Volume 4 (2021) Issue 4 No.4 Pages 92-100
Abstract
Aggressive T and NK/T-cell lymphoma are known to have a high risk of relapse and poor long-term prognosis. Hematopoietic stem cell transplantation has been performed as part of consolidation or salvage treatment. We retrospectively studied the outcomes of autologous (A) and allogeneic (allo) hematopoietic stem cell transplantation (SCT) in aggressive T and NK/T-cell lymphoma at our center between 2010 to 2020. Patients with nodal peripheral T-cell lymphoma (PTCL) that were younger than 65 years old who did not receive upfront autologous SCT (ASCT) at first complete remission were selected from our registry data for further comparison. Thirty-six patients underwent ASCT, and 16 patients underwent alloSCT. In the ASCT cohort, 18 patients with nodal PTCL who underwent upfront ASCT at first complete remission (upfront ASCT) were compared with 15 patients with nodal PTCL who were in first complete remission after single-line induction but did not receive ASCT. The two-year progression-free survival (PFS) and overall survival (OS) rates for the ASCT cohort were 58% and 73%, respectively. The two-year PFS and OS for the alloSCT cohort were 47% (P=0.35, P=0.02, respectively). Twenty-four patients who received SCT at first remission (21 ASCT and three alloSCT) had a two-year PFS and OS of 75% and 89%, respectively. In comparison, 28 patients who received SCT at relapse/refractory (15 ASCT and 13 alloSCT) had a two-year PFS and OS of 40% and 50%, respectively (P=0.047, P=0.024, respectively). Patients in complete remission prior to transplantation (n=42) had a two-year PFS and OS of 59% and 73%, respectively. In contrast, patients in partial remission prior to transplantation (n=10) had a two-year PFS and OS of 40% and 48%, respectively (p>0.05). Non-relapse mortality occurred in 6% and 43% of ASCT and AlloSCT, respectively. Multivariate analysis revealed that EBV-positivity at diagnosis indicated poorer PFS. EBV-positivity at diagnosis and more than two prior lines of treatment at transplant were associated with poorer OS. For nodal PTCL, the two-year PFS and OS were 79% and 100% for the upfront ASCT cohort and 78% and 92% for the non-upfront ASCT cohort, respectively (p>0.05). Hematopoietic SCT is a feasible treatment option for aggressive T and NK/T-cell lymphoma. Patients who underwent SCT at first remission had better survival rates than those who underwent SCT at relapse/refractory. Nevertheless, due to the limited sample size of the current study, the role of upfront ASCT in patients with nodal PTCL who achieved first complete remission remains unclear.
Introduction
Aggressive T and NK-T-cell lymphoma is a group of heterogeneous diseases with a poor long-term prognosis1. Its incidence is high in East Asia, accounting for 22% to 42% of the total number of patients diagnosed with lymphoma2. The risk of relapse is high even with intense chemoimmunotherapy. Based on previous studies, the five-year overall survival (OS) ranges between 30%-50%, with most deaths being disease-related3, 4. To improve long-term survival, hematopoietic stem cell transplantation (HSCT) has been performed in both upfront and relapse-refractory settings. Autologous SCT (ASCT) as consolidation therapy in patients with peripheral T-cell lymphoma (PTCL) who attain first remission following induction chemotherapy is not a universally accepted standard of care. To determine the efficacy and toxicity of HSCT in the management of T and NK-T-cell lymphoma, we analyzed the transplantation results at our institution. Next, we compared the survival outcomes of patients with PTCL who received upfront ASCT at first remission with a historical cohort who did not receive upfront ASCT.
Material and Methods
Patient selection and variables definition
From the hematopoietic SCT registry of Singapore General Hospital, we retrospectively analyzed consecutive T-cell lymphoma and NK-T-cell lymphoma patients who underwent autologous and allogeneic (allo) SCT between January 2010 and June 2020. The decision for transplantation at the upfront or relapse-refractory setting was made by the attending physician and patient. Diagnosis of lymphoma was determined by histological and immunophenotypic analyses and defined according to the 2008 World Health Organization classification system5.
To examine the role of upfront ASCT, a historical cohort of patients aged 18-65 years old with histologically confirmed nodal PTCL (excluding ALK-positive anaplastic large cell lymphoma, ALCL) who achieved first complete remission with single-line curative-intent induction chemotherapy but did not receive upfront ASCT between 2010 and 2020 were selected from the lymphoma registry (non-upfront ASCT). The institutional review board approved the protocols and analyses (2015/2419 and 2018/2520), and informed consent was obtained from all patients for the HSCT and lymphoma registry. Standard definitions were used to determine disease response6.
Statistical analysis
Chi-squared and Fisher's exact two-sided tests were used to compare the categorical variables, and the t-test was used for continuous variables. Two-sided P-values of <0.05 were considered to be statistically significant. The survival probabilities, progression-free survival (PFS), and OS were calculated using the Kaplan-Meier estimator. Log-rank analysis was used to compare the different groups. Cox regression analysis was used for multivariate variable analysis. All analyses were performed using Stata software (StataCorp, College Station, TX, USA).
