Volume 4 (2021) Issue 1 No.1 Pages 1-8
Abstract
Background: Multiple Myeloma (MM) is characterized by the presence of clonal plasma cells. These often result in complications including bone destruction, hypercalcemia, renal insufficiency, and anaemia. Induction with a triplet or quadruplet regimen followed by autologous stem cell transplantation (ASCT) has been the standard of care for transplant eligible patients to achieve durable remission.
Purpose: This is a retrospective analytical study to determine the outcome of Multiple Myeloma patients who underwent ASCT in Ampang Hospital.
Materials and Methods: We included a 5-year cohort of patients transplanted from 1st July 2014 to 30th June 2019. Data were obtained through electronic medical records. Prognostic factors for progression-free survival (PFS) and overall survival (OS) were analyzed using simple and multiple Cox proportional hazard regression analysis. All analyses were done using software R version 3.6.2 with validated statistical packages.
Results: 139 patients were analyzed. The median age at transplant was 56 years old and 56.1% are males (n=78). The most common subtype is IgG Kappa (n=67, 48.2%). Only 93 patients in which the International Staging System (ISS) could be determined, and among them, 33.3% of patients (n=31) have advanced stage ? disease. The most common induction received before ASCT was a bortezomib based regimen and/or an immunomodulatory (IMiD) based regimen. 63.3% of patients achieved at least a very good partial response (VGPR) before ASCT. Most patients received myeloablative conditioning (MAC) (n=119, 85.6%). The mean cell dose is 3.68×106/kg. The median time to engraftment was 11 days for both platelet and absolute neutrophil count (ANC). With the median follow-up of 17.3 (range, 6.2-33.4) months, the median OS and PFS were not reached. The 1-year and 2-year PFS were 75% (95% CI 66-82%) and 52% (95% CI 42-62%), respectively. The 1-year and 2-year OS were 82% (95% CI 74-88%) and 70% (95% CI 60-78%), respectively. 6 patients (4.3%) had transplant-related mortality (TRM). IgA subtype was found to adversely affect PFS. Maintenance therapy and the absence of renal impairment was associated with better PFS and OS.
Discussion and Conclusions: Our study found that ASCT following induction treatment is safe and beneficial to achieve a deeper remission status. In our study, the addition of maintenance therapy is associated with an improved outcome in PFS and OS.
Introduction
Multiple myeloma (MM) is characterized by the malignant proliferation of clonal plasma cells. It is believed that MM cases are preceded by a premalignant state of monoclonal gammopathy of undetermined significance and smoldering multiple myeloma, for which the risk of progression to MM is approximately 1% and 10% per year, respectively1,2. These clonal plasma cells often produce immunoglobulins (Ig), resulting in complications that constitute the diagnostic criteria for MM, such as anemia, bone destruction, renal impairment, and hypercalcemia3,4. MM constitutes about 10% of all hematological malignancies and generally affects the elderly population with a median age of 66 years at diagnosis3. Various staging systems have been established to assess tumor burden and identify high-risk patients based on laboratory markers and cytogenetic abnormalities5-7.
Induction regimens, most commonly with a proteasome inhibitor (PI), an immunomodulator (IMiD), or a combination of both agents, followed by autologous stem cell transplantation (ASCT) for eligible patients has been the standard of care8,9. Following initial treatment, maintenance therapy may be given, especially for high-risk patients8-11. Despite adequate treatment, the majority of MM patients will relapse, and the outcomes of such patients are poor12. Novel therapies such as monoclonal antibodies (e. g., daratumumab) are increasingly being added in the frontline treatment and may improve the outlook for these patients13-15.
In Malaysia, data pertaining to the disease characteristics of MM and long-term outcomes, especially for those who have undergone ASCT, are nonexistent. Hence, a retrospective analytical study on the demographic characteristics and outcomes of transplanted MM patients in a tertiary-level hospital with transplant facilities was carried out to determine the demographic data, risk factors, and outcomes. Understanding the risk factors for treatment outcomes is important to improve the long-term management of these patients.
Patients and Methods
Study design and population
A retrospective analytical study was conducted in a Malaysian tertiary hospital performing hematopoietic stem cell transplantation (HSCT). Our center is the main referral center for ASCT. All patients who were diagnosed with MM and referred to the transplant unit for ASCT within the period from July 1, 2014, to June 30, 2019, were recruited. Sociodemographic data, clinical characteristics, complications, treatment regimens, and outcomes were collected from the medical records using a standardized data collection form. We included all patients aged 18 years and above, and diagnosed with MM, based on the International Myeloma Working Group (IMWG) criteria4, and excluded patients with solitary plasmacytoma without bone marrow involvement. This study was registered with the National Medical Research Register Malaysia, and ethical approval was obtained from the Medical Research and Ethics Committee, Ministry of Health Malaysia (NMRR-20-1332-55374). Written informed consent was obtained from all participants in accordance with the Helsinki Declaration of 1964, as revised in 2000 and 2008. for their participation in these studies.
