Volume 5 (2022) Issue 1 No.2 Pages 16-26
Abstract
Purpose: Autologous stem cell transplantation (ASCT) is an established therapy for many hematological diseases. This study assessed the pattern of ASCTs at a tertiary care center and associated factors, including pre-harvest CD34+ stem cell levels, leading to improved engraftment outcomes.
Methodology: A retrospective study was conducted in India, between February 2009-August 2020. Patients who underwent ASCT for different hematological malignancies (n=65) were included, and the patients' age, sex, type and stage of disease, pre- and post-harvest CD34+ counts, and time to attain platelet/neutrophil engraftment or febrile neutropenia were analyzed. The post-harvest CD34+ dose was calculated. Pre-conditioning was performed using Granulocyte Colony Stimulating Factor (GCSF)±Plerixafor. Progression-free survival (PFS) was calculated using relapse/death as the endpoint.
Results: The median age of the cohort (n=65) was 49 years, with a male preponderance. Multiple myeloma was the most common malignancy (70.8% [46/65]), requiring ASCT. The median time to ASCT was 13 months. All patients had received GCSF, while Plerixafor was used in 17 patients with a pre-harvest CD34+ count of <10 cells/μL. The median pre-harvest CD34+ concentration and post-harvest CD34+ cell dose was 27.54 cells/μL (n=26) and 5.23×106 cells/kg body weight (n=65), respectively. The median time to engraftment was 11 and 12 days, for neutrophils and platelets, respectively. One patient did not engraft and was excluded from the analysis. The time required to attain neutrophil engraftment was significantly lower (p=0.02) among freshly harvested stem cells (n=48) than that of cryopreserved products (n=17). Platelet engraftment associated with CD34+ pre- and post-harvest levels was not significant (p=0.06). The time to attain neutropenia and subsequent febrile neutropenia was significantly lower with an adequate post-harvest CD34+ dose (p=0.009). Febrile neutropenia was seen in 83.1% (54/65) patients. The median time for febrile neutropenia was 4 days post-ASCT. Pre- and post-harvest CD34+ concentrations were directly proportional to each other (p<0.001). The median PFS was 112 months (n=65). Survival was better in males (median PFS: 112 months) vs. females (median PFS: 59 months) (p=0.27). Eight patients relapsed, and eight patients had died.
Conclusion: Although unrelated to age or sex, the post-harvest CD34+ dose was inversely related to febrile neutropenia. As pre- and post-harvest CD34+ levels were directly proportional, pre-harvest CD34+ concentrations may be reliably used to assess engraftment outcomes. Rapid neutrophil engraftment was noted in fresh stem cells with PFS of 112 months, and was better among males, the exact reason being unknown. Thus, a larger number of patients should be followed up to obtain an accurate picture.
Introduction
Autologous stem cell transplantation (ASCT) is a common treatment modality in which the patient's own healthy stem cells are used to replace the diseased stem cells in the bone marrow. ASCT following intensive chemotherapy has been used in patients with hematological malignancies, such as multiple myeloma (MM), lymphoma, or solid tumors1.
In a 2012 survey conducted in Europe, ASCT accounted for 58% of all hematopoietic stem cell transplants (HSCT);2 most of which were performed for plasma cell neoplasms (PCN), non-Hodgkin lymphoma (NHL), Hodgkins lymphoma (HL), and autoimmune disorders2, 3. Most ASCTs utilize peripheral blood instead of bone marrow3. According to CIBMTR data (India also participated in the CIBMTR 2019 analysis), ASCTs are more common (60%) than allogeneic stem cell transplants (AlloSCT)4.
According to the CIBMTR study, the most common cause of death after ASCT is the primary disease, both before (37%) and after 100 days (78%)4. Infections (29%) and organ failure (24%) were the other common causes of death before 100 days post-ASCT4.
Various patient-and disease-related factors are associated with ASCT outcomes. The CD34+ cell dose pre-transplantation is another decisive factor that depends on the ASCT outcome. ASCT conducted with a CD34+ dose of ≥5×106 CD34+ cells/kg body weight leads to rapid engraftment5. The earlier the engraftment and patient's count recovery, the lesser will be the complications.
Very few studies have highlighted the importance of pre-harvest CD34+ cell count with respect to early engraftment and count recovery. Pre-harvest CD34+ counts not only help in predicting the chances of early engraftment, but also helps in reducing the use of expensive therapy with Plerixafor6.
Our study aimed to determine the pattern of ASCTs at our tertiary care center and assess any associated factors leading to improved outcomes, including the importance of pre-harvest CD34+ levels with outcomes on engraftment.
Materials and Methods
Type and duration of study
A retrospective analysis of 65 patients from the ASCT registry of NRS Medical College, a tertiary care hospital in Kolkata, India, was conducted. The study period lasted from February 2009 to August 2020 (11 years and 7 months).
Ethical clearance
All analyses were performed according to the ethical standards of the institutional committee. The consent waiver was obtained from the Ethics Committee due to the retrospective nature of this study.
