Volume 5 (2022) Issue 2 No.4 Pages 61-68
Abstract
Background: Oral busulfan and intravenous cyclophosphamide (Bu/Cy) are common myeloablative preparations used in allogeneic hematopoietic stem cell transplantation (HSCT). Herein, we investigated the safety of (Bu/Cy) administration during HSCT.
Methods: Patients administered Bu/Cy for allogeneic HSCT at Royal Perth Hospital and Fiona Stanley Hospital between 2007 and 2017 were reviewed for inclusion in the study. We performed busulfan pharmacokinetic (PK) testing for a subset of patients and allometric scaling modeling to assess the best method of busulfan dosing in patients at extremes of weight.
Results: Sixty-nine patients were included in the clinical outcome analysis. The median follow-up period was 32 months (range, 9-114 months). The three-year overall survival rate was 62% (95% confidence interval (CI), 51%-75%), and transplant-related mortality was 4% at 6 months (95% CI, 1-7%), with a low rate of sinusoidal obstruction syndrome of the liver being observed. In addition, relapse was 38% (95% CI, 30%-44%) at 3 years. The PK information of 15 patients receiving busulfan was available after oral dosing. The average per-dose busulfan exposure was 1,350 μmol.min/L (range, 878-1,717 μmol.min/L), and the within target range was 1,000-1,500 μmol.min/L in 73% of patients. Of the size measures investigated, ideal and adjusted body weight (ABW40) provided the best fit. No association was observed between busulfan exposure, toxicity, and relapse.
Conclusions: Overall, Bu/Cy administration appeared safe when dosed in relation to weight, showing a low early transplant-related mortality rate following adequate busulfan exposure in majority of the cases. Body size measures, such as ideal body weight or ABW40, are likely more suitable for use during busulfan dosing, particularly at high extremes of the body mass index classification.
Introduction
The alkylating chemotherapy agent busulfan (Bu) is commonly used as a myeloablative conditioning agent in patients undergoing allogeneic hematopoietic stem cell transplantation (HSCT). However, busulfan has a narrow therapeutic window and the risk of toxicity, especially sinusoidal obstruction syndrome (SOS) of the liver, increases with exposure1, 2. It is also likely that reduced exposure to busulfan increases the risk of disease relapse after transplantation3. These observations have led to a proposed target range of 1,000-1,500 μmol.min/L for the area under the plasma concentration-time curve (AUC) per dose of busulfan, when administered four times daily as part of myeloablative conditioning regimens for hematological malignancies4.
Twenty years ago, an intravenous (IV) preparation of busulfan was developed with the purported benefit of more consistent bioavailability and, consequently, a lower risk of toxicity5. Since then, many centers have switched from oral to IV busulfan for use in conditioning for HSCT. While early studies showed an apparent reduction in the incidence of SOS and overall mortality with IV busulfan, these were phase II studies comparing outcomes with historical controls4,6–9. Such comparisons are limited by the inability to account for the effects of other changes in treatment between cohorts, such as better supportive care. Indeed, an improvement in outcomes over time is well documented in HSCT recipients; however, it is not attributed only to changes in conditioning regimens10, 11.
Our center continues to use oral busulfan based on a low incidence of SOS among our patients, a lack of rigorous clinical data to support the switch to IV busulfan, and the lower cost of the dosage form. Herein, we report the clinical outcomes of patients who received oral busulfan and IV cyclophosphamide (Bu/Cy) to assess the safety and efficacy of this regimen when used in a real-world clinical setting using contemporary transplantation techniques and supportive care. Furthermore, we report the pharmacokinetic (PK) properties of oral busulfan in a subset of patients with available information.
Methods
Patients
Patients who underwent allogeneic HSCT with Bu/Cy conditioning between 2007 and December 2017 were included in the study. Busulfan was administered as an oral liquid preparation at a dose of 1 mg/kg four times daily to give a total dose of 16 mg/kg in combination with IV cyclophosphamide at a total dose of 120 mg/kg. Busulfan dosing was based on total body weight (TBW) for non-obese patients and, at the physician's discretion, dosed based on a weight that gave a calculated body mass index (BMI) of 27 kg/m2 in obese patients. A refined disease risk index (DRI) was calculated for each patient using the online tool provided by the Center for International Blood and Marrow Transplant Research to predict the patients' prognosis and survival post-transplant12.