Clinical, sociodemographic characteristics and treatment outcomes
Demographic data, presenting symptoms, laboratory investigations (including myeloma subtypes and cytogenetics), disease staging, treatment plans, and treatment outcomes from patient records were reviewed. Patients were staged according to the International Staging System ( ISS), based on serum albumin and beta-2-microglobulin at diagnosis7.
Pre-transplant treatment modalities, including induction therapy and the number of cycles completed, were recorded. Disease status upon completion of induction and before ASCT were assessed based on IMWG uniform response criteria for multiple myeloma16,17, and these included complete response (CR), very good partial response (VGPR), partial response (PR), stable disease (SD), and progressive disease (PD) or refractory disease.
Transplant details, including conditioning regimen, treatment-related mortality (TRM), post-transplant consolidation, and maintenance therapy, were collected as well. The post-transplant response was assessed using similar response criteria.
Overall survival (OS) was calculated using the time taken from treatment initiation to death from any cause, and progression-free survival (PFS) was calculated using the time from treatment initiation to progression of the disease (biochemical progression or myeloma-defining event), death, or last contact.
Statistical Analysis
The statistical software R version 3.6.2 with validated statistical packages was used to analyze all the collected and extracted data. Descriptive statistics were used to summarize the characteristics of patients. Categorical data were expressed as frequencies and percentages, and continuous data were expressed as means±standard deviations. The normality of continuous variable data was determined using a histogram method. The independent student?s t-test was used to compare group means of continuous dependent variables for normally distributed data, whereas the Mann-Whitney test was used if the normality was not assumed.
The Kaplan-Meier method was used to estimate median survival times, and the log-rank test at a 5% significance level was used to test the equality of survival between groups. Prognostic factors were identified using simple and multiple Cox proportional hazard regression analysis. All p-values were 2-sided and values?0.05 were considered statistically significant.
Results
Sociodemographic data and clinical characteristics
139 patients with a median age of 56 years (range: 30-70) at transplantation were recruited. All patients were deemed to have a good performance status, with Eastern Cooperative Oncology Group status of either 0 or 1. The majority were males (56.1%, n=78), with 57.6% being Malay, followed by Chinese (28.8%) and Indian (12.2%), reflective of the national ethnicity demographics in the country18.
Clinical presentations varied among these patients, with the most common presentation being symptoms of anemia (n=94, 67.6%). The heavy chain subtype of IgG represented the most common subtype (69%), whereas kappa was the most common light chain (66.9%). Sixtyseven patients had a combination of both IgG and kappa. The ISS could be determined for only 93 patients, and among them, 33.3% (n=31) had stage ? disease. Out of the 50 patients for whom cytogenetic data could be obtained, 18% had high-risk cytogenetics of either (t 4;14), (t 14;16), and/or deletion 17p, based on fluorescence in situ hybridization (FISH) studies and karyotyping. Detailed clinical and disease characteristics are illustrated in Table 1.