Results
Baseline characteristics, disease status, and transplant details
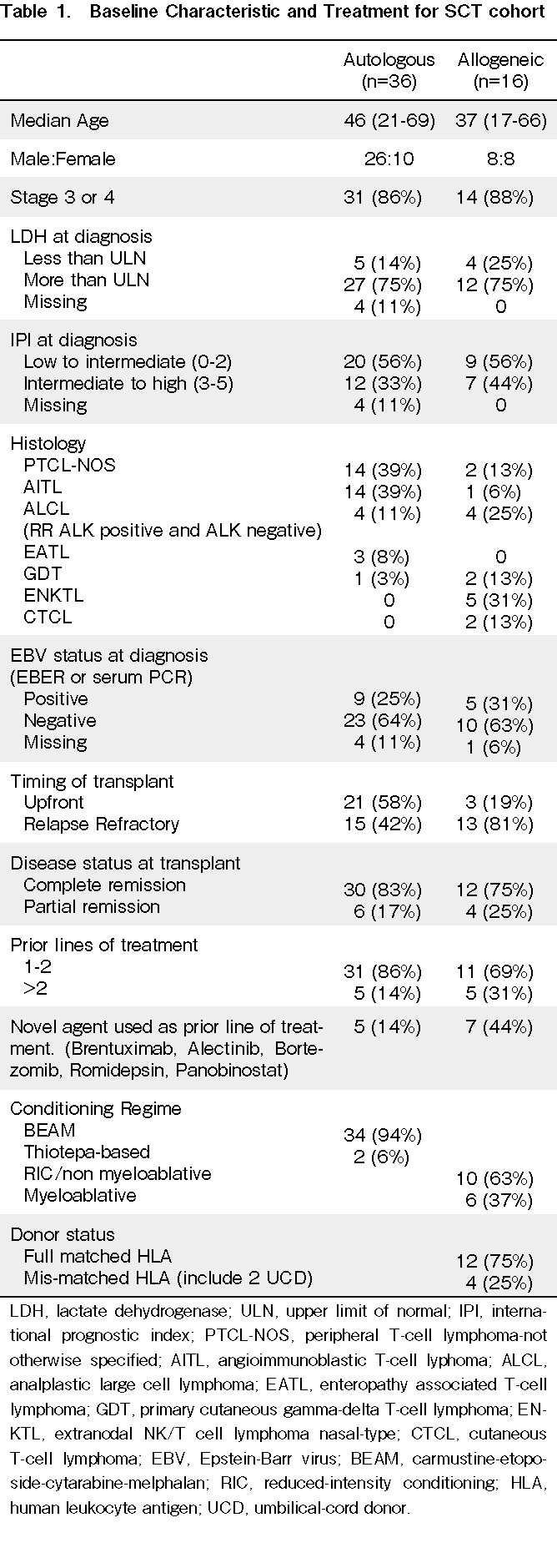
From a total of 52 patients, 32 and 16 underwent autologous SCT and allogeneic SCT, respectively. Four patients relapsed post-autologous SCT and subsequently received allogeneic therapy. These four patients were included in the autologous-only group, and their survival was censored at allogeneic SCT. The median ages of the autologous and allogeneic cohorts were 46 and 37 years old, respectively. More than 80% of the patients were in stages 3-4. In both cohorts, 75% of patients had lactate dehydrogenase (LDH) above the upper limit of normal at diagnosis. Moreover, 33% of patients from the ASCT cohort and 44% from the alloSCT cohort had an intermediate to high international prognostic index (IPI=3-5). Peripheral T-cell lymphoma not otherwise specified (PTCL-NOS) and angioimmunoblastic T-cell lymphoma (AITL) were the two most common subtypes in patients who underwent ASCT, with 14 patients each. In the allogeneic cohort, there were two, one, four, two, two, and five patients with PTCL-NOS, AITL, ALCL, primary cutaneous gamma-delta T-cell lymphoma, cutaneous T-cell lymphoma, and extranodal NK-T-cell lymphoma nasal-type (ENKTL), respectively. Nine (25%) and five (31%) patients from the ASCT and AlloSCT cohorts, respectively, were positive for Epstein-Barr virus (EBV) at diagnosis, defined as EBER (EBV-encoded small ribonucleic acid) positive on histopathology sample or positive plasma EBV polymerase chain reaction.
Five patients (14%) in the ASCT cohort and five patients (31%) in the alloSCT cohort received more than two prior lines of treatment at transplant. Twelve patients (23%), five from the autologous cohort and seven from the allogeneic cohort, received novel agents (one of brentuximab, alectinib, bortezomib, romidepsin, or panobinostat) prior to transplantation. For the autologous SCT cohort, 83% and 17% of patients were in complete remission and partial remission, respectively, prior to transplantation. In the allogeneic SCT cohort, 75% of the patients were in complete remission, and 25% were in partial remission prior to transplantation. More patients in the autologous SCT cohort received HSCT upfront at first remission compared to the allogeneic SCT cohort (58% vs. 19%). The conditioning regimens for patients in the ASCT group were BEAM (carmustine-etoposide-cytarabine-melphalan), except for two who received Thiotepa-based regimens. In the allogeneic SCT group, 63% had RIC/non-myeloablative conditioning, and 37% had myeloablative conditioning. Twelve (75%) patients received full HLA-matched donors, including matched siblings and matched unrelated donors. The baseline characteristics, disease status, and transplant details of all of the included patients are summarized in Table 1.