Inclusion criteria
All patients with the following disease conditions, who underwent ASCT, were included in the study: PCN [MM, plasma cell leukemia (PCL), and light chain deposition disease (LCDD)]; relapsed/refractory HL; NHL in 1st complete remission (CR), including mantle cell lymphoma (MCL), peripheral T-cell lymphoma (PTCL), anaplastic large cell lymphoma (ALCL), relapsed/refractory NHL, such as diffuse large B-cell lymphoma (DLBCL), acute lymphoblastic leukemia (ALL) in CR2, acute myeloid leukemia (AML) in CR1, and chronic myeloid leukemia (CML) in complete molecular remission. Only patients aged ≤65 years were considered for ASCT and were included in the analysis.
Exclusion criteria
Patients with PCN who did not attain CR/VGPR (very good partial remission), patients with follicular lymphoma, DLBCL in CR1, HL in CR1, AML in CR2, patients who refused ASCT, patients aged >65 years, and patients with comorbidities, such as reduced ejection fraction, who were ineligible for ASCT, were excluded from the study.
Parameters tested and methodology
The age, sex, and co-morbidities of the patient, as well as the type and stage of the primary disease, were studied through a thorough history and clinical examination. The primary disease, pre-transplant chemotherapy, remission status, transplant eligibility, and conditioning regimens used for the patient were recorded.
Preconditioning
Patients received Inj. GCSF 300 mcg (@5 mcg/kg/day) twice daily subcutaneously for 5 days, and 2mL of EDTA blood was sent to measure the pre-harvest CD34+ cell count on day 4 of GCSF. Plerixafor was administered at a dose of 0.24 mg/kg (0.16 mg/kg in case of renal impairment) 11 hours prior to stem cell harvest8. However, its use was excluded if the pre-harvest CD34+ count was >10 cells/μL7. Post-harvest, another CD34+ cell count was sent for in 2 mL of EDTA blood on day 5 of GCSF Inj.
CD34+ levels
The pre-harvest CD34+ cell count (cells/μL) and post-harvest CD34+ cell dose (expressed as ×106 cells/kg body weight) were measured from the peripheral blood and harvested product, respectively. The Ishage protocol was used to calculate the CD34+ cell counts9. The final CD34+ cell dose (measured as 106 cells/kg body weight) was calculated from the total nucleated cell count (TNC) (measured as cells/L) in the peripheral blood/harvest product, as follows:
CD34+ cells per kg of patient weight (106/kg)=TNC (per 109/L of product)×bag volume (mL)×viable
The cut-off for pre-harvest CD34+ cells was taken as 10 cells/μL based on established literature7. While published literature suggests that post-harvest CD34+ counts of >2×106 cells/kg body weight are adequate for engraftment, we used a cut-off of 5×106 cells/kg body weight5, 10.
Conditioning
The regimens used were melphalan (Mel) 140-200 mg/m2 for PCN, busulphan-cytarabine (Bu-Cy) for AML and CML, Fludarabine-Melphalan (Flu-Mel) for AML or ALL, and BCNU etoposide cytarabine melphalan (BEAM) for HL and NHL11, 12. Post conditioning, stem cell infusion was performed. In cases of diseases such as NHL, HL, or leukemia, stem cells were initially preserved by cryopreservation and infused at a later date13. Cryopreservation of stem cells was performed using plasma, 5% albumin, and dimethyl sulfoxide (DMSO).
Post-stem cell infusion
The other parameters studied were post-transplant time to attain neutrophil engraftment, post-transplant time to attain platelet engraftment, post-transplant complications, such as febrile neutropenia, and post-transplant delay in engraftment, measured by days of hospital stay14.
Post infusion, the time for engraftment, assessed by absolute neutrophil count (ANC) ≥500/mm3 for 3 consecutive days and platelet count ≥20,000/μL for 3 consecutive days (without platelet transfusion for 7 consecutive days), were calculated by analyzing an EDTA blood sample in a Sysmex XP-200 cell counter and a peripheral blood smear stained with Leishman stain. Delayed engraftment (DE) was defined as platelets
Progression-free survival (PFS) was calculated as the endpoint of relapse or death.
Statistical analysis
The data from the CRF were transcribed into an Excel database and analyzed using R statistical software (Language) version 3.6.3 and R Studio version 1.0.136 (R foundation). Measures such as mean, median, standard deviation, and range were calculated for continuous variables. Kaplan-Meier survival analysis was performed using R Package 'survival' and 'survminer'. The log-rank test was used to test the null hypothesis that there was no difference between the populations in the probability of an event (death or relapse). Differences between the two groups for numerical variables were analyzed using the Wilcoxon rank-sum test for non-parametric distribution. The level of significance was set at 5% for all the comparisons.
