Volume 4 (2021) Issue 4 No.2 Pages 84-87
Abstract
The prevailing corona virus disease 19 (COVID-19) pandemic has adversely affected the healthcare services globally. Hematopoietic cell transplantation (HCT) is considered as the preferred treatment option for several hematological malignancies, and HPC collection facilities have to function continuously along with implementing safety measures. Based on the national and international guidelines, we implemented additional measures and modifications to our standard operating procedure (SOP) to ensure secure HPC collection from patients as well as donors. Here, we report our experience with HPC collection and processing from 1st January, 2020 until 31st December, 2020. We collected 59 HPC products through apheresis and 41 cryopreservation procedures. Compared to 2019, there was a 33% decrease in the number of HPC transplants and 31% reduction in HPC collection procedures. However, we report an 86% (13 procedures) increase in the cryopreservation of HPC products from related donors, as several organizations recommend cryopreservation of HPC products. We report our institutional experience to better understand the impact of COVID-19 on HCT services in a tertiary care center in the developing world. It may also help in being prepared for any future waves of COVID-19 cases.
Introduction
On 11th March, 2020, the WHO declared the corona virus disease 19 (COVID-19) as a pandemic. COVID-19, which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has been reported in over 158 million people and is responsible for more than 3 million deaths globally as on 12th May, 20211. India reported the second highest number of COVID-19 cases, accounting for 22 million cases as of May 2021. The novelty of this disease, lack of understanding of its natural course, and absence of specific antiviral therapy have compounded the challenges in healthcare systems worldwide. Cancer patients are at a higher risk of COVID-19 infection and associated complications due to immunosuppression2. In several hematological malignancies, hematopoietic cell transplantation, including allogeneic and autologous, are the preferred treatment options2. Therefore, despite the rise in COVID-19 cases, hematopoietic progenitor cell (HPC) collection facilities had to function continuously along with implementing safety measures based on the national and international guidelines to ensure secure collection of HPC products from patients as well as donors.
