, Einsele H, Savani BN, Kapp M. Systematic Nutritional Support in Allogeneic Hematopoietic Stem Cell Transplant Recipients. Biol Blood Marrow Transplant. 2015; 21: 1707-13.
4.Kiss N, Loeliger J, Findlay M, Isenring E, Baguley BJ, Boltong A, et al. Clinical Oncology Society of Australia: Position statement on cancer-related malnutrition and sarcopenia. Nutr Diet. 2020; 77: 416-25.
5.Ando T, Fujisawa S, Teshigawara H, Ogusa E, Ishii Y, Miyashita K, et al. Impact of treatment-related weight changes from diagnosis to hematopoietic stem-cell transplantation on clinical outcome of acute myeloid leukemia. Int J Hematol. 2019; 109: 673-83.
6.Ando T, Fujisawa S, Teshigawara H, Matsumura A, Sakuma T, Suzuki T, et al. Computed tomography-defined sarcopenia: prognostic predictor of nonrelapse mortality after allogeneic hematopoietic stem cell transplantation: a multicenter retrospective study. Int J Hematol. 2020; 112: 46-56.
7.Navarro WH, Agovi MA, Logan BR, Ballen K, Bolwell BJ, Frangoul H, et al. Obesity does not preclude safe and effective myeloablative hematopoietic cell transplantation (HCT) for acute myelogenous leukemia (AML) in adults. Biol Blood Marrow Transplant. 2010; 16: 1442-50.
8.Fuji S, Takano K, Mori T, Eto T, Taniguchi S, Ohashi K, et al. Impact of pretransplant body mass index on the clinical outcome after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014; 49: 1505-12.
9.Le Blanc K, Ringdén O, Remberger M. A low body mass index is correlated with poor survival after allogeneic stem cell transplantation. Haematologica. 2003; 88: 1044-52.
10.Hirose EY, de Molla VC, Goncalves MV, Pereira AD, Szor RS, da Fonseca A, et al. The impact of pretransplant malnutrition on allogeneic hematopoietic stem cell transplantation outcomes. Clin Nutr ESPEN. 2019; 33: 213-9.
11.Yang J, Xue SL, Zhang X, Zhou YN, Qin LQ, Shen YP, et al. Effect of body mass index on overall survival of patients with allogeneic hematopoietic stem cell transplantation. Eur J Clin Nutr. 2017; 71: 750-4.
12.Hadjibabaie M, Tabeefar H, Alimoghaddam K, Iravani M, Eslami K, Honarmand H, et al. The relationship between body mass index and outcomes in leukemic patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Transplant. 2012; 26: 149-55.
13.Gleimer M, Li Y, Chang L, Paczesny S, Hanauer DA, Frame DG, et al. Baseline body mass index among children and adults undergoing allogeneic hematopoietic cell transplantation: clinical characteristics and outcomes. Bone Marrow Transplant. 2015; 50: 402-10.
14.Gagelmann N, Eikema DJ, Stelljes M, Beelen D, de Wreede L, Mufti G, et al. Optimized EBMT transplant-specific risk score in myelodysplastic syndromes after allogeneic stem-cell transplantation. Haematologica. 2019; 104: 929-36.
15.Shaffer BC, Ahn KW, Hu ZH, Nishihori T, Malone AK, Valcarcel D, et al. Scoring System Prognostic of Outcome in Patients Undergoing Allogeneic Hematopoietic Cell Transplantation for Myelodysplastic Syndrome. J Clin Oncol. 2016; 34: 1864-71.
16.van Lieshout R, Tick LW, de Laat D, Custers S, Dekker IM, Douma MD, et al. Adherence to guidelines on nutrition support during intensive treatment of acute myeloid leukemia patients: A nationwide comparison. Clin Nutr ESPEN. 2020; 39: 242-50.
17.Nagano A, Nishioka S, Wakabayashi H. Rehabilitation Nutrition for Iatrogenic Sarcopenia and Sarcopenic Dysphagia. J Nutr Health Aging. 2019; 23: 256-65.
18.Baumgartner A, Kägi-Braun N, Tribolet P, Gomes F, Stanga Z, Schuetz P. Individualised nutritional support in medical inpatients – a practical guideline. Swiss Med Wkly. 2020; 150: w20204.
19.Ando T, Fujisawa S, Teshigawara H, Matsumura A, Sakuma T, Suzuki T, et al. Computed tomography-defined sarcopenia: prognostic predictor of nonrelapse mortality after allogeneic hematopoietic stem cell transplantation: a multicenter retrospective study. Int J Hematol. 2020; 112: 46-56.
20.Rondanelli M, Cereda E, Klersy C, Faliva MA, Peroni G, Nichetti M, et al. Improving rehabilitation in sarcopenia: a randomized-controlled trial utilizing a muscle-targeted food for special medical purposes. J Cachexia Sarcopenia Muscle. 2020; 11: 1535-47.