Results
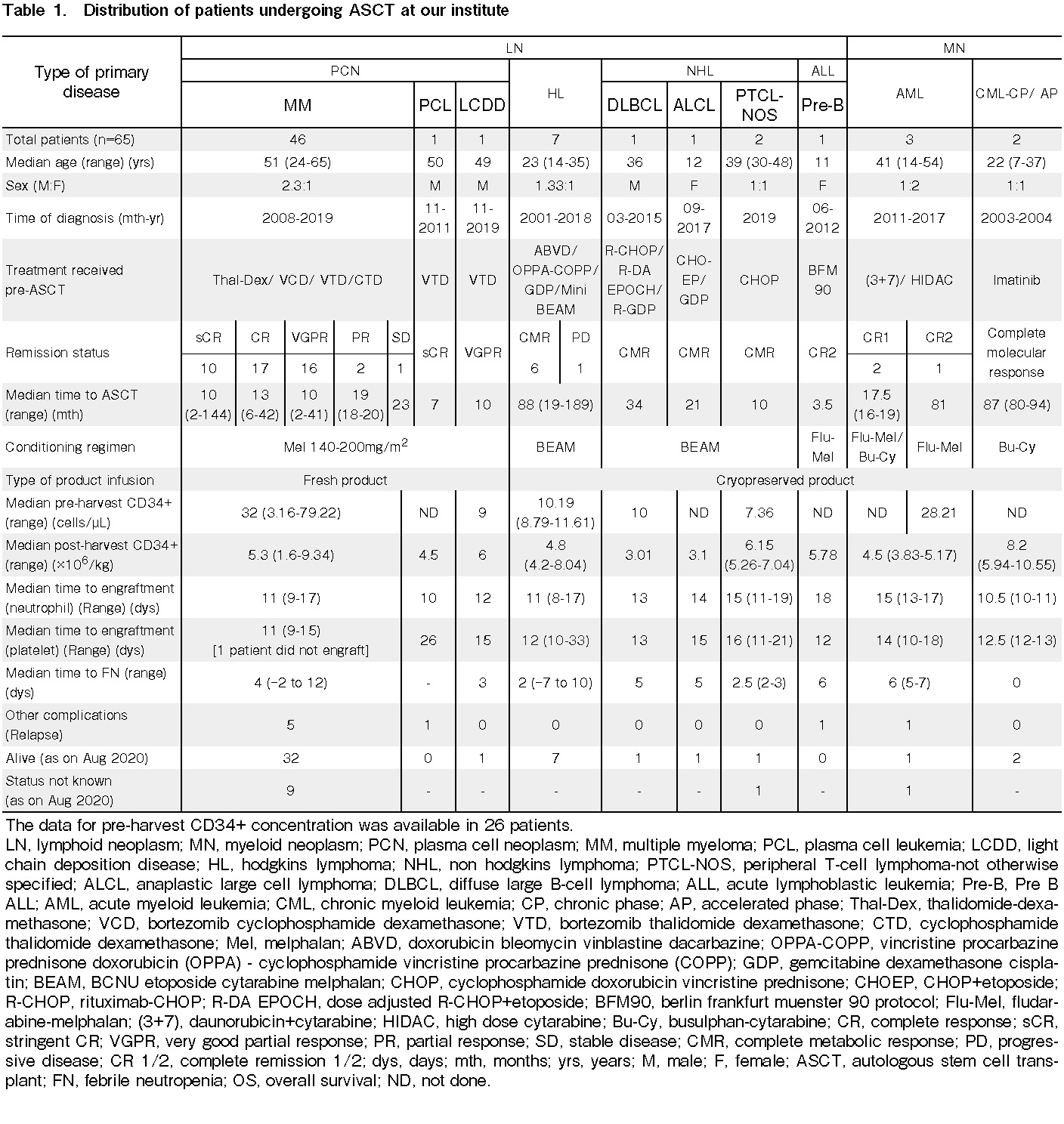
Of the 65 patients who underwent ASCT, patients with MM were the most common (70.8%, 46/65), followed by those with HL (10.8%, 7/65). The median age of our patient population was 49 years (range: 7-65 yrs), with a male: female ratio of 1.9:1. Few patients were initially diagnosed as long ago as 2001 (range: 2001-2019). The median time to ASCT was 13 months (range: 2-189 months). The individual distributions of diseases are shown in Table 1.
Patients with PCN were treated with Thal-Dex/VCD/VTD/CTD regimens (Thal-Dex=thalidomide-
Patients with AML, CML or ALL received therapy with 3+7 and HIDAC [(3+7) = Daunorubicin+ Cytarabine, HIDAC=High dose Cytarabine], Imatinib, and BFM90 (Berlin Frankfurt Muenster 90 protocol), respectively. They were conditioned with Flu-Mel or Bu-Cy regimens (Table 1).
During conditioning, GCSF was administered to all patients (n=65). The data for pre-harvest CD34+ stem cell counts were available for 26 patients. Data for the use of both Plerixafor and GCSF were available for 17 patients. These patients had a pre-harvest CD34+ count of <10 cells/μL.
