Volume 4 (2021) Issue 1 No.2 Pages 9-14
Abstract
Epstein-Barr virus (EBV) is a common virus that latently infects most adults and has a tropism to B lymphocytes. In 1988, two cases of EBV infection were reported to be eradicated by hematopoietic stem cell transplantation from an EBV-negative donor. However, the dynamics of EBV after cord blood transplantation (CBT), namely, the kinetics of anti-EBV antibodies, the incidence of negative/adverse seroconversion (from positive to negative), and the clinical course of re-infection (second primary infection) by EBV, have not yet been characterized in detail. Therefore, we performed a nationwide survey that focused on the dynamics of EBV after CBT 1 year or later after CBT. Negative seroconversion occurred in 23% of previously EBV-infected patients. The incidence of late-onset EBV-associated events was 1.9% (13/674) : 5 infectious mononucleosis, 2 hemophagocytic lymphohistiocytosis (HLH), and 6 remaining typical lymphoproliferative disease. HLH occurred in newly infected patients (primary or second primary) and also in those with reactivation and was fatal. The annual monitoring of anti-EBV antibody titers may facilitate the early detection of these late-onset EBV-associated events and treatment initiation before disease progression.
Introduction
The first successful bone marrow transplantation (BMT) from a sibling to treat a patient with acute leukemia was performed in 19691. The application of allogeneic hematopoietic stem cell transplantation (HSCT) subsequently expanded worldwide. In 1988, the eradication of EBV by BMT from an EBV-negative donor was demonstrated in two recipients previously infected with EBV2. These findings provided proof of the concept of EBV infecting humans and persisting for life as a latent infection concealed in a certain subset of lymphocytes (B cells) after the primary infection (lytic infection).
The Japanese Cord Blood Bank Network was established in 1999. Cord blood transplantation (CBT) has since become popular in Japan. CBT is unique in that an EBV-positive patien (t more than 95% of adults are positive for EBV) receives HSCT from an EBV-negative donor. However, the dynamics of EBV after CBT, namely, the kinetics of the anti-EBV antibody, the incidence of negative/ adverse seroconversion (from positive to negative), and the clinical course of re-infection (second primary infection) by EBV, have not yet been elucidated in detail, there have been except for a few case reports3-5.
Therefore, we performed a nationwide questionnaire survey with a focus on changes in the EBV statu (s anti-EBV antibody titers before and after CBT) and the clinical manifestations of late-onset EBV-associated events. The present study was approved by the Research Ethics Committee of Osaka Women?s and Children?s Hospital (#610).
Patients and Methods
In 2014, letters requesting participation in our survey were sent to 262 institutes in Japan, followed by questionnaires to the 146 institutes that responded.
Data collection and eligibility criteria
Eligibility criteria were as follows: recipients of CBT before December 31st, 2012, CBT as the first allogeneic HCT, complete donor chimerism (?95%), and event-free survivors for more than 1 year after CBT. Events included relapse/progression of the primary disease, second malignancy, any death, and re-transplantation. Patients with congenital immunodeficiency affecting T cells, NK cells, and/or B cells were excluded.
EBV-specific questionnaire items included the following: previous EBV infection before treatment, anti-EBV antibody titers 1 year or later after CBT, late-onset EBVassociated events occurring 1 year or later after CBT, and EBV-related death. As a minimum requirement of anti-EBV antibody titers, information was obtained on the serum level of immunoglobulin G against the viral capsid antigen (VCA IgG) 6,7. A fluorescent antibody test (FA) was commonly used to measure anti-EBV antibody titers, and negative serology was defined as lower than 10.
Definition and statistical analysis
Previous EBV infection before CBT was defined serologically by the detection of the anti-EBV antibody prior to the first blood transfusion. EBV-associated events were as follows: infectious mononucleosis (IM), hemophagocytic lymphohistiocytosis (HLH), and lymphoproliferative disease (LPD). In overlapping cases, patients were diagnosed according to the cardinal symptom. LPD indicates the uncontrolled neoplastic proliferation of lymphoid cells with end-organ manifestations8. However, LPD may also be used as a comprehensive term over a wide variety of EBV-associated diseases. Therefore, LPD without HLH is hereafter described as"typical LPD". The chi-squared test was used in statistical analyses.