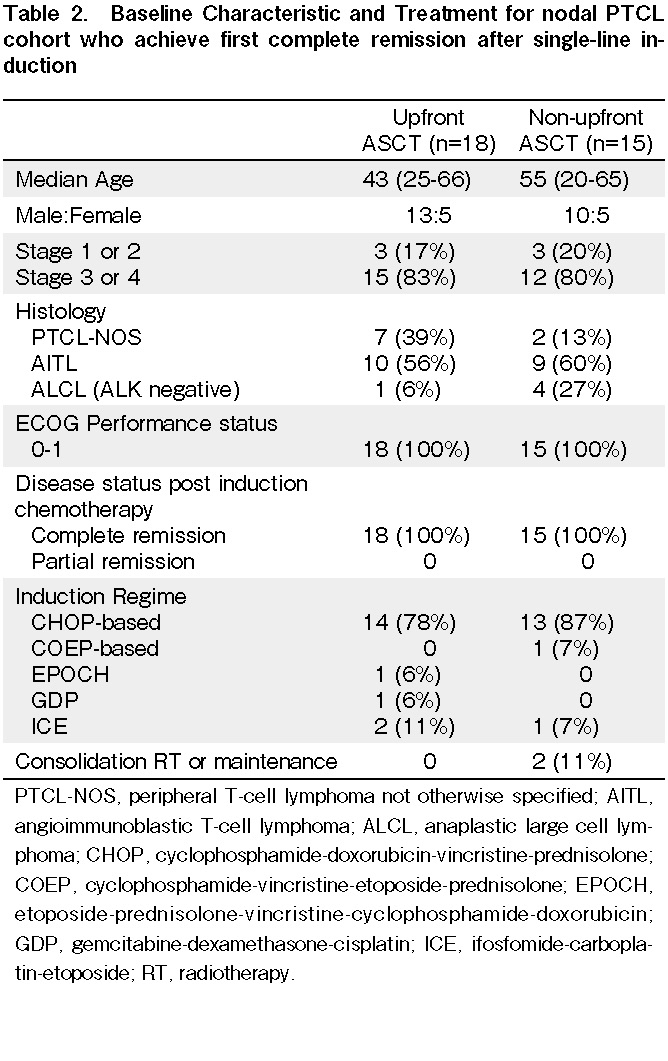
Of the 36 patients in the autologous SCT cohort, 18 had nodal PTCL (excluding ALK-positive ALCL) transplanted upfront at first complete remission (upfront ASCT). These patients were compared to 15 patients with PTCL (excluding ALK-positive ALCL) from a historical cohort that did not undergo ASCT upfront (non-upfront ASCT) at first complete remission. The median age for the upfront ASCT and non-upfront ASCT cohorts was 43 and 55 years old, respectively. These two cohorts did not differ significantly in Eastern Cooperative Oncology Group performance status, PTCL subtype, and stage of disease. The most common induction chemotherapy was cyclophosphamide-doxorubicin-vincristine-prednisolone (CHOP)-based (78% for upfront ASCT and 87% for non-upfront ASCT). For the non-upfront ASCT cohort, one patient received consolidation radiotherapy, and one patient received maintenance methotrexate and prednisolone. These findings are summarized in Table 2.
Autologous and allogeneic transplant survival outcome
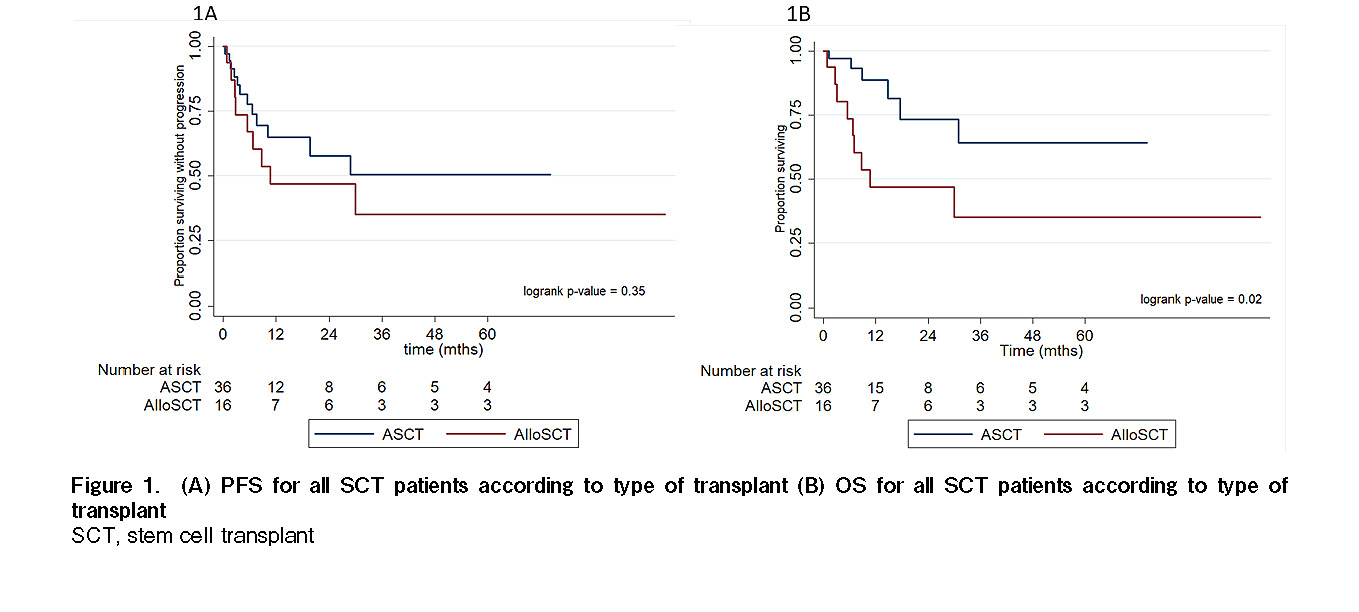
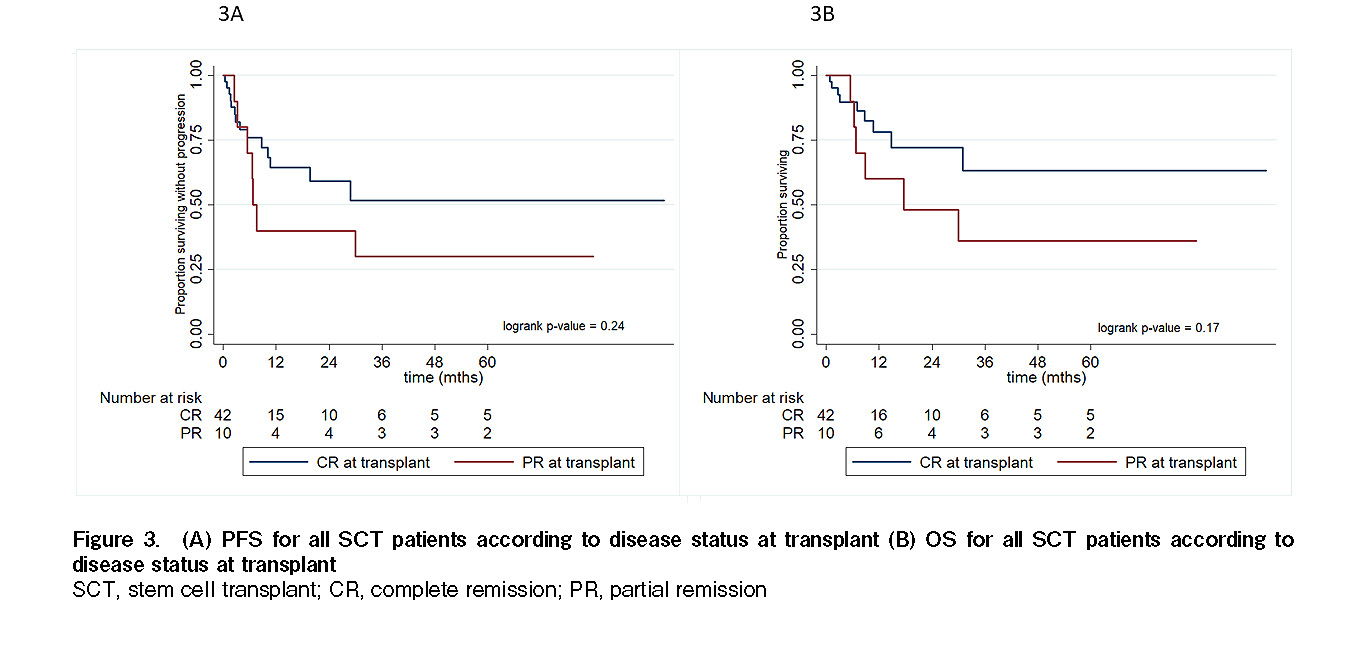
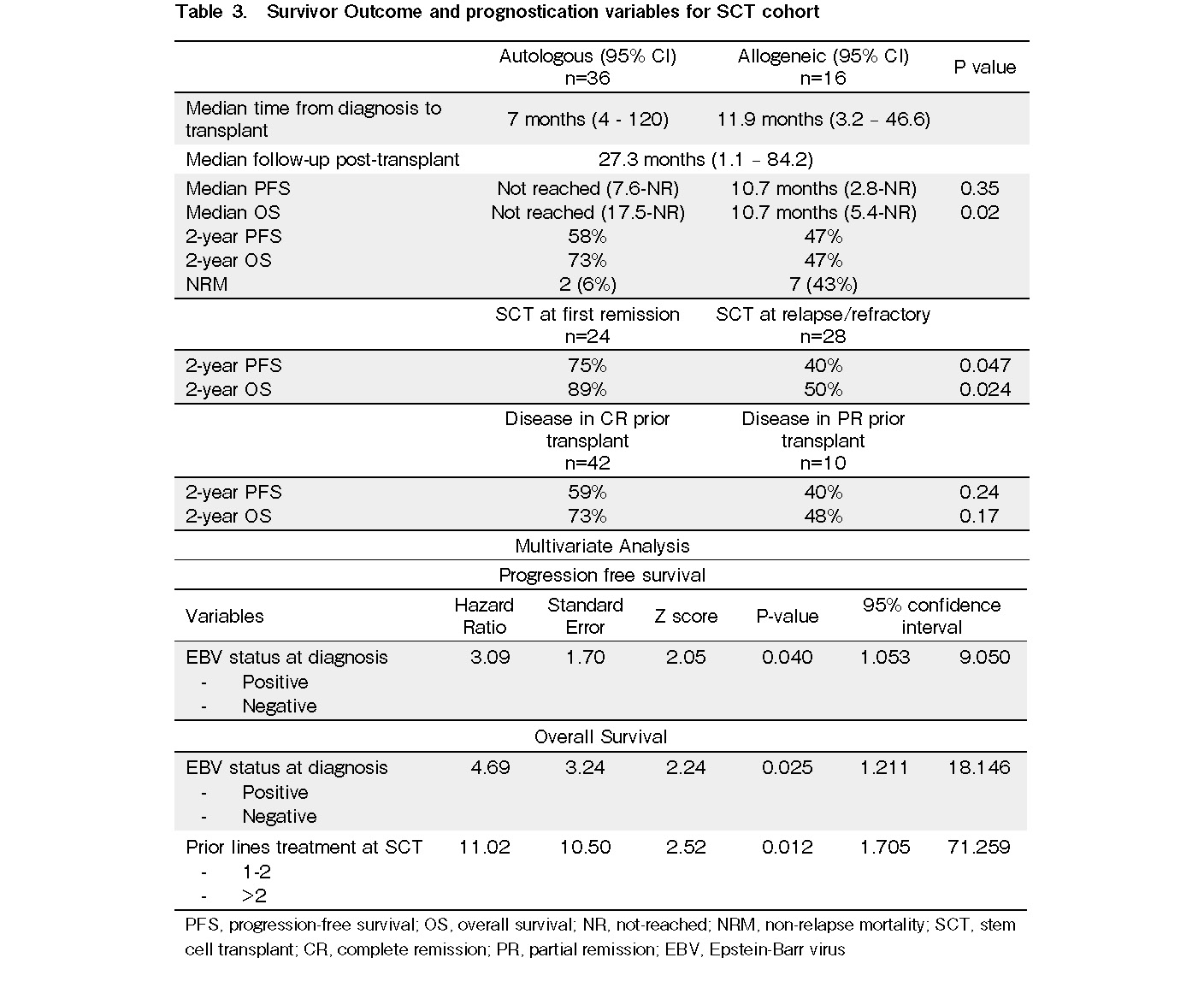
The median time from diagnosis to transplant was seven months (range 4-120 months) for the ASCT cohort and 11.9 months (range 3.2-46.6 months) for the alloSCT cohort. The median follow-up duration post-transplant