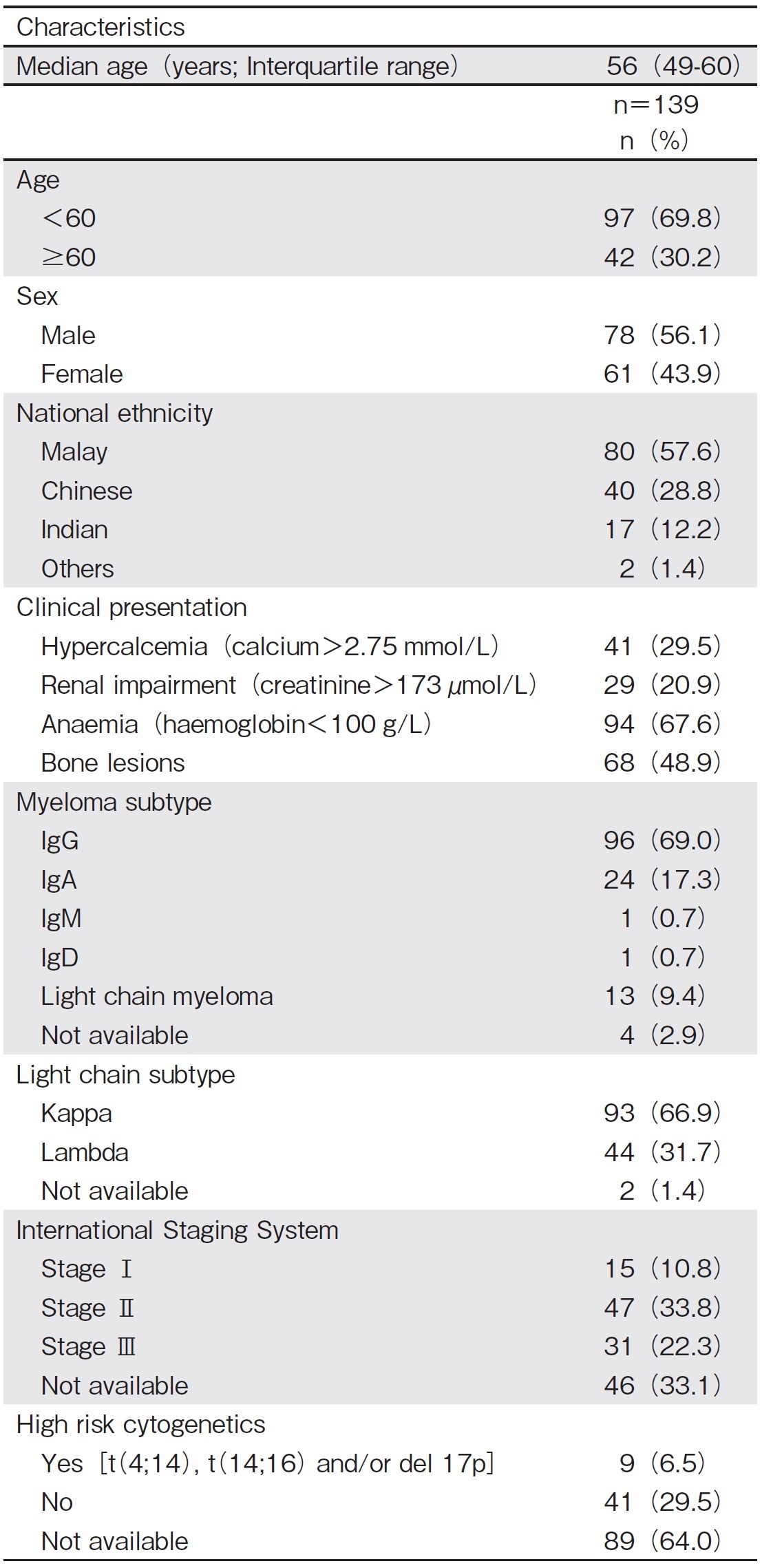
Treatment and outcome before ASCT
Table 1 includes the treatment and response outcome before ASCT. The most common induction received before ASCT was a bortezomib-based regimen (n=55, 39.6%) and an IMiD-based regimen (n=36, 25.9%), with 45 patients receiving a combination of both. Out of the 132 patients for whom the post-induction remission status was known, 68.2% (n=90) achieved either CR or VGPR. 121 patients were transplanted following induction treatment (CR1), whereas 18 patients were transplanted after receiving a salvage treatment for relapsed/refractory disease (CR2). Among those who received salvage treatment before transplant, 6 patients achieved CR, 1 achieved VGPR, and 6 achieved PR. Three patients had a refractory disease and two had unknown disease status.
Transplant and post-transplant assessment
Most patients received myeloablative conditioning (MAC) (n=119, 85.6%). The mean cell dose was 3.68×106/kg. The median time to engraftment was 11 days for both platelet and absolute neutrophil count (ANC). Eight patients had delayed platelet engraftment, whereas one had delayed white cell engraftment. Six patients (4.3%) exhibited TRM, five of them due to sepsis and one of unknown cause. One patient had a tandem autologous and allogeneic stem cell transplant. The treatment and transplant-related details are summarized in Table 2.