Results
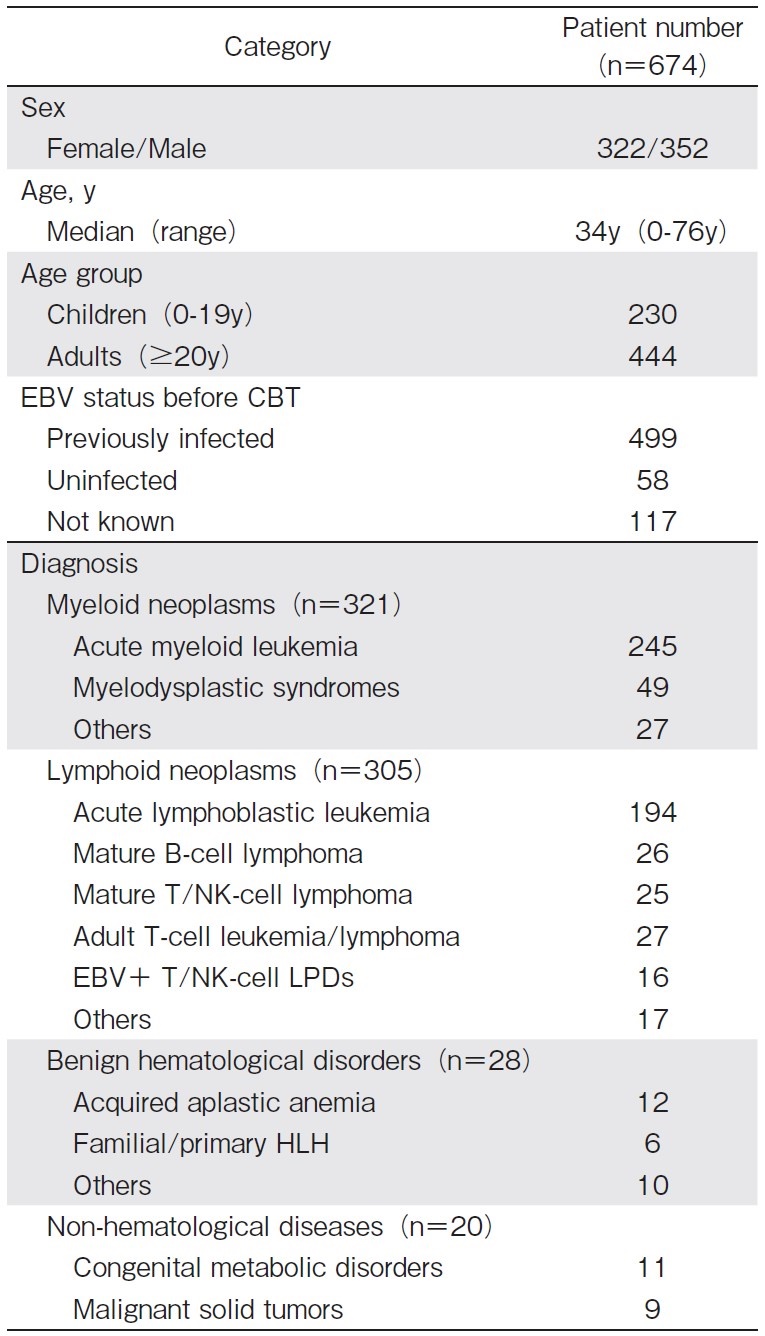
We received case reports from 83 institutes. Incomplete reports (n=4) were excluded, and the remaining 674 patients were analyzed. Patient characteristics are shown in Table 1. As the EBV status before CBT, the number of EBV-positive, EBV-negative, and EBV-indeterminate patients were 499 (74.0%), 58 (8.6%), and 117 (17.4%), respectively.