Results and Discussion
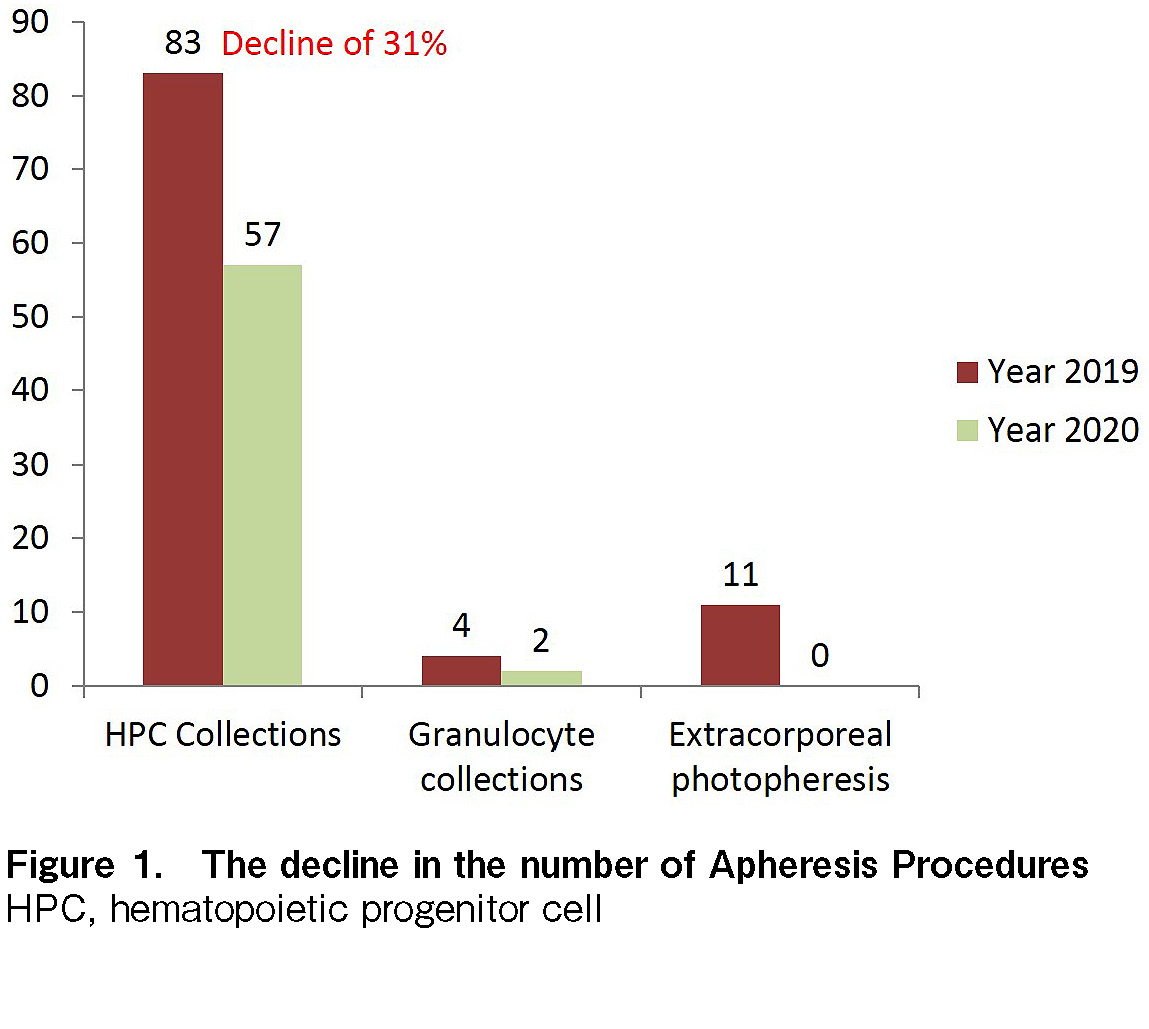
Here, we report our experience with HPC collection and processing from 1st January, 2020 until 31st December, 2020 during this pandemic. We had 59 HPC collections through apheresis and 41 cryopreservation procedures. Compared to 2019, there was a 33% decrease in the number of HPC transplants (75 in 2019 vs. 50 in 2020) and a 31% reduction in HPC collection procedures (30) in 2020, which is attributed to the ongoing pandemic, as shown in Figure 1.
It is noteworthy that DKMS reported a decrease in HPC collections by 15.9% and the decline was significantly high in bone marrow collections, which was estimated to be more than 50%3; it is believed that bone marrow collections have been reserved in cases where there has been a significant benefit over peripheral stem cell collection (PBSC), to reduce logistical constraints (such as operating room availability, manpower hours, cell processing, etc.) on an already over-stretched healthcare facility. The number of PBSC collections at our center was particularly low from April to August 2020, which is most likely due to the different phases of nationwide lockdown and the threat of COVID-19 among patients and donors. However, the increase in this number in September (11 HPC collections) reflected the confidence among patients and health professionals in COVID-19 containment measures at our institution. Based on the national and international guidelines4–5, our Apheresis and Cell Processing unit implemented several interventions. However, with an increase in experience and available literature, there have been revised recommendations and position statements from the national and international transplant fraternity on the measures to deal with COVID-19 pandemic4. The measures and modifications to the standard operating procedure (SOP) are highlighted below. To ensure recipient's safety, all prospective HPC donors were screened for any respiratory symptoms (fever, cough, and cold) and questioned pertaining to the history of exposure to a known COVID-19 case in the donor questionnaire.
