Volume 5 (2022) Issue 1 No.4 Pages 31-34
Abstract
Cytokine release syndrome (CRS), which may be associated with fever, hypotension, hypoxia, and organ damage, is caused by a massive cytokine release after chimeric antigen receptor (CAR)-T cell therapy. We present the case of a patient who developed severe bloody diarrhea due to CRS after CAR-T cell infusion. A 10-year-old boy presented with a second relapse of B-cell precursor acute lymphoblastic leukemia 6 months after hematopoietic stem cell transplantation from an unrelated donor. CAR-T cells (tisagenlecleucel) were infused at the third complete remission after salvage chemotherapy. While fever >39
Introduction
Cytokine release syndrome (CRS), a major acute complication of chimeric antigen receptor (CAR)-T cell therapy, is caused by a massive release of cytokines, which may damage organs, including the gastrointestinal (GI) tract1. However, diarrhea after CAR-T cell infusion has been reported in a few adults and rarely in children2–4. Here, we present the case of a boy with severe bloody diarrhea due to CRS after CAR-T cell infusion for refractory B-cell precursor acute lymphoblastic leukemia (ALL).
Case Presentation
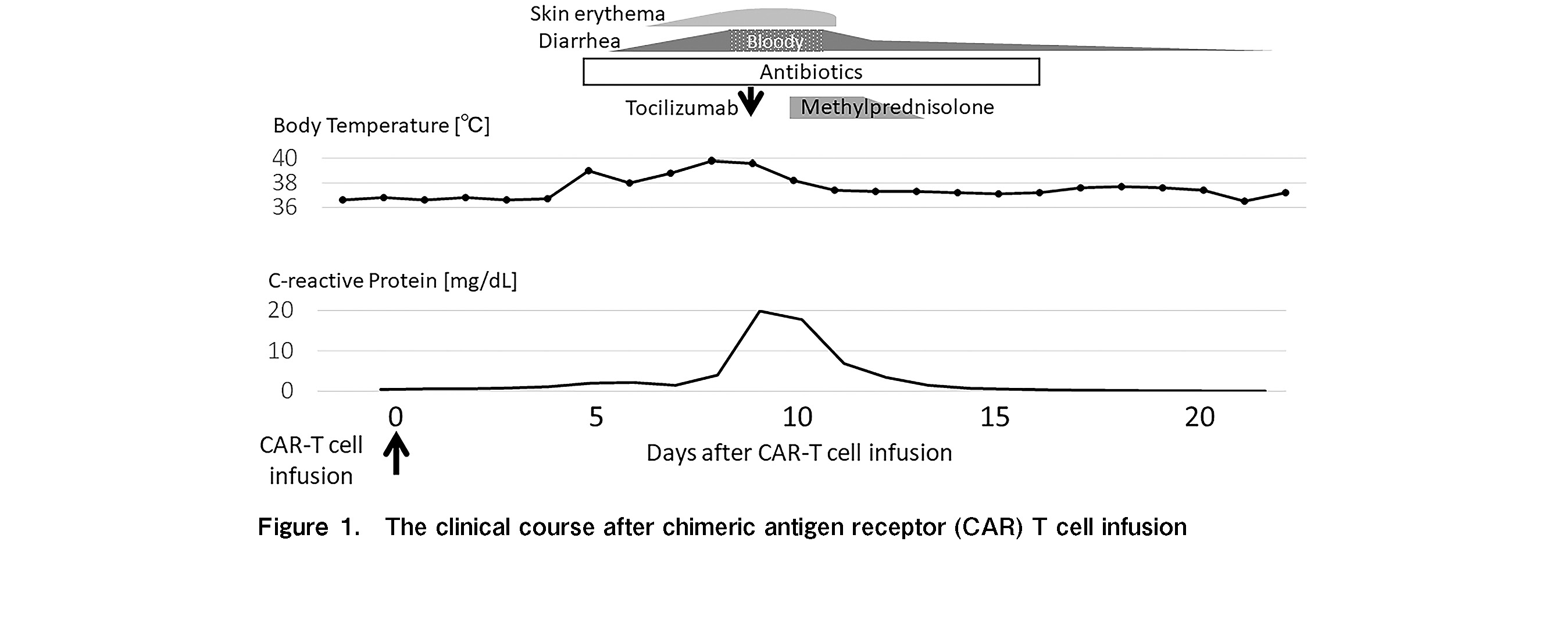
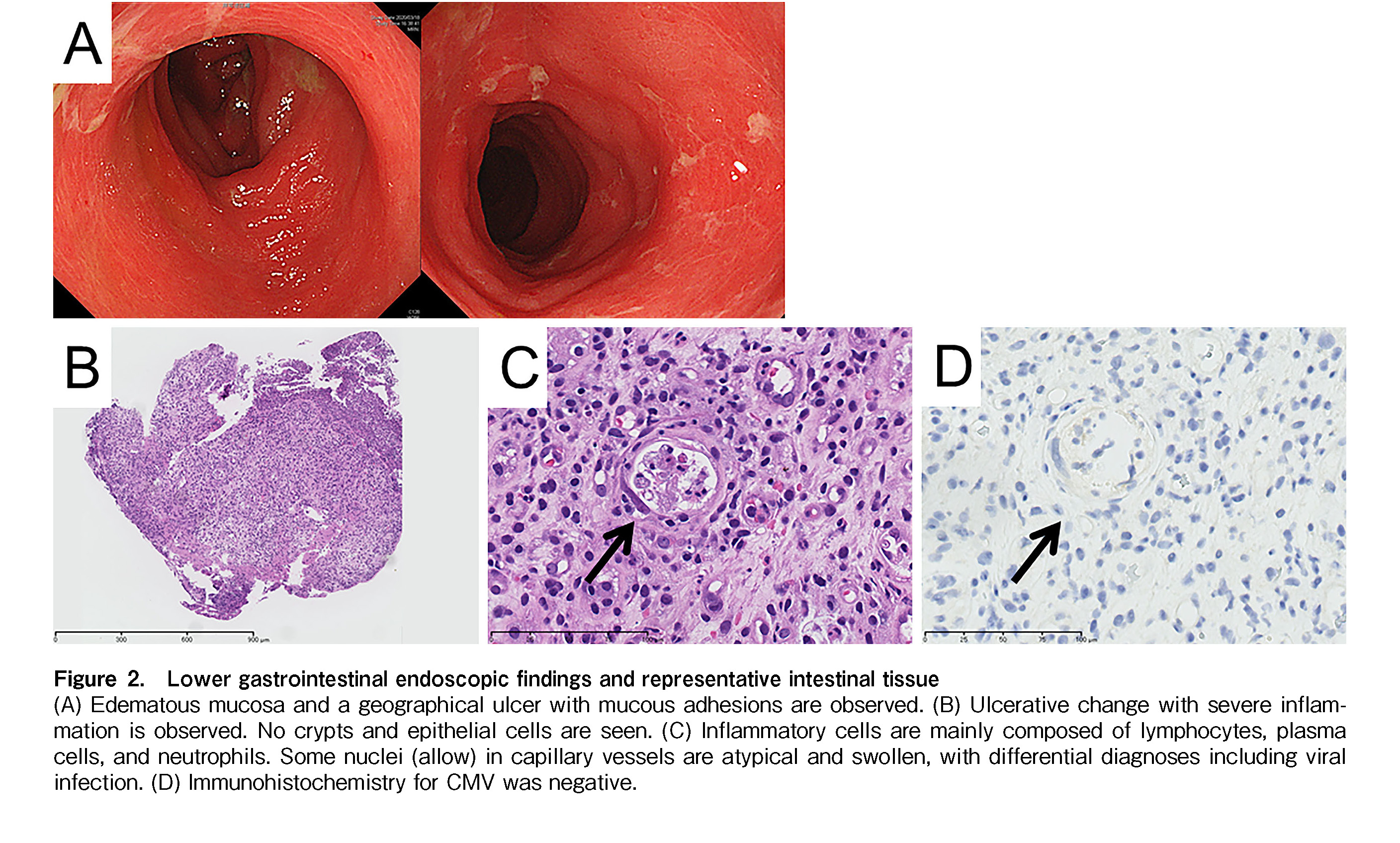
The patient was a 10-year-old boy with a second relapse of B-cell precursor ALL. An isolated bone marrow relapse of CD19-positive ALL harboring P2RY8-CRLF2 fusion was detected 6 months after peripheral blood stem cell transplantation from a 6/8 HLA-allele matched unrelated donor. Donor chimerism in the bone marrow was 100% and 84% at day 60 after transplantation and at relapse, respectively. Tacrolimus, used for GI graft-versus-host disease (GVHD)-associated