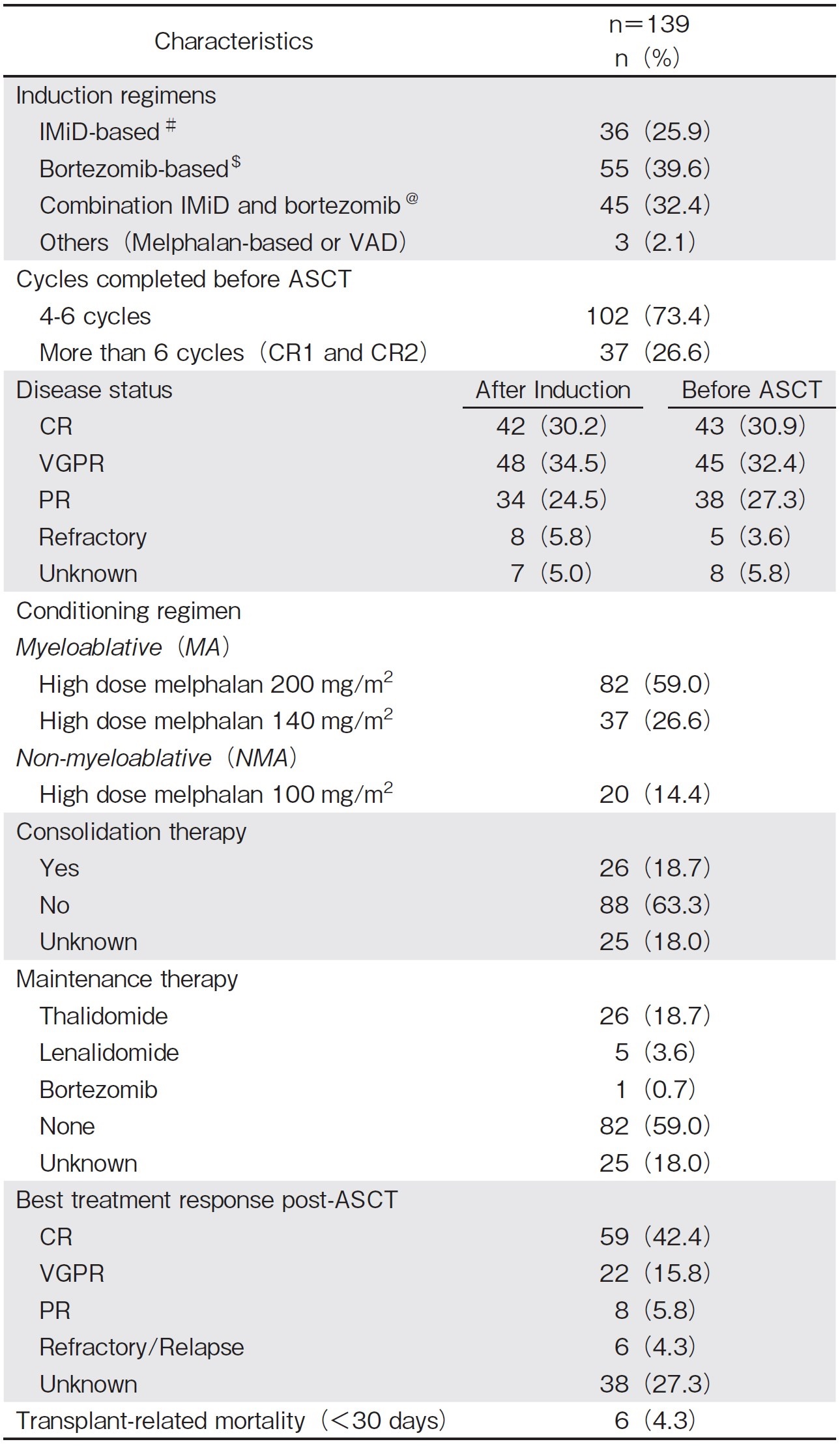
# CTD-Cyclophosphamide, thalidomide, dexamethasone
$ VCD-Bortezomib, cyclophosphamide, dexamethasone
@ VTD-Bortezomib, thalidomide, dexamethasone
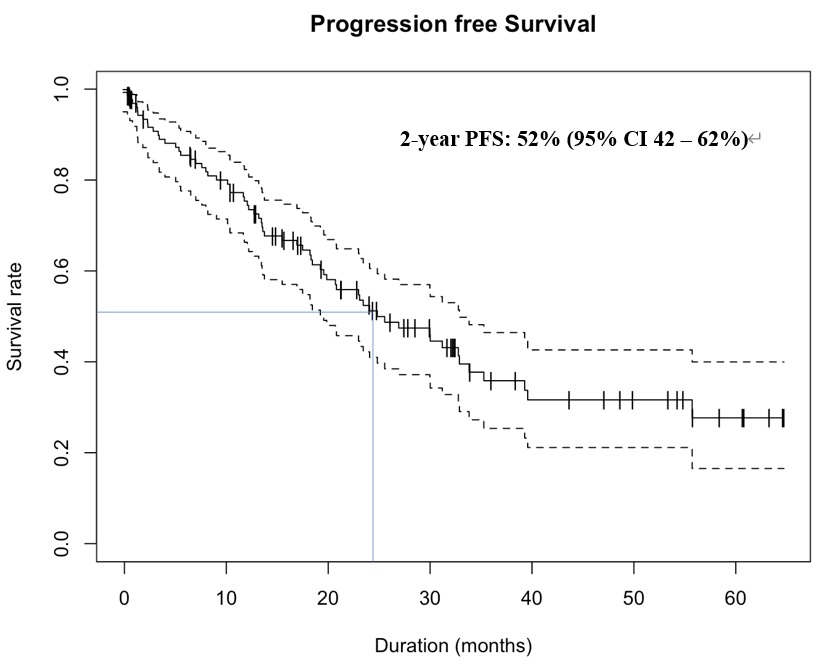
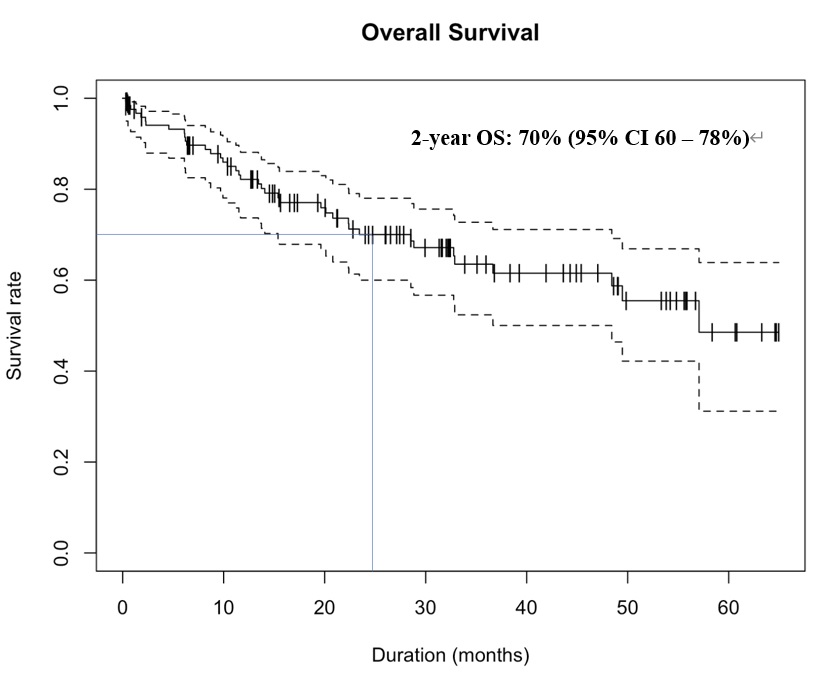
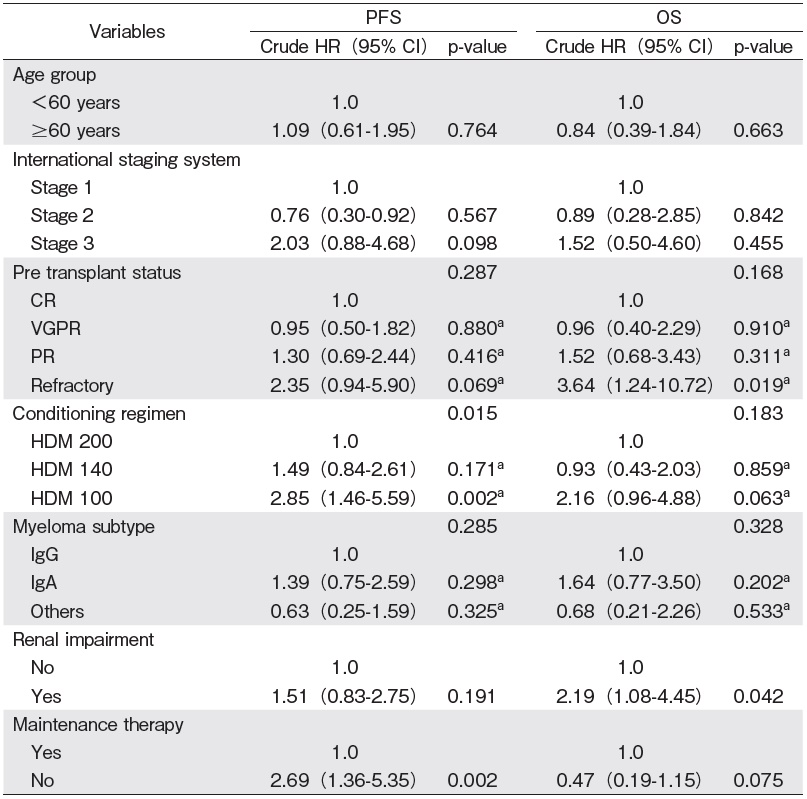
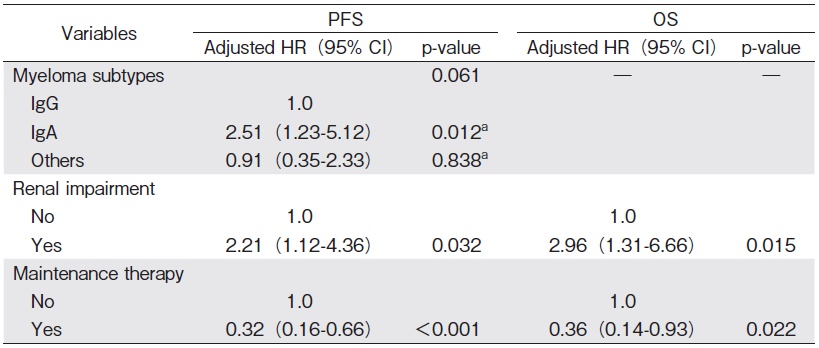
With a median follow-up of 17.3 (range: 6.2-33.4) months, the median OS and PFS were not achieved. The 1- and 2-year PFS were 75% (95% CI 66-82%) and 52% (95% CI 42-62%), respectively. The 1- and 2-year OS were 82% (95% CI 74-88%) and 70% (95% CI 60-78%), respectively (Figures 1 and 2). In multivariate analysis, the IgA subtype was found to adversely affect the PFS, whereas the presence of renal impairment significantly shortened both the PFS and OS. The use of maintenance therapy was associated with better PFS and OS (Tables 3 and 4).
a Z statistic, Z test
a Z statistic, Z test
Discussion
In our cross-sectional study, we analyzed the clinical characteristics of transplant-eligible MM patients who were either treated at our center or referred to us for ASCT from other centers nationwide. MM is a malignant clonal plasma cell disorder with a high risk of disease relapse and progression following initial treatment. Novel therapies such as daratumumab, a monoclonal antibody against