Only the donors with a negative reverse transcription polymerase chain reaction (RT-PCR) report for COVID-19 on the day prior to growth factor (G-CSF) initiation were mobilized, and a repeat test was performed on day 3 of mobilization. Even though there are no reported cases of COVID-19 post-blood transfusion, aggressive COVID-19 testing in HPC donors is recommended due to the theoretical risk of transmission6. This is also because SARS-CoV-2 has been detected at higher concentrations in lymphocytes, and possibly self-limited replication was observed in samples containing high numbers of stem cells and lymphocytes7. All donors were requested to inform the collection center if they developed any COVID-19-like symptoms for up to 14 days after donation. To protect the healthcare providers and donors, we adopted a strict policy on appropriate personal protective equipment (PPE) usage during PBSC collection and cryopreservation. It comprised an N-95 mask, gloves, and diligently practiced hand hygiene. The apheresis staff members were educated on the appropriate PPE usage and personal hygiene. Hospital guidelines ensured that the staff members reported any symptoms of infection and subsequently underwent quarantine. Correct selection and application of an FFP2/3 mask without an exhalation valve was considered very important.
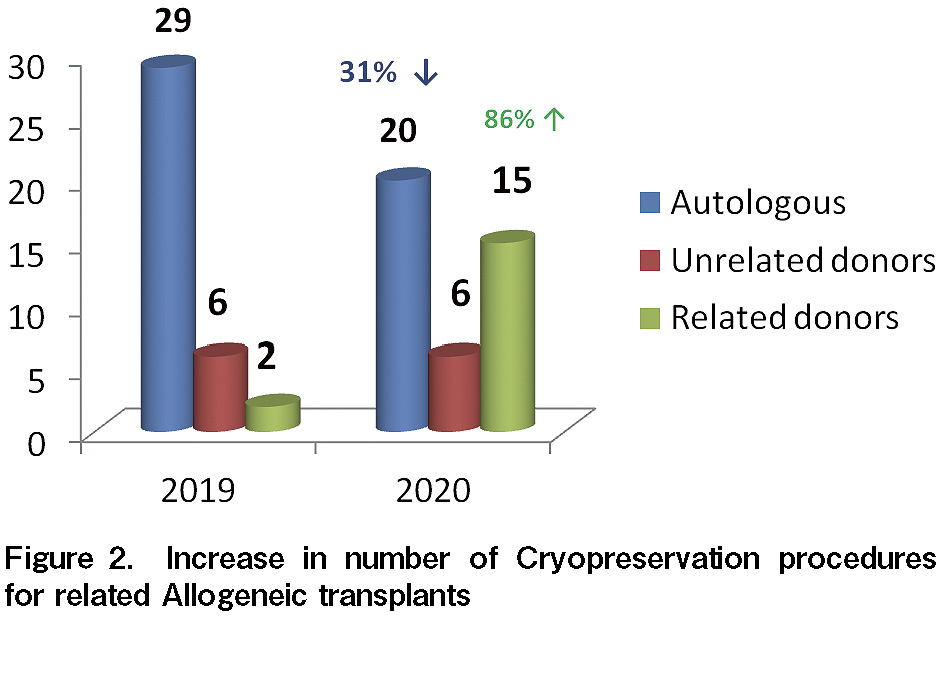
The uncertainty of transport of unrelated HPC products in the initial few months of lockdown led to a delay in unrelated transplants at our center. All transplants from March to October 2020 were either related allogeneic or autologous. According to the standard guidelines, transplants for benign hematological conditions (such as thalassemia) were postponed due to the infection and logistical constrains8 from March to September 2020. Travel restrictions and uncertainty of contracting COVID-19 infection during the period of G-CSF mobilization made it even more challenging to consider using freshly collected HPC products for HCT; in such situations, the physicians preferred to request cryopreservation of all HPC collections before administering preparative chemotherapy to the recipients. ASTCT, NMDP, and ISBMT recommend the cryopreservation of HPC products4–5. As a result, even at our center, the number of cryopreservation procedures exceeded the total number of procedures conducted in 2019 (see Figure 2), and therefore, increased the cost of related allogeneic transplants by almost 1,100 USD. We observed an increase by 86% (13 procedures) in the cryopreservation of HPC products from related donors. In 2019, most of the transplants from related donors were with fresh HPC collections. The practice of cryopreservation was further strengthened during the pandemic, with evidence from a CIBMTR analysis demonstrating that cryopreservation of HPC products in allogeneic HCT with post-transplant cyclophosphamide was not associated with increased graft-vs-host disease risk, delay in hematopoietic recovery, transplant-related mortality, and relapse. However, fresh HPC products still remain the standard of care for bone marrow failures (such as aplastic anemia)6. Several bodies (such as the FDA) do not recommend cryo-quarantine, especially because there is insufficient scientific data to support the transmission of coronavirus via blood, and thus, blood samples or donors are not tested for COVID-19. At our center, 39 of 41 (~95%) cryopreserved products were infused as per the schedule. HPC infusions were rescheduled in two patients- one patient with a non-malignant blood disorder, and the other patient developed