for all SCT patients was 27.3 months. The median PFS and median OS for the autologous SCT cohort were not reached. The median PFS and OS for the allogeneic SCT cohort were the same at 10.7 months. The two-year PFS and OS for the autologous SCT cohort were 58% and 73%, respectively. The two-year PFS and OS for the allogeneic SCT cohort were 47% (Figure 1). Twenty-four patients who received SCT at first remission (21 autologous and three allogeneic) had excellent two-year PFS and OS rates of 75% and 89%, respectively, compared to 28 patients who received SCT at relapse/refractory (15 autologous and 13 allogeneic) that had two-year PFS and OS rates of 40% and 50%, respectively. The differences between these PFS and OS rates were statistically significant (P=0.047, P=0.024, respectively; Figure 2). Patients in complete remission prior to transplantation (n=42) had a two-year PFS and OS of 59% and 73%, respectively. In contrast, patients in partial remission prior to transplantation (n=10) had a two-year PFS and OS of 40% and 48%, respectively. However, these differences were not statistically significant (P=0.24, P=0.17, respectively; Figure 3).
Non-relapse mortality (NRM) due to myocardial infarction and bacterial pneumonia occurred in two patients (6%) in the autologous cohort. Seven patients (43%) in the allogeneic cohort suffered from NRM. Among those, one