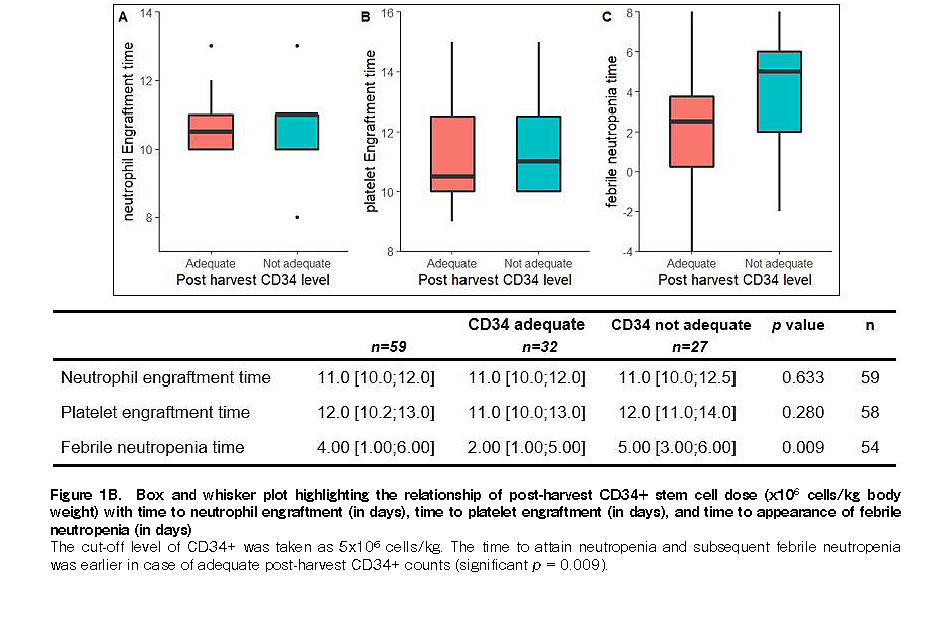
The median pre-harvest and post-harvest CD34+ stem cell counts were 27.54 cells/μL (range: 3.16-113 cells/μL) and 5.23×106 cells/kg body weight (available in all 65 patients; range: 1.6-10.55), respectively. The median time to engraftment was 11 days (range: 8-19 days) and 12 days (range: 9-26 days; one patient did not engraft) for neutrophils and platelets, respectively. Overall, the median neutrophil and platelet engraftment times were 11 days (IQR, 10; 12) and 12 days (IQR, 10.2; 13), respectively (Figure 1A
The cut-off for CD34+ pre-harvest concentration and post-harvest dose were taken as 10 cells/μL and 5×106 cells/kg body weight, respectively. There was no significant relationship between age and post-harvest
Platelet engraftment was associated with adequate CD34+ counts, although it was not statistically significant (p = 0.06, Wilcoxon rank-sum test) (Figure 1A). The time to attain neutropenia and subsequent febrile neutropenia was shorter in the case of adequate post-harvest CD34+ dose (significant p=0.009) (Figure 1B). In the patient who did not engraft, the pre-harvest
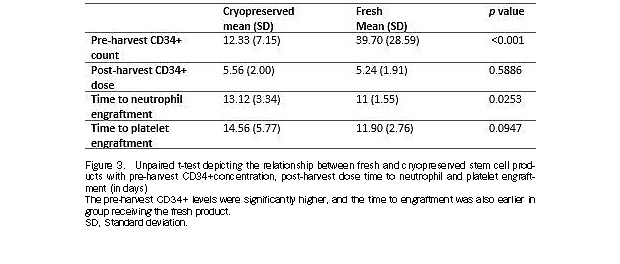
The time required for engraftment was shorter in MM and HL patients (11 days for neutrophil and platelet engraftment), compared to that in patients with NHL (13.5 days for neutrophil and platelet engraftment), and acute leukemia (17 days and 12 days for neutrophil and platelet engraftment, respectively). While fresh stem cell products were infused in the 48 patients with MM, cryopreserved products were infused in the 17 other patients (Table 1). The mean pre-harvest CD34+ level for the fresh products (39.7±28.59 cells/μL) was significantly different (p<0.001) from that of the cryopreserved products (12.33±7.15 cells/μL). The mean post-harvest CD34+ dose for the fresh products [(5.24±2)×106 cells/kg body weight] was not significantly different (p=0.58) from that of the cryopreserved products [(5.56±1.91)×106 cells/kg body weight]. Meanwhile, the mean time for neutrophil engraftment using fresh and cryopreserved products, 11 days (±1.55) and 13.12 days (±3.34), respectively, were significantly different (p=0.02) from each other. Furthermore, although the mean time for platelet engraftment using the fresh products was faster (11.9±2.76 days) than when cryopreserved products were used (14.56±5.77 days), the difference was not statistically significant (p = 0.09) (Figure 3).
Febrile neutropenia was seen in 83.1% (54/65) patients. The median time to febrile neutropenia was 4 days post ASCT [range: day (−7) to (+12)]. The post-harvest CD34+ dose (cut-off: 5×106 cells/kg) was inversely associated with febrile neutropenia (p=0.009) (Figure 1B). Meanwhile, the patients with DE did not have a statistically significant relationship with pre- or post-harvest CD34+ levels (Figures 1A, B).