CD38, either as part of a three-drug or four-drug regimen, have been found to have a better overall response rate (ORR), and more importantly, a CR status13-15. Nevertheless, there is a risk of relapse and progression, and these agents are viewed by many as additions to achieve a deeper remission before transplantation rather than a replacement for it.
Novel therapies including chimeric antigen receptorengineered T cells (CART) targeting BCMA, CD138, CS1 glycoprotein antigen (SLAMF7), and light chains, are in active development for the treatment of relapsed/ refractory MM and are anticipated to have a role in the frontline setting19. In many parts of the world, access to such novel therapies is limited, and therefore, high-dose therapy followed by ASCT as consolidation remains the backbone of treatment20. In the current guideline recommendations, newly diagnosed MM should be assessed for eligibility for transplant, and if suitable, be spared from stem cell-toxic regimens8,9.
Initial treatment
Many options can be potentially used for initial therapy for patients who are transplant-eligible. In countries where access to novel therapies is limited, triplet agents are commonly implemented. These include a PI-based regimen (e. g., bortezomib and carfilzomib), an IMiDbased regimen (e. g., thalidomide and lenalidomide), or a combination of both together with steroids, making up the triplet therapy. A prolonged initial therapy may impair the ability for stem cell collection in a manner similar to using a stem cell toxic agent21. Hence, stem cell collections are generally performed after 4 cycles of initial therapy at our center, keeping in mind the need to reduce the burden of malignant plasma cells in the bone marrow.
In our cohort of patients, cyclophosphamide (C) was often used with either bortezomib (Velcade©) or thalidomide ( T) and dexamethasone (D), i. e., VCD or CTD. Another common regimen used was the combination of bortezomib and thalidomide, i. e., VTD (Table 1). The choice of the initial induction therapy was mainly at the discretion of the respective treating physician at each treatment center, with considerations for individual disease characteristics, i. e., high-risk cytogenetics, comorbidities ( e. g., renal impairment, thrombotic risks, and peripheral neuropathy), and feasibility of outpatient visits. One patient received melphalan as she was initially deemed transplant-ineligible, but later had an improvement in functional status, and hence, was transplanted accordingly. We employed a granulocyte colony-stimulating factor-primed peripheral blood stem cell collection and chemotherapy mobilization for those who failed this initial approach.