SARS-CoV-2 infection. The DKMS registry fears that 5-10% of all cryopreserved products may not have been transfused during the ongoing pandemic3. PBSC collections in our center were delayed (5% of total collections) in two patients undergoing autologous transplantation and one allogeneic donor because of COVID-19 infection. PBSC collections were performed after 14 days, only when the patients/donors tested negative for COVID-19 on two separate occasions. The adaptation of the guidelines to our protocols have improved our preparedness and systematic management of any future waves of COVID-19 cases. SARS-CoV-2 testing and use of appropriate PPE, such as N95 mask and head shield, contributed to a marginal increase in institutional cost in adherence to COVID-19 protocols. None of the apheresis staff or patients in the transplantation ward acquired SARS-CoV-2 infection. The median neutrophil engraftment period was 10 days (range, 9-12 days), and the median platelet engraftment period was 11 days (range, 7-33 days) among our patients who underwent transplantation.
Conclusion
The prevailing pandemic has profoundly affected HCT services globally. We consider sharing real-world data based on our institutional experience to better understand the impact of COVID-19 on HCT services in a tertiary care center in the developing world. Testing for COVID-19, PPE use, and cryopreservation of all PBSC collections had financial implications. However, it is imperative that HPC collection centers should develop practical and locally adaptable strategies in these constantly evolving times to minimize the uncertainties that may adversely affect HCT services.
Author Contributions
RJ conducted the procedures, the literature search on the topic, and drafted the initial version of the manuscript. MR provided nursing care and critical revision of the manuscript for intellectual content. JK and SB supervised the procedures and provided critical revision of the manuscript for intellectual content. SB supervised the Covid-19 testing and Infectious disease control measures. AN, VR, RKS, NG, DKM, RN, and MC reviewed the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. Disclosure forms provided by the authors are available on the website.
References
1.WHO Coronavirus (COVID-19) Dashboard available at
2.Samaha R, Kattan J. Hematopoietic stem cell transplantation dilemma during the COVID-19 era. Future Oncol. 2020; 16: 1569-73.
3.Mengling T, Rall G, Bernas SN, Astreou N, Bochert S, Boelk T, et al. Stem cell donor registry activities during the COVID-19 pandemic: a field report by DKMS. Bone Marrow Transplant. 2021; 56: 798-806.
4.Ljungman P, Mikulska M, de la Camara R, Basak GW, Chabannon C, Corbacioglu S, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020; 55: 2071-6.
5.Waghmare A, Abidi MZ, Boeckh M, Chemaly RF, Dadwal S, El Boghdadly Z, et al. Guidelines for COVID-19 Management in Hematopoietic Cell Transplantation and Cellular Therapy Recipients. Biol Blood Marrow Transplant. 2020; 26: 1983-94.
6.Algwaiz G, Aljurf M, Koh M, Horowitz MM, Ljungman P, Weisdorf D, et al; WBMT and the CIBMTR Health Services and International Studies Committee. Real-World Issues and Potential Solutions in Hematopoietic Cell Transplantation during the COVID-19 Pandemic: Perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant. 2020; 26: 2181-9.
7.Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerg Infect Dis. 2020; 26: 1631-3.
8.Dutheil F, Baker JS, Navel V. The SARS-Cov-2 Pandemic: A Good Time for Stem Cell Transplantation? Biol Blood Marrow Transplant. 2020; 26: e239-40.
Search
News