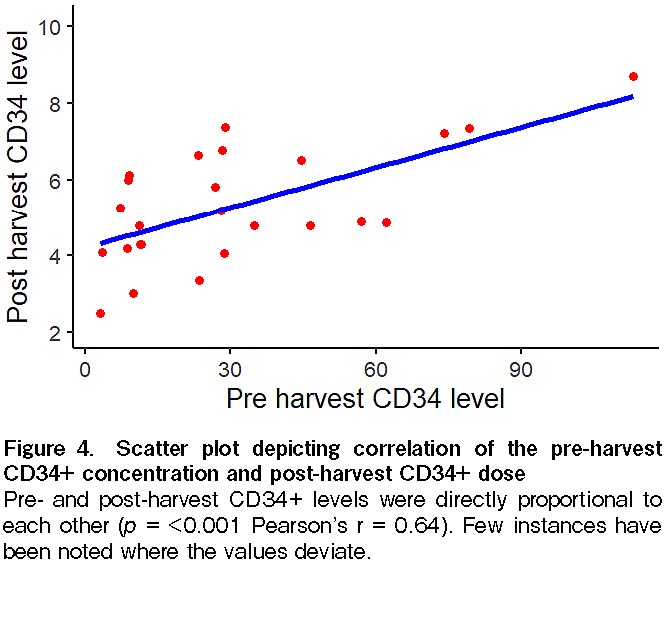
The pre- and post-harvest CD34+ levels were directly proportional to each other (p<0.001, Pearson's r=0.64). However, there were a few instances noted where the values deviated (Figure 4).
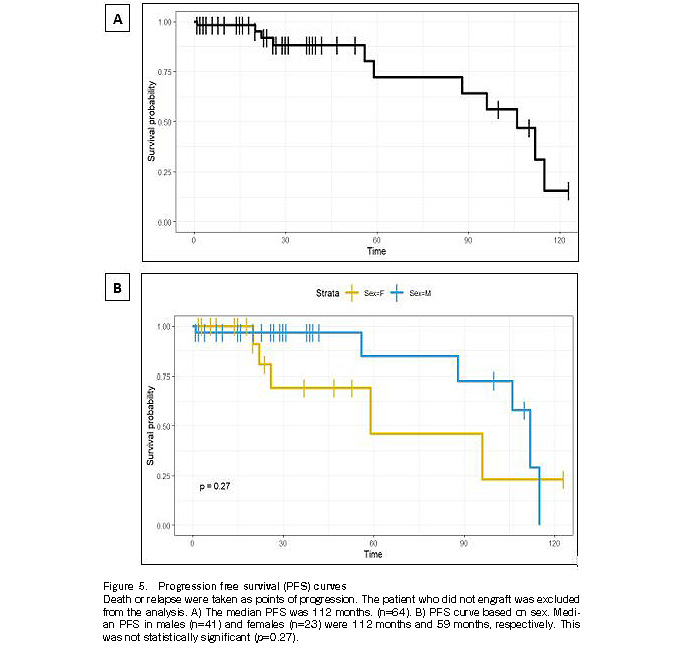
The median PFS was 112 months (n=65). Survival was better in males (median PFS: 112 months) than in females (median PFS: 59 months), although the difference was not statistically significant (p=0.27) (Figure 5). Furthermore, eight patients relapsed (five with MM, one with PCL, and one each with ALL and AML) and eight patients died (five with MM, one with PCL, one with ALL, and one with AML).
Discussion
ASCTs are very common, and PCN was the most common indication for ASCT in 73.8% (48/65) of patients, followed by HL (10.8%; 7/65) and NHL (6.2%; 4/65). This is similar to the trend seen in other studies2, 4. In a study done in India, MM was the most common disease for which ASCT was conducted6. In another study, HL (38%) was the most common indication of ASCT, followed by NHL and MM1. In our analysis, ASCT was conducted in 65 patients, of which, 4.6% (3/65) had AML, 3.1% (2/65) had CML-accelerated phase (AP), and 1.5% (1/65) had precursor B cell (Pre-B) ALL. It has been observed that ASCT may be a good option in AML-intermediate risk (IR) patients who do not have a provision for allogeneic HSCT16. Two patients had AML-IR, while one had relapsed and chose ASCT for logistic reasons. ASCT led to lower relapse rates as compared to chemotherapy and lesser mortality than allogeneic HSCTs in AML17. Though the exact role of ASCT in CML remains undefined, ASCT has been used to reverse patients in transformation back to Chronic phase (CP)18. In addition, ASCT is rarely considered in Philadelphia chromosome+ALL19, 20. In our patient with Pre-B ALL, ASCT was considered in CR2 due to logistic reasons.