In addition, a subset of the Bu/Cy group was exposed to additional busulfan, and clinical outcomes were analyzed prospectively in a separate clinical trial (Australian Clinical Trials Registry number: ACTRN12616000709448). The aim of this investigation was to assess inter-patient variability to busulfan exposure, the effect of extreme weight (obesity) on exposure, and whether there was any association between toxicity and exposure. Institutional review board approval for this study was obtained following consent received for the collection of patient samples. Samples were de-identified when processed in the laboratory.
The subgroup analyzed for busulfan exposure had busulfan concentrations measured in plasma. Blood was collected for the busulfan assay at 30 min, 2 h 15 min, 3 h, 4 h, and 6 h after the first busulfan dose was administered at 6:00 am. Patients had further blood concentrations measured at four hours after the 6:00 am morning dose on days 3 and 4 of busulfan administration. Busulfan concentrations were determined by liquid chromatography with tandem mass spectrometry (LC-MS/MS) at the PathWest Clinical Pharmacology and Toxicology Laboratory. The method was validated according to the current FDA guidelines for the validation and verification of LC-MS/MS bioanalytical methods. The method was linear from 40-9,000 μg/L with demonstrated accuracy and precision across this concentration range. All measured concentrations were within the measurement range used in the assay.
Pharmacokinetic modeling
Busulfan concentrations measured in our PK subgroup were analyzed using population pharmacokinetics within the NONlinear Mixed Effects Modeling (NONMEM) software. Further details of the modeling procedure are provided in the supplementary material (
One key aspect of the applied PK modeling in this study was its use in the investigation of the effect of body size on PK parameters. Allometric scaling, namely adjusting the patient's actual weight, was based on body size and was performed on clearance and volume parameters. Several measures of size were investigated during the model-building process to identify the most appropriate descriptor. These were TBW, ideal body weight (IBW), adjusted body weight with a 25% or 40% correction factor [ABW25 = IBW +0.25*(TBW-IBW) and ABW40 = IBW +0.4*(TBW-IBW)], and lean body mass13. Further information about the modelling performance for each body weight model are presented in
Pharmacokinetic simulations
Simulations using the final model were performed to explore the potential effects of different dose regimens. One thousand individuals in each BMI group (15, 20, 25, 30, 35, and 40 kg/m2) were simulated with different weights; however, their heights were fixed at 170 cm to allow for comparability between the simulated groups. Two different dose regimens were simulated, viz. i) 1 mg/kg using total body weight and ii) 1 mg/kg using adjusted body weight (ABW40). The results of the simulations were plotted as box and whisker plots and the percentage of patients with overall average exposure within the target (1,000-1,500 μmol.min/L) is detailed in
Study end points and assessments
The study endpoints for the overall Bu/Cy group included overall survival, relapse rate, mortality, and cause of death. In the subgroup investigated for busulfan exposure, additional endpoints included rates of SOS, transplant-associated thrombotic microangiopathy (TA-TMA), admission to the intensive care unit (ICU), the need for total parenteral nutrition (TPN), and duration of hospital admission. These additional endpoints represent surrogate markers of toxicity, possibly resulting from exposure to chemotherapy.
Statistical analysis
Overall survival was calculated using the Kaplan-Meier method. The cumulative incidence of transplant-related mortality was calculated using the method of Fine and Grey, were relapse was a competing risk14. The cumulative incidence of relapse was calculated using the same method, and death without relapse was the competing risk. Multivariate analysis was performed by the Cox proportional hazards method, using a limited number of variables with clinical rationale for inclusion in the model. Pre-planned subgroup analysis assessed the association between busulfan exposure and patient outcomes. The correlation between BMI and busulfan exposure was investigated using linear regression analysis. IBM SPSS Statistics version 24 (IBM, Chicago, IL, USA) was used for all analyses.