Kinetics of the anti-EBV antibody after CBT
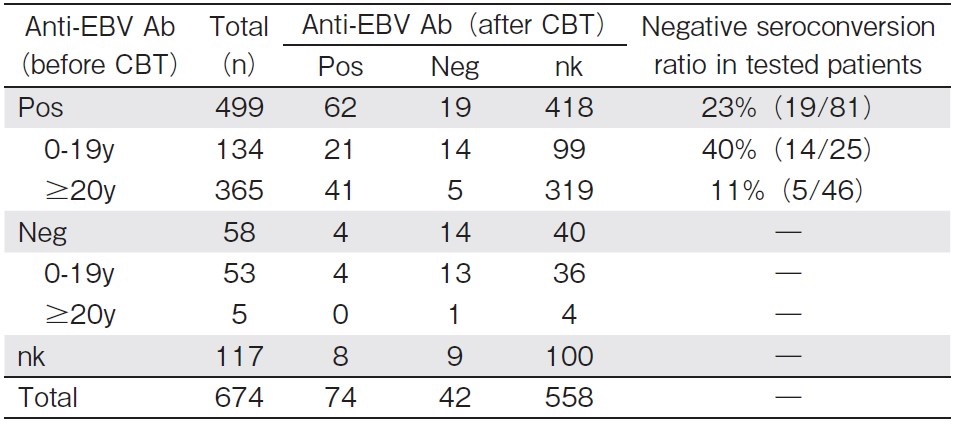
Among EBV-positive patients before CBT, the anti-EBV antibody was measured 1 year or later after CBT in 81 patients (Table 2), with negative seroconversion of the anti-EBV antibody titer being observed in 19 (23%). In the age-oriented analysis, the ratio of negative seroconversion was as high as 40% (14/35) in children (0-19 years old at CBT) and 11% (5/46) in adults (?20 years old at CBT) (P<0.01). However, anti-EBV antibody titers were not measured in 82.8% (558/674) of all patients and in 83.8% (418/499) of EBV-positive patients before CBT.