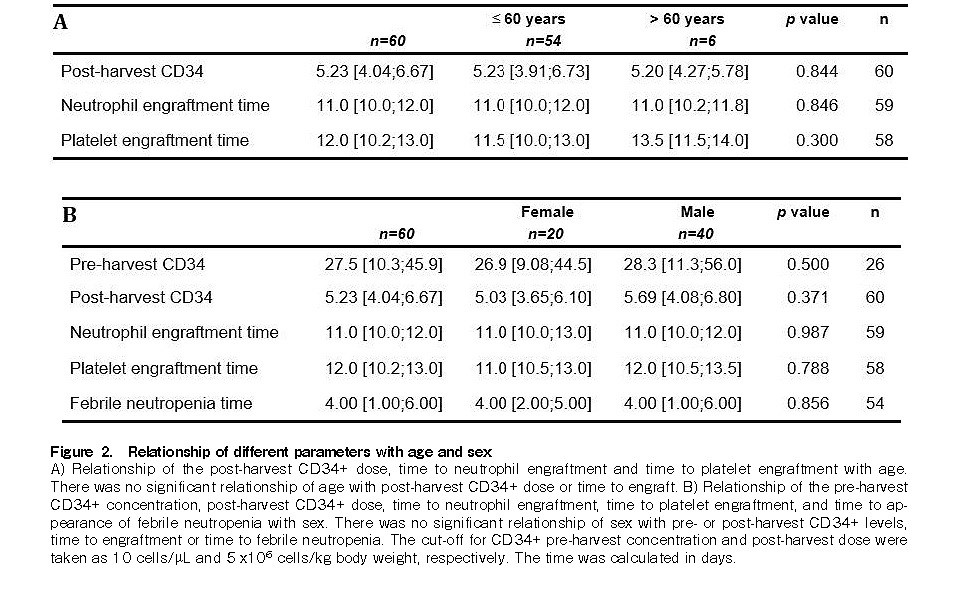
The median age of our patients was 49 years (range: 11-65 years) with a male predominance (male: female ratio=1.9:1). In another study, out of 69 patients who developed ASCT, the median age was 34 years (range: 4-64 years) with a male: female ratio of 2.5:11. In another study by Turk et al., the median age, which was 31.5 years (range: 13-70 years), was comparable, and there was a mild male preponderance (male: female ratio=1.4:1)21. In our findings, there was no association of the time to engraft with respect to age or sex of the patient. In a study by Zhu et al., the age of donors was found to be the main factor related to CD34+ cell count, while sex seemed to be unrelated22. The optimal time for cell collection in the study was day 4 of GCSF, unlike in our study where apheresis was conducted on the 5th day. In another study, the age of the patient, pre-harvest CD34+ cell count on day 2 of GCSF, and platelet counts were directly associated with CD34+ cell yield23. In this study, the GCSF dose was 10 mcg/kg, like in our study.
In different studies, patients received conditioning regimens with ICE/BEAM/CNV regimens21. Bu and Flu-Cy have been used in myeloid leukemias, while Mel and BEAM/BEAC have been used for PCN patients and in lymphomas, respectively11. In cases of lymphomas other than BEAM, Bu-Cy or irradiation have also been used as pre-conditioning agents12. No relationship of time to attain engraftment was associated with the type of conditioning regimen used15. Different conditioning regimens have been used depending on the primary disease in our study.
In a previous study, a pre-harvest CD34+ count of
The minimum CD34+ cell dose required for a successful stem cell engraftment after ASCT is 2 million/kg body weight10. As the normal CD34+ cell count in peripheral blood ranges between 0.01-0.05%, it is necessary to mobilize the stem cells using GCSF and Plerixafor6. For our analysis, the cut-off of the post-harvest CD34+ dose was taken as 5×106 cells/kg body weight.
Among our patients, there were some who were not able to send their pre-harvest CD34+ count due to logistic reasons, while in a few cases, the data could not be traced. Thus, a pre-harvest CD34+ count was only available for 26 patients. The median CD34+ count was 27.54 cells/μL (range: 3.16-113 cells/μL). The post-harvest CD34+ count was available for all 65 patients, and the median count was 5.23 ×106 cells/kg body weight (range: 1.6-10.55 cells/kg body weight). In patients whose post-harvest CD34+ cell count was <2×106 cells/kg body weight, the harvest was carried out for 2 days.
In a study by Liang et al., the CD34+ count was 4.91×106/kg body weight, and depended on the administration schedule and dose of GCSF24. In another study by Ali et al., the mean CD34+ cells infused was 4.7×108±1.7 mononuclear cells/kg1. A minimal CD34+ cell count of ≥8×106 CD34+ cells/kg seemed to be optimal for a successful ASCT5. Armitage et al. opined that a mobilization sample of stem cells ≥20×106 cells/kg resulted in an acceptable post-harvest stem cell count of ≥2×106 cells/kg25.
In our findings, the time required to attain neutrophil engraftment was significantly shorter among freshly harvested stem cells vs. cryopreserved products (p=0.02), and although not significant, the time required to attain platelet engraftment was also lower among the freshly harvested stem cells (p=0.09). The mean post-harvest CD34+ stem cell count was higher in the cryopreserved products than in the freshly harvest products. Meanwhile, in a study by Humpe et al., the median post-harvest CD34+ dose was lower prior to cryopreservation compared to that of a cryopreserved product26.
The median time for platelet and neutrophil engraftment was 15 days (range: 12-40 days) and 18.2±5.34 days, respectively, in one study1. In our study, the neutrophil and platelet engraftment times were shorter: 11 days (range 8-19 days) and 12 days (9-26 days, one patient did not engraft), respectively. In another study, the neutrophil and platelet engraftment times were nearly similar in all patients with a CD34+ cell count of >2×106 cells/kg27. In a study by Lutfi et al., 22.6% of patients had DE and most cases had delayed platelet recovery15. The study also showed that DE was more common among NHL than MM15. In our study, patients who did not have platelet engraftment had MM. Kiss et al. have shown that most patients had prompt neutrophil recovery, but there was delayed or no platelet recovery, especially in patients who were heavily pre-treated28.