Results
Patient characteristics
A total of 69 patients were included in the overall outcome analysis, of which 15 had additional PK and clinical information available. Patients were included from 2007 to 2017, and all these patients received Bu/Cy conditioning. Demographic and baseline disease characteristics are detailed in Table 1. The group included a greater percentage of men than women (64% vs. 36%), and the most common indication for transplant was acute myeloid leukemia (80%). The typical source of stem cells was peripheral blood stem cells (93%) and 25 patients (36%) were classified as being of high or very high risk based on the refined DRI.
Between February 2016 and January 2017, 15 patients were included in the busulfan PK subgroup. This group included a greater percentage of men than women (64% vs. 36%), and 93% of these patients were at intermediate or high risk according to the refined DRI. All patients received stem cells derived from a peripheral blood stem cell source. The demographic and baseline disease characteristics for this subgroup are detailed in Table 1.
Outcome in entire cohort
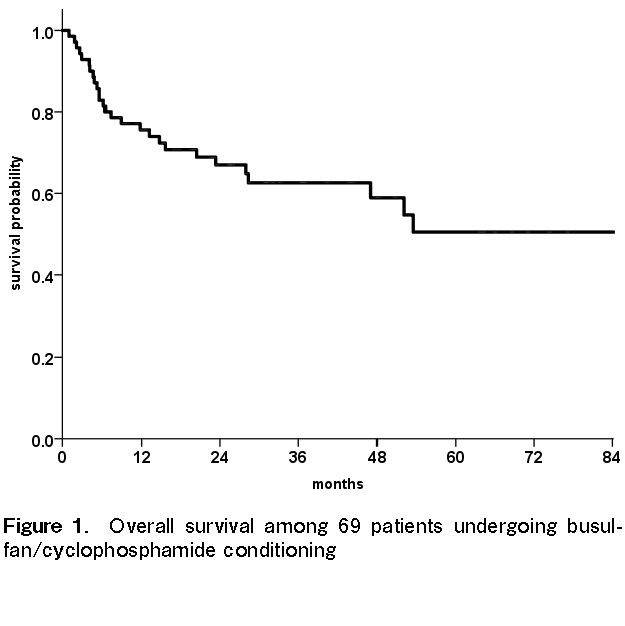
The median follow-up period was 32 months (range, 9-114 months). Overall survival at 3 years was 62% (95% CI, 51%-75%), as shown in Figure 1. Four patients died of transplant-related causes, resulting in a cumulative incidence of transplant-related mortality of 4% at 6 months. Three patients died after primary graft failure and one died of multi-organ failure in the context of thrombotic microangiopathic anemia, which occurred 126 days after transplant. Of those with graft failure, one was a cord blood transplant recipient, one was undergoing a second allogeneic transplant with relapse after an initial reduced intensity transplant, while the third developed SOS of the liver and died 31 days after transplant without evidence of neutrophil recovery. Of the patients with graft failure, PK data were only available for those who underwent a second allogeneic transplant.
The cumulative incidence of grade II-IV acute graft-versus-host disease (aGVHD) was 34% (95% CI, 26%-42%) at 6 months. The cumulative incidence of chronic GVHD (cGVHD) of any severity was 76% (95% CI, 66%-83%) at 3 years, and 33% (95% CI, 25%-42%) for moderate to severe cGVHD.
Twenty-six patients relapsed, and the cumulative incidence of relapse at three years post-transplant was 38% (95% CI, 27%-49%). For those who relapsed, the median time to relapse from transplant was five months (range, 2-96 months). Following multivariate analysis after adjusting for age at transplant and donor type (related vs. unrelated), a high or very high disease risk index was the only factor significantly associated with an increased risk of relapse (hazard ratio 2.4 compared with low/intermediate DRI; 95% confidence interval, 1.1-5.3, P=0.03). The estimated 3-year relapse incidence was 28% (95% CI, 16-40%) for low/intermediate DRI compared with 53% (95% CI, 43-63%) for high/very high DRI (P=0.04).