patient with a full HLA-matched donor died of severe graft versus host disease (GVHD) refractory to multiple lines of immunosuppressant/immunomodulator. Two, two, one, and one patient died of fungal pneumonia, bacterial pneumonia, pneumonia with underlying chronic lung GVHD, and pneumonia with concomitant diffuse alveolar hemorrhage and thrombotic microangiopathy, respectively.
Multivariate analysis was performed on the LDH, stage of disease, IPI, EBV status at diagnosis, number of prior line treatments, and use of novel agents. EBV-positivity at diagnosis had a poor prognosis in terms of PFS with a hazard ratio of 3.09 (P=0.040). For OS, EBV-positivity and more than two prior lines of treatment at transplant were associated with a poor prognosis with hazard ratios of 4.69 (P=0.025) and 11.02 (P=0.012), respectively. The above findings are summarized in Table 3.
Survival outcome among PTCL compared to upfront ASCT vs. non-upfront ASCT
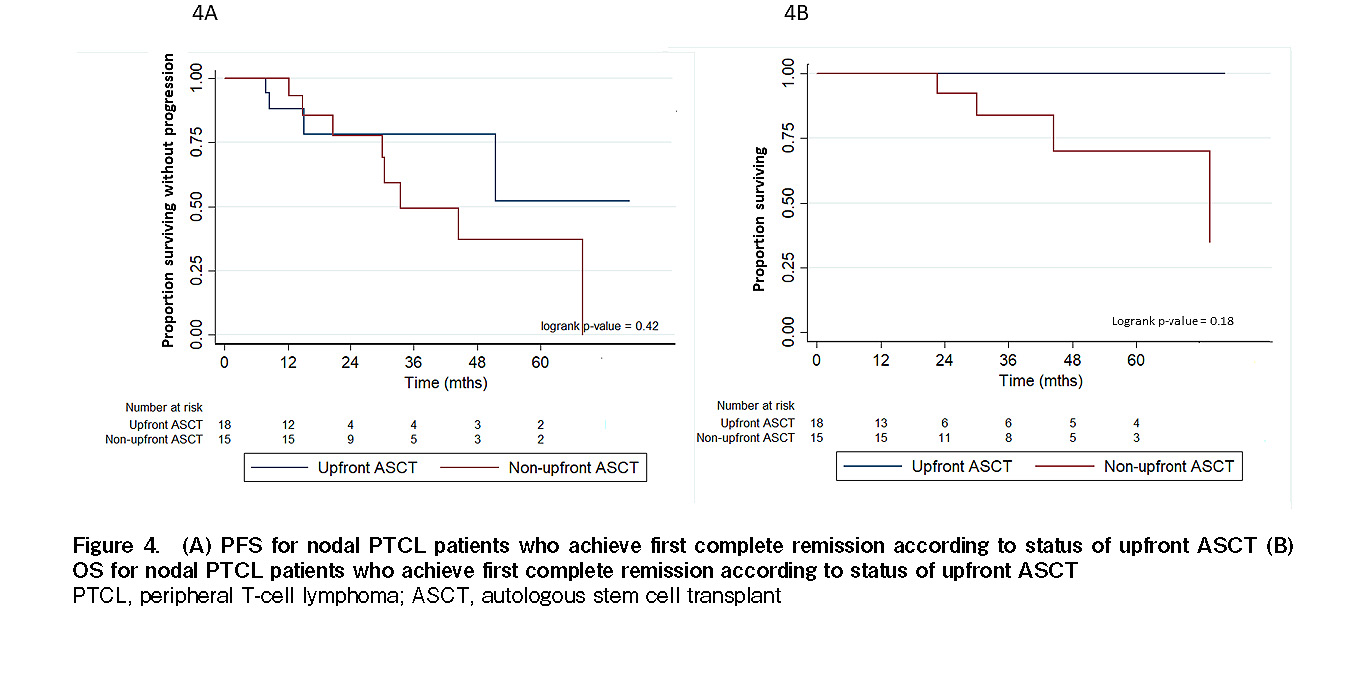
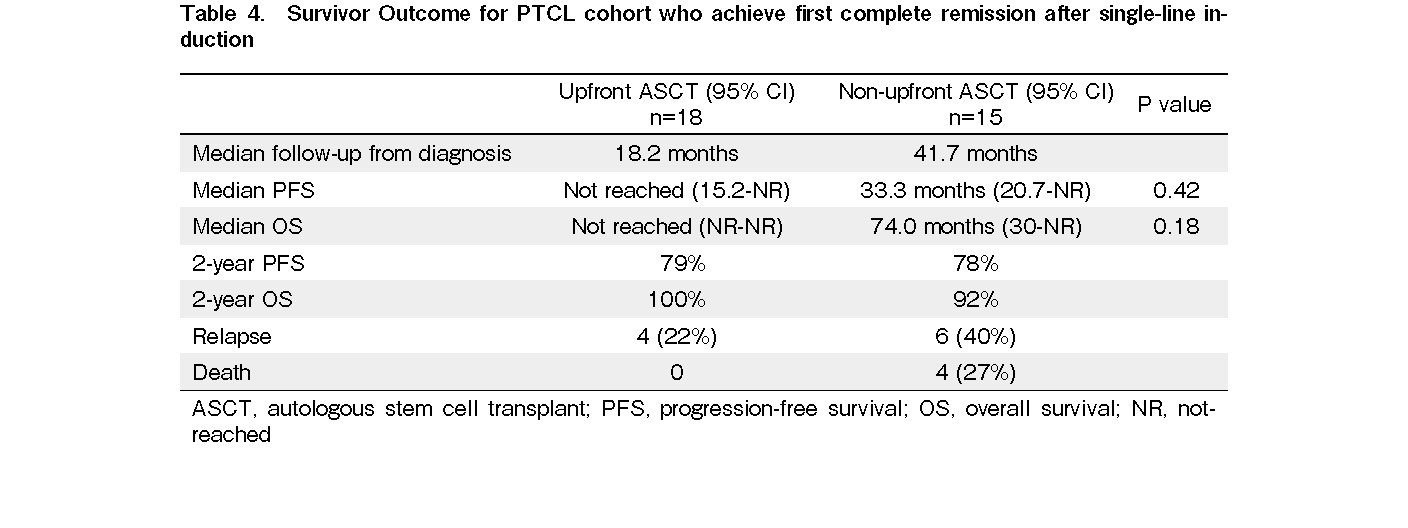
The median follow-up from diagnosis was 18.2 months for the upfront ASCT cohort and 41.7 months for the non-upfront ASCT cohort. Both the median PFS and median OS were not reached in the upfront ASCT cohort. The median PFS and OS were 33.4 months and 73 months in the non-upfront ASCT cohort. The two-year PFS and OS rates were 79% and 100% for the