mild nausea, was immediately discontinued for lymphocyte apheresis. Following lymphocyte apheresis, he was treated with one cycle of chemoimmunotherapy with inotuzumab ozogamicin and mini hyper-CVD and achieved a third complete remission with negative P2RY8-CRLF2 transcripts (donor chimerism 98.8%). During this period, there was no recurrence of symptoms related to GI GVHD. CAR T-cells (tisagenlecleucel) were infused after completion of lymphodepleting chemotherapy consisting of fludarabine and cyclophosphamide. The clinical course of the patient is shown in Figure 1. The patient sustained a fever of >39
Discussion
Diarrhea may occur after CAR-T cell therapy as a manifestation of CRS1. However, previous studies reported only two adult patients and no pediatric patients presenting with diarrhea after CAR-T cell therapy2–4. Furthermore, the severity or clinical course of GI symptoms in these few cases has not been described. In addition to CRS, the main differential diagnoses for the causes of bloody diarrhea after CAR-T cell therapy are infections and GVHD in those with a history of hematopoietic stem cell transplantation. Diarrhea is a typical symptom of GI GVHD, and in severe cases, bloody stools may be present. Although GVHD risk is relatively low with tisagenlecleucel6, a higher incidence of GVHD has been reported in patients treated with the new-generation CAR-T products6, 7. Furthermore, active GVHD at lymphocyte apheresis is a risk factor for worsening pre-existing GVHD. Activation of residual host-derived T cells in the intestine may also aggravate GI GVHD8; therefore, the possibility of GVHD should be considered after CAR-T cell therapy in patients who experience relapse after transplantation.