Pharmacokinetic modeling
In the busulfan PK subgroup (N=15), the average per-dose busulfan exposure was 1,350 μmol.min/L (range, 878-1,717 μmol.min/L). This corresponded to an inter-patient coefficient of variability of 17% in clearance and 12.8% in volume of distribution. Three patients had an average per-dose busulfan AUC > 1,500 μmol.min/L, one had a per-dose AUC < 1,000 μmol.min/L, and four patients (27%) had BMI values greater than 30 kg/m2. These patients, who underwent dose adjustment, demonstrated busulfan AUC values across the target range (1,043-1,717 μmol.min/L) and no correlation between BMI and exposure was observed (P=0.28).
The coefficient of variability of busulfan exposure between doses in the same patient was 16%. The busulfan concentrations from days 3 and 4 of treatment showed that busulfan levels may vary on a day-to-day basis, with some patients having higher and others lower levels compared to those observed on day 1. Further details of the PK modeling results are included in the supplementary material (
Clinical outcomes in the PK subgroup
There was no indication that a higher busulfan level was associated with increased toxicity. Two patients (13%) required TPN, most often due to severe mucositis, and both patients had AUC targets that were within range. Only one patient was admitted to the ICU but this was a result of graft failure and sepsis rather than as a direct complication of the conditioning regimen. Two patients (13%) developed TA-TMA and one of them recovered after treatment with eculizumab, a humanized monoclonal IgG antibody that blocks the formation of the complement factor C5b, while the other succumbed to a number of transplant-related complications. There were no cases of SOS in this group. The average length of hospital stay was 32 days (range, 21-65 days).
Relapse in the PK subgroup occurred in four patients (27%) and five patients (33%) died. Grade II-IV acute GVHD occurred in six patients (40%) and chronic GVHD occurred in eight patients (60%). No association was observed between busulfan levels during transplant and subsequent GVHD. Of the five patients in the PK subgroup who died, one died from graft failure (busulfan AUC: 1,461 μmol.min/L), one from multi-organ failure (busulfan AUC: 1,717 μmol.min/L), and three from relapsed or persistent disease (busulfan AUCs: 878, 1,226, and 1,563 μmol.min/L).
Simulations from the final PK model
Overall, adjusted body weight (ABW40) performed best as an allometric size measure to account for the effect of body weight on PK parameters and was thus included in the final model. Simulations demonstrated that dosing based on TBW performed similarly to ABW40 based dosing for simulated patients with BMI values of 20 and 25 kg/m2, with approximately 80% of simulated individuals within the desired target.
The simulations also identified an increased number of doses that would deliver a below-target average AUC for patients with a lower BMI if TBW dosing was used compared to the ABW40 based dosing (68% vs. 11.4%). As BMI increased, the risk of TBW-only dosing resulting in an AUC per dose over the target also increased, with approximately 9 in 10 patients being above the target in the ABW40 simulations. Across the BMI groups, the ABW40 dose simulations resulted in a similar percentage of patients within the target range. The results from the allometric scaling models are depicted in Figure 2.
Discussion
In this retrospective analysis, the outcomes of 69 patients that underwent allogeneic HSCT with Bu/Cy conditioning at our center were comparable with those of patients that received IV Bu/Cy reported in the literature15–17. Reassuringly, we did not observe high rates of SOS in our cohort, suggesting the absence of significant toxicity or high rates of graft failure pointing to inadequate drug exposure. Issues such as graft failure and SOS were generally observed in patients who had been heavily pre-treated, such as those undergoing a second HSCT, or those with limited hematopoietic progenitor cell dose, such as a recipient of a cord blood transplant. Despite concerns about the variable bioavailability and toxicity of oral busulfan, we found acceptable outcomes in our cohort.
In the entire investigated cohort, the relapse rate was moderately high at 38% (95% CI, 27%-49%). However, the relapse rate was higher in patients with a high/very high DRI, suggesting that the underlying disease characteristics contributed significantly to this risk. Furthermore, our PK data did not suggest that relapse was related to inadequate busulfan exposure. The relapse rate observed herein was similar to that reported in a comparably high-risk cohort with a high pre-transplant risk of relapse12.