21.Liao CD, Tsauo JY, Wu YT, Cheng CP, Chen HC, Huang YC, et al. Effects of protein supplementation combined with resistance exercise on body composition and physical function in older adults: a systematic review and meta-analysis. Am J Clin Nutr. 2017; 106: 1078-91.
22.Arends J, Bachmann P, Baracos V, Barthelemy N, Bertz H, Bozzetti F, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017; 36: 11-48.
23.Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN expert group recommendations for action against cancer-related malnutrition. Clin Nutr. 2017; 36: 1187-96.
24.Sorror ML. How I assess comorbidities before hematopoietic cell transplantation. Blood. 2013; 121: 2854-63.
25.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005; 106: 2912-9.
26.Takano K, Fuji S, Uchida N, Ogawa H, Ohashi K, Eto T, et al. Pre-transplant diabetes mellitus is a risk factor for non-relapse mortality, especially infection-related mortality, after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2015; 50: 553-8.
27.Steinberg A, Van Cleave JH, Parikh AB, Moshier E, Ru M, Lawson M, et al. The Effect of Glucose Levels Prior to Hematopoietic Cell Transplantation on Post-Transplant Complications and Health Resource Utilization. Int J Hematol Oncol Stem Cell Res. 2019; 13: 122-31.
28.Sopfe J, Pyle L, Keating AK, Campbell K, Liu AK, Wadwa RP, et al. Malglycemia is associated with poor outcomes in pediatric and adolescent hematopoietic stem cell transplant patients. Blood Adv. 2019; 3: 350-9.
29.Wake AD. Antidiabetic Effects of Physical Activity: How It Helps to Control Type 2 Diabetes. Diabetes Metab Syndr Obes. 2020; 13: 2909-23.
30.Toi PL, Anothaisintawee T, Chaikledkaew U, Briones JR, Reutrakul S, Thakkinstian A. Preventive Role of Diet Interventions and Dietary Factors in Type 2 Diabetes Mellitus: An Umbrella Review. Nutrients. 2020; 12: 2722.
31.Baumgartner A, Schuetz P. Nutritional Support. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies. Cham (CH), Springer Copyright 2019, EBMT and the Author(s), 2019; p. 171-6
32.McMillen KK, Coghlin-Dickson T, Adintori PA. Optimization of nutrition support practices early after hematopoietic cell transplantation. Bone Marrow Transplant. 2021; 56: 314-26.
33.Fuji S, Kim SW, Fukuda T, Kamiya S, Kuwahara S, Takaue Y. Positive impact of maintaining minimal caloric intake above 1.0 x basal energy expenditure on the nutritional status of patients undergoing allogeneic hematopoietic stem cell transplantation. Am J Hematol. 2009; 84: 63-4.
34.Schuetz P, Fehr R, Baechli V, Geiser M, Deiss M, Gomes F, et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. The Lancet. 2019; 393: 2312-21.
35.Seguy D, Berthon C, Micol JB, Darre S, Dalle JH, Neuville S, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 2006; 82: 835-9.
36.Seguy D, Duhamel A, Rejeb MB, Gomez E, Buhl ND, Bruno B, et al. Better outcome of patients undergoing enteral tube feeding after myeloablative conditioning for allogeneic stem cell transplantation. Transplantation. 2012; 94: 287-94.
37.Beckerson J, Szydlo RM, Hickson M, Mactier CE, Innes AJ, Gabriel IH, et al. Impact of route and adequacy of nutritional intake on outcomes of allogeneic haematopoietic cell transplantation for haematologic malignancies. Clin Nutr. 2019; 38: 738-44.
38.Peled JU, Gomes ALC, Devlin SM, Littmann ER, Taur Y, Sung AD, et al. Microbiota as Predictor of Mortality in Allogeneic Hematopoietic-Cell Transplantation. N Engl J Med. 2020; 382: 822-34.
39.Peled JU, Jenq RR, Holler E, van den Brink MR. Role of gut flora after bone marrow transplantation. Nat Microbiol. 2016; 1: 16036.
40.D'Amico F, Biagi E, Rampelli S, Fiori J, Zama D, Soverini M, et al. Enteral Nutrition in Pediatric Patients Undergoing Hematopoietic SCT Promotes the Recovery of Gut Microbiome Homeostasis. Nutrients. 2019; 11: 2958.
41.Schorghuber M, Fruhwald S. Effects of enteral nutrition on gastrointestinal function in patients who are critically ill. Lancet Gastroenterol Hepatol. 2018; 3: 281-7.
42.Andersen S, Staudacher H, Weber N, Kennedy G, Varelias A, Banks M, et al. Pilot study investigating the effect of enteral and parenteral nutrition on the gastrointestinal microbiome post-allogeneic transplantation. Br J Haematol. 2020; 188: 570-81.
43.Mattsson J, Westin S, Edlund S, Remberger M. Poor oral nutrition after allogeneic stem cell transplantation correlates significantly with severe graft-versus-host disease. Bone Marrow Transplant. 2006; 38: 629-33.