neg, negative; nk, not known; pos, positive; y, years old at CBT.
The anti-EBV antibody was monitored in 13 out of 19 patients with negative seroconversion, with 8 subsequently showing re-infection (positive seroconversion) by EBV: the clinical manifestation was IM in one and asymptomatic in seven.
Incidence of late-onset EBV-associated events
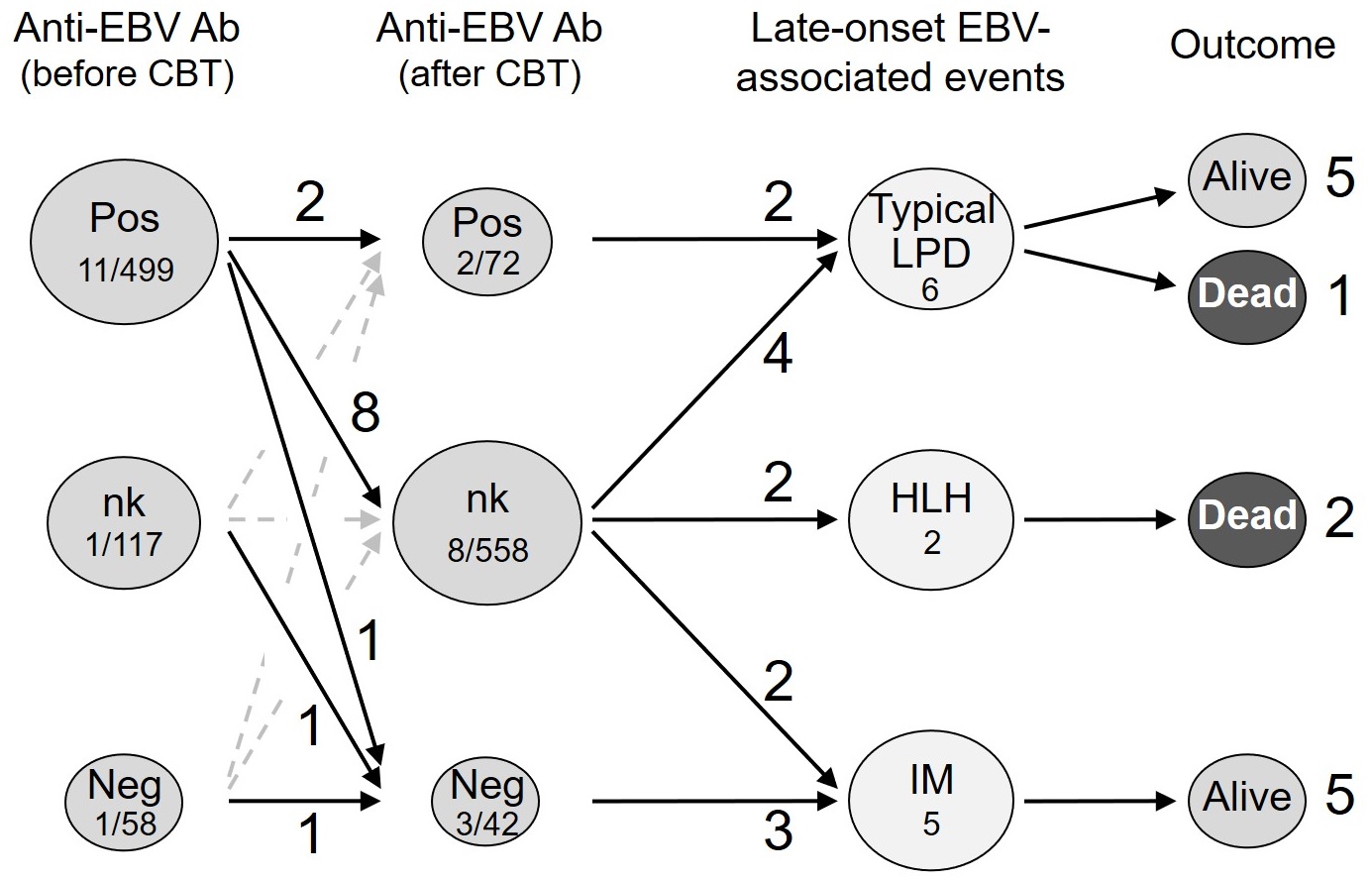
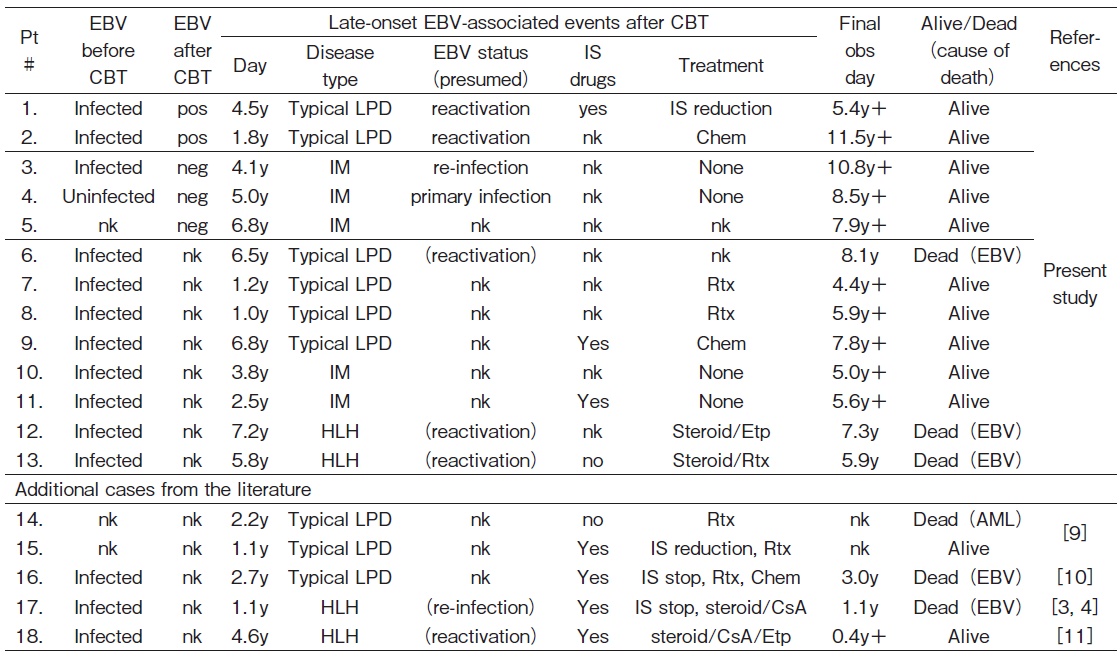
Late-onset EBV-associated events were documented in 13 out of 674 patients (1.9%) more than 1 year after CBT (Figure 1).