As numerous studies have correlated busulfan blood concentration levels and PK data with therapeutic outcome, we also analyzed busulfan levels in a small cohort to compare drug exposure with patient outcomes4, 6, 18. The majority of analyzed patients had average per-dose busulfan AUC levels within the target range of 1,000-1,500 μmol.min/L, indicating that most patients receiving oral busulfan were exposed to an adequate drug dose. There was no evident correlation between the exposure and relapse rates. Although one of the patients with a busulfan AUC value of 878 μmol.min/L relapsed, none of the other three patients who relapsed had AUC values < 1,000 μmol.min/L. This illustrates the limitations of busulfan levels in predicting toxicity and relapse, as there are undoubtedly other individual factors involved.
The inter-dose variability in AUC observed in this study (16%), which corresponds to variability in clearance or bioavailability, was similar to that previously reported in patients who received intravenous busulfan1, 19–24. These aforementioned studies reported inter-dose coefficients of variability in clearance between 11% and 16%. These estimates are prone to underestimation (i.e., shrinkage; noting this was not high in the population PK model) due to the small sample size. In the current investigation, it was not possible to further delineate the source of variability; however, bioavailability and changes in daily clearance were found to be the main contributors.
It is reassuring that despite oral dosing, the variability was similar to reports of IV dosing. Despite concerns regarding variable intestinal absorption, our data suggest that the variability between doses was not dissimilar to what might be achieved with IV dosing.
Likewise, inter-patient variability for clearance in our cohort (17%) was similar when compared with that observed in larger population studies, where the range was between 16% and 34%20–23. This, coupled with the fact that we did not observe significant toxicity in patients with higher busulfan exposure, is reassuring that the oral preparation is predictably eliminated.
When postulating why the inter-dose and inter-patient variability in AUC and clearance in our PK cohort were similar to published reports of patients who received IV busulfan, we considered a number of possible variables. The AUC achieved with oral busulfan may be affected by multiple factors, such as gastrointestinal permeability, loss of drug fraction, and metabolism. In our cohort, it did not appear that these factors produced significant variability as seen in other published series, which may be due to careful patient selection, the relatively young age of our patients, and management of nausea and vomiting during busulfan administration.
As busulfan is metabolized in the liver via glutathione conjugation by glutathione-S-transferase (GST) enzymes followed by oxidation, genetic polymorphisms in these GST enzymes may affect metabolism and contribute to variability in IV busulfan metabolism and clearance. In a meta-analysis by Kim et al., GST enzyme polymorphisms appeared to affect IV busulfan AUC and clearance, while other factors, such as gastrointestinal absorption, were more important in the variability of oral busulfan25. In our PK cohort, we confirmed that factors such as GST enzyme polymorphisms were not a significant factor in causing inter-dose or inter-patient variability in AUC or clearance.
Furthermore, most patients who received oral busulfan achieved adequate busulfan levels. Our results suggest that variability in bioavailability was not significantly influence by exposure. Moreover, altering the busulfan dose in obese patients appeared to be appropriate, resulting in adequate busulfan levels. Our results emphasize the importance of busulfan dose adjustment in obese patients to achieve safe and adequate exposure.
In our simulations, ABW40 was the overall best method for allometric scaling, as it reduced the between-subject variability for both clearance and volume of distribution. The difference between ABW40 and ABW25 in our testing was small, suggesting that either method is appropriate for busulfan dosing, although the number of patients tested in our cohort was small. Both methods performed better than the TBW method alone. The American Society for Blood and Marrow Transplantation Practice Guidelines Committee recommends using ABW25 for oral busulfan dosing in adults, both obese and non-obese, who are dosed per kilogram or by body surface area based on TBW for m2 dosing26. Further research may help to better elucidate the best method for dosing busulfan in patients of extreme weight.
Although our observations were generally reassuring, the presence of a single patient with a busulfan AUC < 1,000 μmol.min/L suggests that despite acceptable PK parameters and appropriate weight-based dosing, some risk of below-target busulfan exposure is possible in the absence of real-time PK-adjusted dosing. It is not routine to alter oral dosing by using PK monitoring, and many centers may choose to use the IV preparation with PK monitoring to mitigate this risk.