Therapeutic interventions for CRS of GI damage without other organ dysfunction may vary depending on the grading system used as a reference. For example, the American Society for Transplantation and Cellular Therapy grading system is based on fever, hypotension, and hypoxia. Organ toxicity was not included because it is thought to be associated with severe respiratory failure and hypotension. Management of organ toxicities is based on specific standard guidelines, and it is not thought to influence the decision to use CRS-specific therapies9. Other CRS grading systems include organ toxicities, and therapeutic interventions with tocilizumab +/− corticosteroids are recommended for patients with ≥ grade 2 organ toxicities1, 10. Despite a trend towards the early use of tocilizumab, the administration of corticosteroids is considered cautiously because high cumulative doses of corticosteroids may affect clinical outcomes and induce disease recurrence1, 10, 11.
In our case, although the circulatory and respiratory statuses were unremarkable, high fever persisted and GI symptoms worsened. As no evidence of infection or GVHD was obtained, we concluded that the bloody diarrhea was due to CRS. Since grade 3 GI dysfunction corresponded to grade 2 CRS, tocilizumab was administered according to the algorithm1. A single dose of tocilizumab was highly effective not only for the persistent fever but also for bloody diarrhea and erythema, and the concomitant corticosteroids was discontinued after 4 days. The fact that the immunosuppressive drugs could be completely discontinued within a few days suggests that the GI symptoms in this case were more likely to be associated with CRS.
Severe bloody diarrhea is a rare but important complication of CRS. For non-infectious severe GI symptoms after CAR-T cell therapy, we recommend the early use of tocilizumab, as it may prevent the long-term use of corticosteroids.
Author Contributions
H.S., K.E., and H.S. wrote the manuscript, H.S, T.I., J.I., T.K., D.K., F.Y., H.G., and H.S. contributed to the management of the patient.
Informed Consent
Informed consent was obtained from the patients’ guardians.
Conflicts of Interest
H.G. has been on the advisory board of Novartis. The other authors declare no conflicts of interest. Disclosure forms provided by the authors are available on the website.
References
1.Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014; 124: 188-95.
2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011; 365: 725-33.
3.Brentjens RJ, Rivière I, Park JH, Davila ML, Wang X, Stefanski J, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011; 118: 4817-28.
4.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018; 378: 439-48.
5.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol. 2009; 40:909-17.
6.Levine JE, Grupp SA, Pulsipher MA, Dietz AC, Rives S, Myers GD, et al. Pooled safety analysis of tisagenlecleucel in children and young adults with B cell acute lymphoblastic leukemia. J Immunother Cancer. 2021; 9: e002287.
7.Sanber K, Savani B, Jain T. Graft-versus-host disease risk after chimeric antigen receptor T-cell therapy: the diametric opposition of T cells. Br J Haematol. 10.1111/bjh.17544
8.Divito SJ, Aasebø AT, Matos TR, Hsieh PC, Collin M, Elco CP, et al. Peripheral host T cells survive hematopoietic stem cell transplantation and promote graft-versus-host disease. J Clin Invest. 2020; 130: 4624-36.
9.Pennisi M, Jain T, Santomasso BD, Mead E, Wudhikarn K, Silverberg ML, et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 2020; 4:676-86.
10.Brudno JN, Kochenderfer JN. Recent advances in CAR T-cell toxicity: Mechanisms, manifestations and management. Blood Rev. 2019; 34: 45-55.
11.Davila ML, Riviere I, Wang X, Bartido S, Park J, Curran K, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014; 6: 224ra25.
Search
News