Timing and benefit of transplantation
After successful stem cell collection, many of our patients were transplanted following an initial response to treatment, either after recovery from stem cell collection or after another 1-2 cycles of initial induction therapy. Eighteen out of the 139 patients had their transplant delayed after response to salvage treatment for relapsed or refractory disease (CR2). In a randomized controlled trial that evaluated early and delayed transplant (at the time of relapse), early transplantation resulted in deeper responses and improved PFS, but not better OS22. However, in this trial, more than 20% of the patients were not transplanted mainly due to progression of the disease and were deemed unfit for transplants, a common situation in clinical practice. This may potentially deprive patients of an opportunity for better disease control.
Achieving a good remission status following initial therapy and transplant has been viewed as an important predictor of disease progression and survival23. In our study, we aimed to analyze pre-transplant remission status to outline the value of a deep remission. There is a trend towards poorer outcomes for PFS and OS in patients who failed to achieve at least a VGPR status in our study. However, our study failed to demonstrate a statistically significant benefit of PFS and OS for those attaining CR over those achieving VGPR or PR. This is likely due to a high number of unknown responses posttransplant with a short median follow-up duration. The greater number of patients who attained a CR post-transplant ( n=43, pre-transplant vs. n=59, post-transplant) suggested the benefit of ASCT to render deeper remissions. Our cohort had similar reported outcomes for transplanted patients and demonstrated a benefit in OS compared to non-transplanted patients across all ISS stages6.
Transplantation measures and post-transplant treatment
The standard conditioning regimen used for ASCT in MM is melphalan at a dose of 200 mg/m2 (HDM 200). This is based on the findings of French and Italian studies24,25. In our cohort of patients, melphalan dose adjustments were made for those with renal impairment at the time of transplantation and elderly patients aged 65 years and above, to 140 mg/m2 (HDM 140), or 100 mg/m2 (HDM 100) if both risk factors were present. Based on this approach, the median time to engraftment of 11 days is reasonable. The TRM of 4.3% (n=6, 5 in HDM 200 and 1 in HDM 100) was higher compared to the results of both these studies. Retrospectively, these patients had multiple comorbidities, including diabetes mellitus, hypertension, and chronic kidney disease, and were deemed to be high-risk patients before ASCT. This suggests that we need to adopt a better selection criterion for transplant eligibility that can potentially reduce the TRM.
Maintenance therapy post-ASCT is common, especially for high-risk patients. IMiD agents such as thalidomide and lenalidomide are used in this setting10,26-28. Such trials have demonstrated a survival benefit in terms of PFS and/or OS, at the expense of adverse events and secondary malignancy. Similarly, in our study, the implementation of maintenance therapy did confer a 68% reduction in the risk of progression and a 64% reduction in the risk of mortality (Table 3). However, given the small numbers of those on maintenance treatment (n= 32), further validation of this finding with a prospective trial is required. Advanced-stage disease ISS ? was identified as a factor with a trend toward poorer PFS[crude HR (95% CI), 2.03 (0.88-4.68) ] (P=0.098) and poorer OS[crude HR (95% CI), 1.52 (0.50-4.60) ] (P=0.455) when comparing patients with early-stage (stage ?) disease in univariate analysis. A larger patient cohort with longer follow-up duration can potentially illustrate this significance.
Tandem transplants (double ASCT or autologous followed by allogeneic) have been considered a strategy for better disease remissions29,30. We consider this approach for young patients (<40 years old) with high-risk disease and allogeneic SCT if a suitable donor is identified. Our only patient with a tandem transplant was a 33-year-old gentleman with a revised International Staging System (R-ISS) stage ?, who underwent VCD induction therapy. He received a reduced-intensity conditioning allogeneic transplantation (allo-HSCT) from a matched sibling donor 4 months following recovery from ASCT. At the time of writing, he remained in stringent complete response (sCR) 2 years post allo-HSCT with mild chronic graft-versus-host disease of the liver.
This study is limited by its lack of comprehensive data (e. g., revised ISS staging and treatment responses) and its retrospective nature of analysis. Although the analysis was conducted at a major transplant center in our country, a comprehensive multicenter prospective study for the entire nation will be beneficial. Nonetheless, our study has provided an insight into the demographics of the MM population in this region and highlighted the importance and feasibility of ASCT as a consolidative treatment, especially in a resource-limited setting.
Acknowledgements
The authors would like to thank the Director-General of Health Malaysia for his permission to publish this article.
Author Contributions
C. C. K. L, Y. L. L, K. W. H, S. A. K. S. S, and J. T. C. T performed the study design.
C. C. K. L and Y. L. B wrote the manuscript and review of the literature.
C. C. K. L, Y. L. B, K. W. H, S. A. K. S. S, T. C. O, and S. M. T contributed information and data for manuscript writing.