Our study was limited by the small sample size in both the overall busulfan cohort and the busulfan PK subgroup. Due to the sample size of the PK cohort, we were unable to draw firm conclusions on the associations between exposure/dose and clinical outcomes. Furthermore, the 69 patients that received Bu/Cy had been selected by physicians as being fit enough to undergo this intensive myeloablative regimen. The overall cohort group was relatively young, with an average age of 43 years. This may denote a group with few comorbidities and a better pre-morbid state. Thus, the low TRM observed in this group compared to earlier literature may reflect careful patient selection and a younger age, rather than improved safety of the investigated Bu/Cy regimen.
We conclude that oral busulfan appears to be safe and effective in the context of careful patient selection and modern supportive care. For transplant units for whom the IV formulation is prohibitively expensive or are unable to set up PK monitoring to allow dose modification when IV busulfan is used, oral busulfan still provides a safe option as part of a myeloablative chemotherapy regimen. In centers where busulfan PK monitoring is not available, either IV or oral busulfan may be considered, and we do not draw a firm conclusion from our data regarding the superiority of either preparation, as our analysis only included patients treated with oral busulfan.
Author Contributions
R.S. and S.S. analyzed results and wrote the manuscript; N.M. collected data and contributed to the manuscript; M.W., P.C., J.C. contributed to the manuscript; S.O. and D.J. performed experiments, analyzed results and contributed to the manuscript; D.P. designed the research, analyzed results and contributed to the manuscript.
Ethical Approval and Informed Consent
Institutional review board approval for this testing was obtained with informed consent obtained for collection of patient samples. Samples were de-identified when processed in the laboratory. Ethics approval granted by the Royal Perth Hospital ethics review board (Approval number: 15-187).
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Nguyen L, Leger F, Lennon S, Puozzo C. Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: A population pharmacokinetic study. Cancer Chemother Pharmacol. 2006;57: 191-8.
2.Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17: 225-30.
3.Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, et al. Marrow transplantation for chronic myeloid leukemia: The influence of plasma busulfan levels on the outcome of transplantation. Blood. 1997;89: 3055-60.
4.Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, et al. Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: Defining a therapeutic window for IV BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant. 2002;8: 477-85.
5.Bhagwatwar HP, Phadungpojna S, Chow DS, Andersson BS. Formulation and stability of busulfan for intravenous administration in high-dose chemotherapy. Cancer Chemother Pharmacol. 1996;37: 401-8.
6.Geddes M, Kangarloo SB, Naveed F, Quinlan D, Chaudhry MA, Stewart D, et al. High busulfan exposure is associated with worse outcomes in a daily i.v. busulfan and fludarabine allogeneic transplant regimen. Biol Blood Marrow Transplant. 2008;14: 220-8.
7.Aggarwal C, Gupta S, Vaughan WP, Saylors GB, Salzman DE, Katz RO, et al. Improved outcomes in intermediate- and high-risk aggressive non-hodgkin lymphoma after autologous hematopoietic stem cell transplantation substituting intravenous for oral busulfan in a busulfan, cyclophosphamide, and etoposide preparative regimen. Biol Blood Marrow Transplant. 2006;12: 770-7.
8.Dean RM, Pohlman B, Sweetenham JW, Sobecks RM, Kalaycio ME, Smith SD, et al. Superior survival after replacing oral with intravenous busulfan in autologous stem cell transplantation for non-Hodgkin lymphoma with busulfan, cyclophosphamide and etoposide. Br J Haematol. 2010;148: 226-4.
9.Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: Decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mo. Biol Blood Marrow Transplant. 2002;8: 493-500.
10.Gooley TA, Chien JW, Pergam SA, Hingorani S, Sorror ML, Boeckh M, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363: 2091-101.