upfront ASCT cohort and 78% and 92% for the non-upfront ASCT cohort, respectively. There was no statistically significant difference in PFS and OS (P=0.42, P=0.18, respectively; Figure 4). Four patients from the upfront ASCT cohort relapsed. Of these, two received subsequent allogeneic HSCT with their survival censored at the time of allogeneic transplant. Six patients from the non-upfront ASCT cohort relapsed. Three of these patients received subsequent ASCT after attaining CR2 (two patients) and CR3 (one patient). No deaths occurred in the ASCT cohort. Four deaths occurred in the non-upfront ASCT cohort, two from lymphoma, one from pneumonia, and one from end-stage renal disease. The above findings are summarized in Table 4.
Discussion
Our two-year PFS and OS rates of 58% and 73% for autologous SCT and 47% for both PFS and OS for allogeneic SCT were comparable, if not better, to several retrospective studies. Smith et al. reported a three-year PFS rate of 47% and OS rate of 59% for autologous SCT, plus a three-year PFS rate of 37% and OS rate of 46% for allogeneic SCT3. Feyler from BSBMT/ABMTRR group reported a three-year PFS rate of 50% and OS rate of 53% for autologous SCT, plus a three-year PFS of 33% and OS rate of 39% for allogeneic SCT7. Moreover, our study included non-nodal T and NK/T-cell lymphoma, which is generally associated with poorer outcomes.