Ab, antibody; neg, negative; nk, not known; pos, positive.
(?) Patients persistently positive for EBV before and after CBT
Among 62 patients who were persistently positive for the anti-EBV antibody before and after CBT, 2 (3.3%) developed late-onset EBV-associated events, with both showing typical LPD as the reactivation of EBV.
(?) Patients negative for EBV after CBT
Three (7.1%) out of 42 patients who were negative for the anti-EBV antibody after CBT (regardless of the EBV status before CBT) developed late-onset EBV-associated events. The EBV status before CBT was positive in one patient, negative in one, and unknown in one. All three patients showed IM as the new infection (primary infection or re-infection/second primary infection) by EBV.
(?) Patients with an indeterminate EBV status after CBT
Eight (1.4%) out of 558 patients, whose anti-EBV antibody titers were not measured after CBT, developed late-onset EBV-associated events. All patients were positive for EBV before CBT. EBV-associated events were typical LPD in 4 patients, IM in 2, and HLH in 2. Among the 4 patients with typical LPD, the EBV status in one patient was presumed to be reactivation based on the anti-EBV antibody titer measured after its occurrence, and remained unknown in the remaining 3 patients.
Mortality of late-onset EBV-associated events
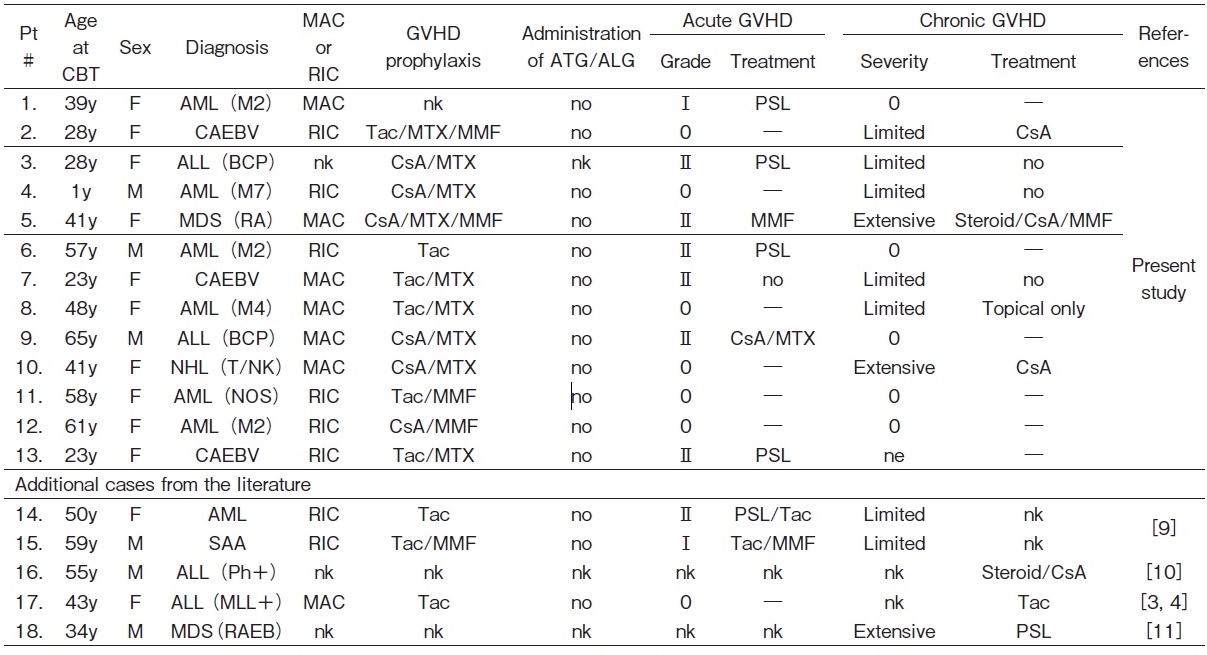
Late-onset EBV-associated events were fatal in 3 out of 13 patients (23%) (Table 3 and 4).