11.Canaani J, Beohou E, Labopin M, Ghavamzadeh A, Beelen D, Hamladji RM, et al. Trends in patient outcome over the past two decades following allogeneic stem cell transplantation for acute myeloid leukaemia: an ALWP/EBMT analysis. J Intern Med. 2019;285: 407-18.
12.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123: 3664-71.
13.Green B, Duffull SB. What is the best size descriptor to use for pharmacokinetic studies in the obese? Br J Clin Pharmacol. 2004;58: 119-33.
14.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94: 496-509.
15.Lee JH, Joo YD, Kim H, Ryoo HM, Kim MK, Lee GW, et al. Randomized trial of myeloablative conditioning regimens: Busulfan plus cyclophosphamide versus busulfan plus fludarabine. J Clin Oncol. 2013;31: 701-9.
16.Liu H, Zhai X, Song Z, Sun J, Xiao Y, Nie D, et al. Busulfan plus fludarabine as a myeloablative conditioning regimen compared with busulfan plus cyclophosphamide for acute myeloid leukemia in first complete remission undergoing allogeneic hematopoietic stem cell transplantation: A prospective and multicen. J Hematol Oncol. 2013;6: 15.
17.Nagler A, Rocha V, Labopin M, Unal A, Ben Othman T, Campos A, et al. Allogeneic hematopoietic stem-cell transplantation for acute myeloid leukemia in remission: comparison of intravenous busulfan plus cyclophosphamide (Cy) versus total-body irradiation plus Cy as conditioning regimen. J Clin Oncol. 2013;31: 3549-56.
18.Pidala J, Kim J, Anasetti C, Kharfan-Dabaja MA, Field T, Perkins J, et al. Targeted i.v. BU and fludarabine (t-i.v. BU/Flu) provides effective control of AML in adults with reduced toxicity. Bone Marrow Transplant. 2011;46: 641-9.
19.Bartelink IH, van Kesteren C, Boelens JJ, Egberts TC, Bierings MB, Cuvelier GD, et al. Predictive performance of a busulfan pharmacokinetic model in children and young adults. Ther Drug Monit. 2012;34: 574-83.
20.Trame MN, Bergstrand M, Karlsson MO, Boos J, Hempel G. Population pharmacokinetics of busulfan in children: Increased evidence for body surface area and allometric body weight dosing of busulfan in children. Clin Cancer Res. 2011;17: 6867-77.
21.McCune JS, Bemer MJ, Barrett JS, Scott Baker K, Gamis AS, Holford NH. Busulfan in infant to adult hematopoietic cell transplant recipients: A population pharmacokinetic model for initial and bayesian dose personalization. Clin Cancer Res. 2014;20: 754-63.
22.Diestelhorst C, Boos J, McCune JS, Hempel G. Population pharmacokinetics of intravenous busulfan in children: Revised body weight-dependent NONMEMⓇ model to optimize dosing. Eur J Clin Pharmacol. 2014;70: 839-47.
23.Hadjibabaie M, Rahimian S, Jahangard-Rafsanjani Z, Amini M, Alimoghaddam K, Iravani M, et al. Population pharmacokinetics of oral high-dose busulfan in adult patients undergoing hematopoietic stem cell transplantation. DARU, J Pharm Sci. 2011;19: 216-23.
24.Paci A, Vassal G, Moshous D, Dalle JH, Bleyzac N, Neven B, et al. Pharmacokinetic behavior and appraisal of intravenous busulfan dosing in infants and older children. Ther Drug Monit. 2012;34: 198-208.
25.Kim MG, Kwak A, Choi B, Ji E, Oh JM, Kim K. Effect of glutathione S-transferase genetic polymorphisms on busulfan pharmacokinetics and veno-occlusive disease in hematopoietic stem cell transplantation: A meta-analysis. Basic Clin Pharmacol Toxicol. 2019;124: 691-703.
26.Bubalo J, Carpenter PA, Majhail N, Perales MA, Marks DI, Shaughnessy P, et al. Conditioning chemotherapy dose adjustment in obese patients: a review and position statement by the American Society for Blood and Marrow Transplantation Practice Guideline Committee. Biol Blood Marrow Transplant. 2014;20: 600-16.
Search
News