We observed that patients who received upfront SCT at first remission had excellent outcomes, with a two-year PFS of 75% and OS of 89%. In contrast, those that received SCT at relapse/refractory had a two-year PFS of 40% and OS of 50%. Beitinjaneh et al. supported this finding, with four-year OS rates of 76% and 54% for upfront autologous and allogeneic SCT, respectively, compared to four-year OS rates of 50% and 36% for autologous and allogeneic SCT, respectively, at relapse8. Rodriguez from the GELTAMO group demonstrated a five-year OS of 80% for autologous SCT at first remission compared to a five-year OS of 50% for autologous SCT at the second or more remission9. The Nordic Lymphoma Group reported a five-year PFS of 44% and OS of 51% in patients that initially underwent autologous SCT10. Wilhelm et al. examined the role of upfront autologous SCT at first remission for PTCL in five prospective studies based in Germany and found a five-year PFS rate of 39% and OS of 44%11. This is related to the nature of the disease. Patients who experienced relapse-refractory were likely to have more aggressive disease and hence had worse outcomes despite transplantation. This indicates that better salvage or consolidative treatment is required in this group of patients.
All our patients were in remission (complete or partial) prior to transplantation. This is due to patient selection and physician recognition that disease status at transplantation greatly determines the outcome. Hwang et al. demonstrated that patients with PTCL and NK/T-cell lymphoma who underwent transplantation in remission had superior five-year OS rates of 76% and 53%, respectively, compared with those not in remission, with five-year OS rates of 25% and 20%, respectively12. We did not observe any significant difference in survival between complete remission and partial remission, although a trend of better survival was observed in the complete remission cohort. With the use of novel agents, it is expected that more patients will achieve a certain degree of remission, which would enable them to receive SCT when possible. The impact of a novel agent upfront, such as the use of brentuximab vedotin in the first-line treatment of CD30-positive PTCL as described by Howitz et al. on SCT consolidation at first remission, remains unclear13. A proportion of our patients with relapse-refractory disease received novel agents followed by allogeneic SCT, indicating that chemo-responsiveness does affect SCT choice.
Based on multivariate analysis, EBV-positivity at diagnosis appeared to be a poor prognostic marker in PFS and OS with hazard ratios of 3.09 and 4.69, respectively. This suggests that EBV-associated T-cell lymphoma is more aggressive and that transplantation cannot entirely negate the negative impact of this disease biology. Dupuis et al. had previously reported adverse prognostic outcomes in EBV-positive lymphoma, especially in elderly patients14. Haverkos et al. reported a two-year OS rate of 26% for EBV-positive patients compared to 55% for EBV-negative PTCL patients15. We believe that our study results were affected by patients with EBV-positive extranodal NK/T-cell lymphoma in the allogeneic cohort who did not have favorable outcomes. A recent phase 2 study by Porcu et al. looking at the novel agent Nanotinostat combined with Valganciclovir showed promising efficacy in relapse-refractory EBV-positive T and NK/T-cell lymphoma cohorts with a documented overall response rate of 80% and a complete remission rate of 40%16. The role of EBV-targeted novel therapy incorporated into peri-transplantation is worth exploring in the future. In addition to EBV status, patients with more than two prior lines of treatment also had poorer OS, with a hazard ratio of 11.02. Better salvage and consolidation in patients with multiple relapse-refractory T and NK/T-cell lymphoma is required.
The above finding of excellent survival for upfront SCT at first remission prompted us to look further into the benefit of upfront ASCT in nodal PTCL. The median follow-up duration between our upfront ASCT and non-upfront ASCT cohorts was quite different (18.2 months vs. 41.7 months), making it challenging to compare survival. There was no statistically significant difference in survival outcome between the two cohorts. Patients in the non-upfront ASCT cohort received other consolidation and maintenance therapy methods, which might have benefited them without the need for SCT. Some patients appeared to benefit from the defer-ASCT approach. This was observed when three patients in the non-upfront ASCT cohort received ASCT at relapse. Two of the three patients remained alive and progression-free at the data cut-off. Fossard from the LYSA group conducted an analysis using propensity score matching to compare a group that underwent ASCT and a group that did not. They found that both showed similar survival outcomes, with five-year PFS and OS rates of 41% and 60% vs. 46% and 59%, respectively17. Park et al. reported that the COMPLETE study demonstrated that upfront autologous SCT is associated with superior survival in patients with advanced-stage disease and higher IPI and AITL subtypes18.
There are several limitations to our study. First, because this is a retrospective analysis, patient selection bias and physician preference bias may have occurred. The cohorts of HSCT patients were those that fit and were chemosensitive. It does not reflect the actual demographics of aggressive T and NK/T-cell lymphoma populations in which a proportion of patients would be elderly, unfit, or have refractory disease. For the historical nodal PTCL cohort patients, no data could be found on why patients did not receive upfront ASCT at first remission. Although we selected patients less than 65 years old, those who might be comorbidly unfit for transplantation may have been included. Tan et al. suggested that AITL histology pattern-1 is associated with better prognoses, which might affect decisions regarding upfront ASCT19. Second, this transplantation data covered ten years from 2010 to 2020, but two-thirds of the autologous HSCTs were performed after 2016. Therefore, the follow-up duration for the HSCT cohort was relatively short. This reflected the change in practice by our institution in which upfront HSCT consolidation in PTCL is being performed more often since the last American Society for Blood and Marrow Transplantation and European Society for Blood and Marrow Transplantation recommendations20, 21. This explains the discrepancy in follow-up duration between the upfront ASCT and non-upfront ASCT groups. Lastly, our sample size was small, and collaboration with other institutions is required to improve our analysis power.
Conclusion
Our study shows that HSCT is a feasible treatment option for aggressive T and NK/T-cell lymphoma in both upfront and relapse-refractory settings with acceptable survival outcomes and toxicity profiles. EBV-positivity at diagnosis and more than two prior lines of treatment at transplantation are associated with a poorer prognosis. The role of upfront ASCT in first complete remission for nodal PTCL is unclear and is limited by the small sample size. Nevertheless, we believe there is a role for upfront ASCT in young-fit PTCL patients with higher disease burden stratified by staging and IPI and possibly EBV-positivity. Further studies with a larger number of patients are required.