44.Iyama S, Tatsumi H, Shiraishi T, Yoshida M, Tatekoshi A, Endo A, et al. Possible clinical outcomes using early enteral nutrition in individuals with allogeneic hematopoietic stem cell transplantation: A single-center retrospective study. Nutrition. 2021; 83: 111093.
45.Yoshifuji K, Inamoto K, Kiridoshi Y, Takeshita K, Sasajima S, Shiraishi Y, et al. Prebiotics protect against acute graft-versus-host disease and preserve the gut microbiota in stem cell transplantation. Blood Adv. 2020; 4: 4607-17.
46.Iyama S, Sato T, Tatsumi H, Hashimoto A, Tatekoshi A, Kamihara Y, et al. Efficacy of Enteral Supplementation Enriched with Glutamine, Fiber, and Oligosaccharide on Mucosal Injury following Hematopoietic Stem Cell Transplantation. Case Rep Oncol. 2014; 7: 692-9.
47.Fuji S, Kim SW, Mori S, Fukuda T, Kamiya S, Yamasaki S, et al. Hyperglycemia during the neutropenic period is associated with a poor outcome in patients undergoing myeloablative allogeneic hematopoietic stem cell transplantation. Transplantation. 2007; 84: 814-20.
48.Engelhardt BG, Savani U, Jung DK, Powers AC, Jagasia M, Chen H, et al. New-Onset Post-Transplant Diabetes Mellitus after Allogeneic Hematopoietic Cell Transplant Is Initiated by Insulin Resistance, Not Immunosuppressive Medications. Biol Blood Marrow Transplant. 2019; 25: 1225-31.
49.Aberer F, Kremser S, Mader JK, Zinke-Cerwenka W, Greinix H, Tripolt NJ, et al. Hyperglycaemia within the first month after allogeneic haematopoietic stem-cell transplantation is an independent risk factor for overall survival in patients with acute myeloid leukaemia. Diabetes Metab. 2017; 43: 560-2.
50.Hammer MJ, Casper C, Gooley TA, O'Donnell PV, Boeckh M, Hirsch IB. The contribution of malglycemia to mortality among allogeneic hematopoietic cell transplant recipients. Biol Blood Marrow Transplant. 2009; 15: 344-51.
51.Kawajiri A, Fuji S, Tanaka Y, Kono C, Hirakawa T, Tanaka T, et al. Clinical impact of hyperglycemia on days 0-7 after allogeneic stem cell transplantation. Bone Marrow Transplant. 2017; 52: 1156-63.
52.Mizrahi N, Braun M, Ben Gal T, Rosengarten D, Kramer MR, Grossman A. Post-transplant diabetes mellitus: incidence, predicting factors and outcomes. Endocrine. 2020; 69: 303-9.
53.Hecking M, Sharif A, Eller K, Jenssen T. Management of post-transplant diabetes: immunosuppression, early prevention, and novel antidiabetics. Transpl Int. 2021; 34: 27-48.
54.Fuji S, Kim SW, Mori S, Kamiya S, Yoshimura K, Yokoyama H, et al. Intensive glucose control after allogeneic hematopoietic stem cell transplantation: a retrospective matched-cohort study. Bone Marrow Transplant. 2009; 44: 105-11.
55.Fuji S, Kim SW, Kamiya S, Nakane T, Matsumoto K, Onishi Y, et al. A multi-center prospective study randomizing the use of fat emulsion in intensive glucose control after allogeneic hematopoietic stem cell transplantation using a myeloablative conditioning regimen. Clin Nutr. 2018; 37: 1534-40.
56.Farag SS, Abu Zaid M, Schwartz JE, Thakrar TC, Blakley AJ, Abonour R, et al. Dipeptidyl Peptidase 4 Inhibition for Prophylaxis of Acute Graft-versus-Host Disease. N Engl J Med. 2021; 384: 11-9.
57.Fuji S, Mori T, Khattry N, Cheng J, Do YR, Yakushijin K, et al. Severe weight loss in 3 months after allogeneic hematopoietic SCT was associated with an increased risk of subsequent non-relapse mortality. Bone Marrow Transplant. 2015; 50: 100-5.
58.Smith J, Poon C, Gilroy N, Kabir M, Brice L, Dyer G, et al. Nutritional issues and body weight in long-term survivors of allogeneic blood and marrow transplant (BMT) in NSW Australia. Support Care Cancer. 2017; 25: 137-44.
59.Chen G, Kendall PA, Hillers VN, Medeiros LC. Qualitative studies of the food safety knowledge and perceptions of transplant patients. J Food Prot. 2010; 73: 327-35.
60.Feliu J, Clay J, Raj K, Barber L, Devlia V, Shaw B, et al. Transplant-acquired food allergy (TAFA) following cord blood stem cell transplantation in two adult patients with haematological malignancies. Br J Haematol. 2014; 167: 426-8.
61.Sakashita K, Nakazawa Y, Yanagisawa R, Tanaka M, Saito S, Yoshikawa K, et al. Food allergy after cord blood transplantation in children. Br J Haematol. 2012; 158: 672-6.
Search
News