Chem, chemotherapy; Etp, etoposide; HLH, hemophagocytic lymphohistiocytosis; IM, infectious mononucleosis; IS, immunosuppressive/immunosuppressant; LPD, lymphoproliferative disease; neg, negative; nk, not known; pos, postive; Rtx, rituximab; y, year(s)/year(s) old.
(?) Patients persistently positive for EBV before and after CBT
Both patients with typical LPD were successfully treated and are alive.
(?) Patients negative for EBV after CBT
All three patients with IM recovered and are alive.
(?) Patients with an indeterminate EBV status after CBT
Among the 4 patients with typical LPD, one died and the remaining 3 were successfully treated. Two patients with IM recovered without any disease-specific treatment, and are currently disease-free. On the other hand, two patients with HLH died despite treatments.
Discussion
In the present study, HLH was presumedly due to EBV reactivation and was fatal in both patients. There were 2 additional cases of HLH among 5 Japanese patients with late-onset EBV-associated events in the literature, as shown in Table 3 and 43,4,9-11. One case of HLH was attributed to the re-infection by EBV after negative seroconversion, and the patient died3,4. The other case was presumedly due to the reactivation of EBV (with coinfection with varicella-zoster virus) and the patient is currently alive11. Therefore, HLH may occur in patients with new EBV infection and EBV reactivation, and its mortality was as high as 75% (3/4).
The negative seroconversion of the anti-EBV antibody might indicate the eradication of EBV after CBT. We used anti-EBV titer at least 1 year after CBT, based on our previous study5, to avoid a false positive by residual antibodies early after CBT and the adoptive antibodies from blood transfusions. Negative seroconversion, in the present study, was estimated to occur in 23% of previously EBV-infected patients, which was lower than that in the previous report5. In that report, the eradication rate was as high as 43%, however, the patient number was small, and the patient age was mostly under 10 years old. Indeed, in the present study, the rate of EBV eradication was as high as 40% in children (0-19 years old at CBT), comparing 11% in adults. One explanation of the different rate by age may be attributed to the difference between adults and children in personal activities such as deep kissing. Also, acute lymphoblastic leukemia is common in children, and the drugs for ALL have much suppressive effect on lymphocytes (including EBV-infected B cells). Another explanation may be the spread of EBV to organs other than B cells with aging, such as EBV infection to gastric epithelial cells, which accounts for 7.2% of gastric cancer12. In addition, the intensity of conditioning regimen can affect the rate of negative seroconversion, because the incidence of PTLD was reported to be higher for patients receiving reduced-intensity conditioning than that for patients receiving myeloablative conditioning13. Intensive conditioning might help eradicating EBV-infected B cells and EBV itself.
Among 42 patients negative for EBV after CBT regardless of the EBV status before CBT, the anti-EBV antibody titer was monitored in 28 patients. Sixteen of these patients were newly infected by EBV more than 1 year after CBT: 3 (11%) showed IM and 13 (89%) were asymptomatic, and generally recovered with no treatment or only supportive care. However, it is important to note that although it is rare, life-threatening (primary and second primary) EBV infection may occur3,4.
Late-onset EBV-associated events were observed in 5 out of 116 patients (4.3%) whose anti-EBV antibody titers were measured after CBT, and in 8 out of 558 patients (1.4%) whose titers were not. The incidence of these events was significantly lower in the latter group (P= 0.04). In contrast, none of the 5 patients whose titers were measured died of EBV-associated events, while 3 out of 8 patients whose titers were not evaluated died. Although the mortality rate was high in the latter group, it was not significant (P>0.1). Therefore, monitoring of the EBV status may have led to early detection and treatment initiation, whereas a delayed diagnosis resulted in severe illness.
In conclusion, among EBV-positive patients before CBT, negative seroconversion of the anti-EBV antibody was observed in 23% (19/81) more than 1 year after CBT. The incidence of late-onset EBV-associated events was 1.9% (13/674), and the mortality rate was 23% (3/13). Due to the life-threatening late events of EBV, it is important to monitor anti-EBV antibody titers annually after CBT. When EBV-associated events are suspected, the EBV-DNA load needs to be measured. The identity of the EBV-infected subset of lymphocytes and whether its origin is recipient- or donor-derived also need to be clarified. Early diagnosis and treatment initiation may prevent late-onset EBV-associated mortality.