Author Contributions
LCKN and CSWT collected data, YC, SYO, CSWT and LCKN analysed data, LCKN wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.Vose J, Armitage J, Weisenburger D; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008; 26: 4124-30.
2.Park S, Ko YH. Peripheral T cell lymphoma in Asia. Int J Hematol. 2014; 99: 227-39. doi: 10.1007/s12185-014-1520-3. Epub 2014 Jan 31. PMID: 24481942.
3.Smith SM, Burns LJ, van Besien K, Lerademacher J, He W, Fenske TS, et al. Hematopoietic cell transplantation for systemic mature T-cell non-Hodgkin lymphoma. J Clin Oncol. 2013; 31: 3100-9.
4.Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: a study from the Swedish Lymphoma Registry. Blood. 2014; 124: 1570-7.
5.Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. The 2008 WHO classification of lymphoid neoplasms and beyond: evolving concepts and practical applications. Blood. 2011; 117: 5019-32.
6.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of hodgkin and non-hodgkin lymphoma: The lugano classification. J Clin Oncol. 2014; 32: 3059-67.
7.Feyler S, Prince HM, Pearce R, Towlson K, Nivison-Smith I, Schey S, et al. The role of high-dose therapy and stem cell rescue in the management of T-cell malignant lymphomas: a BSBMT and ABMTRR study. Bone Marrow Transplant. 2007; 40: 443-50.
8.Beitinjaneh A, Saliba RM, Medeiros LJ, Turturro F, Rondon G, Korbling M, et al. Comparison of survival in patients with T cell lymphoma after autologous and allogeneic stem cell transplantation as a frontline strategy or in relapsed disease. Biol Blood Marrow Transplant. 2015; 21: 855-9.
9.Rodríguez J, Caballero MD, Gutiérrez A, Marín J, Lahuerta JJ, Sureda A, et al. High-dose chemotherapy and autologous stem cell transplantation in peripheral T-cell lymphoma: the GEL-TAMO experience. Ann Oncol. 2003; 14: 1768-75.
10.d'Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012; 30: 3093-9.
11.Wilhelm M, Smetak M, Reimer P, Geissinger E, Ruediger T, Metzner B, et al. First-line therapy of peripheral T-cell lymphoma: extension and long-term follow-up of a study investigating the role of autologous stem cell transplantation. Blood Cancer J. 2016; 6: e452.
12.Hwang WYK, Koh LP, Lim ST, Linn YC, Loh YS, Koh MBC, et al. Multicenter study of comparative outcomes of hematopoietic stem cell transplant for peripheral T cell lymphoma and natural killer/T-cell lymphoma. Leuk. Lymphoma. 2011; 52: 1382-6.
13.Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet. 2019; 393: 229-40.
14.Dupuis J, Emile JF, Mounier N, Gisselbrecht C, Martin-Garcia N, Petrella T, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d'Etude des Lymphomes de l'Adulte (GELA) study. Blood. 2006; 108: 4163-9.
15.Haverkos BM, Huang Y, Gru A, Pancholi P, Freud AG, Mishra A, et al. Frequency and clinical correlates of elevated plasma Epstein-Barr virus DNA at diagnosis in peripheral T-cell lymphomas. Int J Cancer. 2017; 140: 1899–906.
16.Porcu P, Haverkos BM, Alpdogan O, Baiocchi R, Brammer JE, Feldman TA, et al. Oral nanatinostat (Nstat) and valganciclovir (VGCV) in patients with recurrent Epstein-Barr virus (EBV)-positive lymphomas: initial Phase 2 results. 62nd American Society of Hematology (ASH) Annual Meeting and Exposition. December 5-8, 2020.
17.Fossard G, Broussais F, Coelho I, Bailly S, Nicolas-Virelizier E, Toussaint E, et al. Role of up-front autologous stem-cell transplantation in peripheral T-cell lymphoma for patients in response after induction: an analysis of patients from LYSA centers. Ann Oncol. 2018; 29: 715-23.
18.Park SI, Horwitz SM, Foss FM, Pinter-Brown LC, Carson KR, Rosen ST, et al. The role of autologous stem cell transplantation in patients with nodal peripheral T-cell lymphomas in first complete remission: Report from COMPLETE, a prospective, multicenter cohort study. Cancer. 2019; 125: 1507-17.
19.Tan LH, Tan SY, Tang T, Lim ST, Tan D, Lim LC, et al. Angioimmunoblastic T-cell lymphoma with hyperplastic germinal centres (pattern 1) shows superior survival to patterns 2 and 3: a meta-analysis of 56 cases. Histopathology. 2012; 60: 570-85. doi: 10.1111/j.1365-2559.2011.04097.x. Epub 2012 Jan 17. PMID: 22251198.
20.Kharfan-Dabaja MA, Kumar A, Ayala E, Hamadani M, Reimer P, Gisselbrecht C, et al. Clinical Practice Recommendations on Indication and Timing of Hematopoietic Cell Transplantation in Mature T Cell and NK/T Cell Lymphomas: An International Collaborative Effort on Behalf of the Guidelines Committee of the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2017; 23: 1826-38.
21.Duarte RF, Labopin M, Bader P, Basak GW, Bonini C, Chabannon C, et al. European Society for Blood and Marrow Transplantation (EBMT). Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019; 54: 1525-52.
Search
News