The number of CD34+ stem cells infused was significantly related to the time to neutrophil or platelet recovery28. In our study, an adequate CD34+ concentration (pre- or post-harvest) was not significantly associated with the time to attain engraftment. However, the pre-harvest concentration and post-harvest cell dose were directly proportional to each other. This was also seen in a study by Lemos et al. where they have highlighted a moderate positive correlation between peripheral blood CD34+ cell count and total CD34+ cell count/kg (r=0.596; p <0.001)29.
Ali et al. showed that the median time to febrile neutropenia was 13 days, unlike in our study, where the median time to febrile neutropenia was 4 days (IQR 1; 6)1. It maybe opined that in our study, the adequate
In our analysis, very few patients relapsed after ASCT (n=8), of which there were five patients with MM. Early relapse within a year of ASCT is indicative of poor PFS30. We have also found that most patients who relapsed ultimately succumbed to the disease. In a study by Ali et al., an overall survival of 86% after a median follow-up of 104 months was recorded1. In MM, transplant-related mortality (TRM) occurred in one patient, and the overall survival (OS) was 93%. Meanwhile, in NHL, the TRM and OS were 25% and 70%, respectively, while in HL, the TRM and OS were 12% and 71%, respectively1.
ASCT is an established therapy for various hematological malignancies, the most common of which is MM. Although unrelated to age or sex, the post-harvest CD34+ dose was found to be inversely proportional to the time to febrile neutropenia. In addition, the pre- and post-harvest CD34+ concentrations were directly proportional to each other, and hence pre-harvest CD34+ levels may be reliably used to assess the engraftment outcome. The mean time for neutrophil and platelet engraftment was shorter in the case of fresh stem cell harvest products in comparison to cryopreserved products. The PFS was 112 months, and few patients suffered from relapse/death. The PFS was better in males than in females; however, the exact reason for this is unknown. Therefore, a larger number of patients need to be followed up to obtain a more accurate picture.
Acknowledgments
Prof (Dr) Malay Ghosh, Prof (Dr) Maitreyee Bhattacharya, Prof (Dr) Prantar Chakrabarti, Dr Tusti Ganguly, Dr Soumya Mukherjee, Dr Vinodhini, Dr Abhijit Phukan, Dr Pritish Patra, Dr Shazia Gulshan, Dr Ankit Jitani, Dr Bijita Dutta, Dr Manisha Jain, Dr Karthika V, Dr Prakash Shekhawat, Dr Avriti Baveja, Dr Malini Garg, Dr Rishu Vidhatri
Author Contributions
TKD reviewed the data and manuscript. RD helped in patient management. AS participated in data collection, analyzed data, and prepared the manuscript. SNB, SM, SB helped in patient management. IM helped in pathological investigations. KM did the statistical analysis. AC, SD helped in pathological investigations. PKM helped in patient management.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Ali N, Adil SN, Shaikh MU. Autologous Hematopoietic Stem Cell Transplantation-10 Years of Data from a Developing Country. Stem Cells Transl Med. 2015; 4:873-7.
2.Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone Marrow Transplantation. 2014; 49: 744-50.
3.Passweg JR, Baldomero H, Bregni M, Cesaro S, Dreger P, Duarte RF, et al.; European Group for Blood and Marrow Transplantation. Hematopoietic SCT in Europe: data and trends in 2011. Bone Marrow Transplant. 2013; 48: 1161-7.
4.D'Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant. 2020; 11: S1083-8791(20)30225-1.
5.Siena S, Schiavo R, Pedrazzoli P, Carlo-Stella C. Therapeutic Relevance of CD34 Cell Dose in Blood Cell Transplantation for Cancer Therapy. Journ of Clin Oncol. 2000; 18: 1360-77.
6.Agarwal P, Tejwani N, Pathak A, Kumar D, Agrawal N, Mehta A. Benefits of Pre-harvest Peripheral Blood CD34 Counts Guided Single Dose Therapy with PLERIXAFOR in Autologous Hematopoietic Stem Cell Transplantation: A Retrospective Study at a Tertiary Care Institute in India. Ind Journ of Hemat and Blood Transfus. 2019; 35: 72-6.
7.Szwajcer D, Jennings-Coutts A, Giftakis A, Wall DA. Identification of the CD34 enumeration on the day before stem cell harvest that best predicts poor mobilization. Transfusion. 2011; 51: 587-90.
8.Kouroukis CT, Varela NP, Bredeson C, Kuruvilla J, Xenocostas A. Plerixafor for autologous stem-cell mobilization and transplantation for patients in Ontario. Curr Oncol. 2016; 23: e409-30.
9.Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996; 5: 213-26.
10.Barnett D, Janossy G, Lubenko A, Matutes E, Newland A, Reilly JT. Guideline for the flow cytometric enumeration of CD34+ haematopoietic stem cells. Prepared by the CD34+ haematopoietic stem cell working party. General Haematology Task Force of the British Committee for Standards in Haematology. Clin Lab Haematol. 1999; 21: 301-8.
11.Gyurkocza B, Sandmaier BM. Conditioning regimens for hematopoietic cell transplantation: one size does not fit all. Blood. 2014; 124: 344-53.