Acknowledgements
The authors would like to thank Dr. Koyama-Sato M for helpful comments on second primary EBV infection. We thank Matsumoto K and Tomura M for their secretarial help. We would also like to give special thanks to all the doctors who participated in this survey for their co-operation.
Author Contributions
A. S. designed and performed the research, analyzed data, and wrote the manuscript. K. K. designed the research, analyzed data, and supervised the manuscript. S. T. and S. T. analyzed data and provided helpful comments. M. I . , Y. O., M. T. , H. H., M. K., and A. N. reported data and provided helpful comments.
Conflicts of Interest
The authors declare no conflict of interest associated with this article. Disclosure forms provided by the authors are available here.
References
1. Buckner CD, Epstein RB, Rudolph RH, Clift RA, Storb R, Thomas ED. Allogeneic marrow engraftment following whole body irradiation in a patient with leukemia. Blood. 1970; 35: 741-50.
2. Gratama JW, Oosterveer MA, Zwaan FE, Lepoutre J, Klein G, Ernberg I. Eradication of Epstein-Barr virus by allogeneic bone marrow transplantation: implications for sites of viral latency. Proc Natl Acad Sci USA. 1988; 85: 8693-6.
3. Kawabata Y, Hirokawa M, Saitoh Y, Kosugi S, Yoshioka T, Fujishima M, et al. Late-onset fatal Epstein-Barr virus-associated hemophagocytic syndrome following cord blood cell transplantation for adult acute lymphoblastic leukemia. Int J Hematol. 2006; 84: 445-8.
4. Kawa K, Sawada A, Koyama M, Inoue M. Epstein-Barr virus infection after unrelated cord blood transplantation: reactivation or reinfection? Int J Hematol. 2007; 85: 267-9.
5. Sawada A, Inoue M, Koyama-Sato M, Kondo O, Yamada K, Shimizu M, et al. Umbilical cord blood as an alternative source of reduced-intensity hematopoietic stem cell transplantation for chronic Epstein-Barr virus-associated T or natural killer cell lymphoproliferative diseases. Biol Blood Marrow Transplant. 2014; 20: 214-21.
6. Balfour HH Jr, Odumade OA, Schmeling DO, Mullan BD, Ed JA, Knight JA, et al. Behavioral, virologic, and immunologic factors associated with acquisition and severity of primary Epstein-Barr virus infection in university students. J Infect Dis. 2013; 207: 80-8.
7. Balfour HH Jr, Dunmire SK, Hogquist KA. Infectious mononucleosis. Clin Transl Immunology. 2015; 4: e33.
8. Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, et al.; Sixth European Conference on Infections in Leukemia, a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP), the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer (EORTC-IDG), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016; 101: 803-11.
9. Nishida A, Yamamoto H, Ohta Y, Shimazu H, Ishiwata K, Nakano N, et al. Incidence and clinical features of EBV-PTLD following CBT. Rinsho Ketsueki. 2009; 50: 210.
10. Sakamoto K, Takase K, Saito N, Kawano I, Henzan H, Eto T. Report of two cases of EBV-associated lymphoproliferative disorder with intestinal ulcer after cord blood transplantation. The 34th annual meeting of the Japanese society for hematopoietic cell transplantation. 2012; 34: 266.
11. Ono K, Murata K, Miyazaki A, Tachibana N, Nakamura T, Nishimura R, et al. Late-onset hemophagocytic lymphohistiocytosis with varicella zoster virus and Epstein-Barr virus coinfection after umbilical cord blood transplantation. Ann Hematol. 2018; 97: 1493-5.
12. van Beek J, zur Hausen A, Klein Kranenbarg E, van de Velde CJ, Middeldorp JM, van den Brule AJ, et al. EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement. J Clin Oncol. 2004; 22: 664-70.
13. Sanz J, Arango M, Senent L, Jarque I, Montesinos P, Sempere A, et al. EBV-associated post-transplant lymphoproliferative disorder after umbilical cord blood transplantation in adults with hematological diseases. Bone Marrow Transplant. 2014; 49: 397-402.
Search
News