12.Chen YB, Lane AA, Logan B, Zhu X, Akpek G, Aljurf M, et al. Impact of conditioning regimen on outcomes for patients with lymphoma undergoing high-dose therapy with autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2015; 21: 1046-53.
13.Hornberger K, Yu G, McKenna D, Hubel A. Cryopreservation of Hematopoietic Stem Cells: Emerging Assays, Cryoprotectant Agents, and Technology to Improve Outcomes. Transfus Med Hemother 2019; 46: 188-96.
14.Turk HM, Komurcu S, Arpaci F, Ozet A, Kilic S, Kuzhan O, et al. Factors affecting engraftment time in autologous peripheral stem cell transplantation. Asian Pac J Cancer Prev. 2010; 11: 697-702.
15.Lutfi F, Skelton IV WP, Wang Y, Rosenau E, Farhadfar N, Murthy H, et al. Clinical predictors of delayed engraftment in autologous hematopoietic cell transplant recipients. Hematol Oncol Stem Cell Ther. 2020; 13: 23-31.
16.Li Z, Liu Y, Wang Q, Chen L, Ma L, Hao S: Autologous Stem Cell Transplantation Is a Viable Postremission Therapy for Intermediate-Risk Acute Myeloid Leukemia in First Complete Remission in the Absence of a Matched Identical Sibling: A Meta-Analysis. Acta Haematol 2019; 141: 164-75.
17.Ganzel C, Rowe JM. Revisiting autologous transplantation in acute myeloid leukemia. Curr Opin Hematol. 2018; 25: 95-102.
18.Olavarria E. Autologous stem cell transplantation in chronic myeloid leukemia. Semin Hematol. 2007; 44: 252-8.
19.Gorin NC, Giebel S, Labopin M, Savani BN, Mohty M, Nagler A. Autologous stem cell transplantation for adult acute leukemia in 2015: time to rethink? Present status and future prospects. Bone Marrow Transplant. 2015; 50: 1495-502.
20.Wetzler M, Watson D, Stock W, Koval G, Mulkey FA, Hoke EE, et al. Autologous transplantation for Philadelphia chromosome-positive acute lymphoblastic leukemia achieves outcomes similar to allogeneic transplantation: results of CALGB Study 10001 (Alliance). Haematologica. 2014; 99: 111-5.
21.Turk HM, Komurcu S, Arpaci F, Ozet A, Kilic S, Kuzhan O, et al. Factors Affecting Engraftment Time in Autologous Peripheral Stem Cell Transplantation. Asian Pacific Journal of Cancer Prevention. 2010; 11: 697-702.
22.Zhu L, Zhou LK, Xue M, Yan HM, Liu J, Wang ZD, et al. [Factors impacting yield of CD34(+) cells from healthy donors mobilized with rhG-CSF]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009; 17: 1541-5. Chinese.
23.Suzuya H, Watanabe T, Nakagawa R, Watanabe H, Okamoto Y, Onishi T, et al. Factors associated with granulocyte colony-stimulating factor-induced peripheral blood stem cell yield in healthy donors. Vox Sang. 2005; 89: 229-35.
24.Liang ZY, Ren HY. [Factors affecting mobilization of peripheral blood stem/progenitor cells and apheresis efficiency from healthy donors by rhG-CSF]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2008; 16: 847-51. Chinese.
25.Armitage S, Hargreaves R, Samson D, Brennan M, Kanfer E, Navarrete C. CD34 counts to predict the adequate collection of peripheral blood progenitor cells. Bone Marrow Transplant. 1997; 20: 587-91.
26.Humpe A, Riggert J, Vehmeyer K, Troff C, Hiddemann W, Kohler M, et al. Comparison of CD34+ cell numbers and colony growth before and after cryopreservation of peripheral blood progenitor and stem cell harvests: influence of prior chemotherapy. Transfusion. 1990; 37: 1050-7.
27.Stiff PJ, Micallef I, Nademanee AP, Stadtmauer EA, et al. Transplanted CD34+ Cell Dose Is Associated with Long-Term Platelet Count Recovery following Autologous Peripheral Blood Stem Cell Transplant in Patients with Non-Hodgkin Lymphoma or Multiple Myeloma. Biology of Blood and Marrow Transplantation. 2011; 17: 1146-53.
28.Kiss JE, Rybka WB, Winkelstein A, de Magalhaes-Silverman M, Lister J, D'Andrea P, et al. Relationship of CD34+ cell dose to early and late hematopoiesis following autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997; 19: 303-10.
29.Lemos NE, Farias MG, Kubaski F, Scotti L, Onsten TGH, Brondani LA, et al. Quantification of peripheral blood CD34+ cells prior to stem cell harvesting by leukapheresis: a single center experience. Hematol Transfus Cell Ther. 2018; 40: 213-8.
30.Bygrave C, Pawlyn C, Davies F, Craig Z, Cairns D, Hockaday A, et al. Early relapse after high-dose melphalan autologous stem cell transplant predicts inferior survival and is associated with high disease burden and genetically high-risk disease in multiple myeloma. British Journ of Hemat. 2021; 193: 551-5.
Search
News