C. C. K. L, Y. L. L, and K. B. L performed the data analysis.
T. C. O, J. T. C. T, N. S. L, S. M. T, and J. S supervised the manuscript writing.
Conflicts of Interest
The authors declare no conflict of interest.Disclosure forms provided by the authors are available here.
References
1. Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009; 113: 5412-7.
2. Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002; 346: 564-9.
3. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78: 21-33.
4. Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15: e538-48.
5. Durie BG, Salmon SE. A clinical staging system for multiple myeloma correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975; 36: 842-54.
6. Greipp PR, San Miguel J, Durie BG, Crowley JJ, Barlogie B, Bladé J, et al. International staging system for multiple myeloma. J Clin Oncol. 2005; 23: 3412-20.
7. Palumbo A, Avet-Loiseau H, Oliva S, Lokhorst HM, Goldschmidt H, Rosinol L, et al. Revised international staging system for multiple myeloma: a report from International Myeloma Working Group. J Clin Oncol. 2015; 33: 2863-9.
8. Kumar SK, Callander NS, Hillengass J, Liedtke M, Baljevic M, Campagnaro E, et al. NCCN Guidelines Insights: Multiple Myeloma, Version 1.2020. J Natl Compr Canc Netw. 2019; 17:1154-65.
9. Moreau P, San Miguel J, Sonneveld P, Mateos MV, Zamagni E, Avet-Loiseau H, et al. Multiple myeloma: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2017; 28 (Suppl 4) : iv52-iv61.
10. Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012; 366: 1759-69.
11. Palumbo A, Gay F, Cavallo F, Di Raimondo F, Larocca A, Hardan I, et al. Continuous therapy versus fixed duration of therapy in patients with newly diagnosed multiple myeloma. J Clin Oncol. 2015; 33: 3459-66.
12. Harousseau JL, Attal M. How I treat first relapse of myeloma. Blood. 2017; 130: 963-73.
13. Voorhees PM, Kaufman JL, Laubach J, Sborov DW, Reeves B, Rodriguez C, et al. Daratumumab, lenalidomide, bortezomib, & dexamethasone for transplant-eligible newly diagnosed multiple myeloma: GRIFFIN. Blood. 2020; 136: 936-45.
14. Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA) : a randomised, open-label, phase 3 study. Lancet. 2019; 394: 29-38.
15. Facon T, Kumar SK, Plesner T, Orlowski RZ, Moreau P, Bahlis N, et al. Phase 3 randomized study of daratumumab plus lenalidomide and dexamethasone (D-Rd) versus lenalidomide and dexamethasone (Rd) in patients with newly diagnosed multiple myeloma (NDMM) ineligible for transplant (MAIA). Blood. 2018; 132 (Suppl. 1) : LBA-2.
16. Durie BG, Harousseau JL, Miguel JS, Bladé J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006; 20: 1467-73.
17. Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011; 117: 4691-5.
18. Current Population Estimates Malaysia 2019". Department of Statistics, Malaysia. http://pqi.stats.gov.my/searchBI.php?tahun=2019&kodData=2&kodJadual=1&kodCiri=4&kodNegeri=Semua. [Accessed June 29, 2020]
19. Lin Q, Zhao J, Song Y, Liu D. Recent updates on CAR T clinical trials for multiple myeloma. Mol Cancer. 2019; 18: 154.
20. Al Hamed R, Bazarbachi AH, Malard F, Harousseau JL, Mohty M. Current status of autologous stem cell transplantation for multiple myeloma. Blood Cancer J. 2019; 9: 1-10.
21. Kumar S, Giralt S, Stadtmauer EA, Harousseau JL, Palumbo A, Bensinger W, et al. Mobilization in myeloma revisited: IMWG consensus perspectives on stem cell collection following initial therapy with thalidomide-, lenalidomide-, or bortezomib-containing regimens. Blood. 2009; 114: 1729-35.
22. Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017; 376: 1311-20.
23. Kapoor P, Kumar SK, Dispenzieri A, Lacy MQ, Buadi F, Dingli D, et al. Importance of achieving stringent complete response after autologous stem-cell transplantation in multiple myeloma. J Clin Oncol. 2013; 31: 4529-35.
24. Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, et al. Comparison of 200 mg/m2 melphalan and 8 Gy total body irradiation plus 140 mg/m2 melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002; 99: 731-5.
25. Palumbo A, Bringhen S, Bruno B, Falcone AP, Liberati AM, Grasso M, et al. Melphalan 200 mg/m2 versus melphalan 100 mg/m2 in newly diagnosed myeloma patients: a prospective, multicenter phase 3 study. Blood. The Journal of the American Society of Hematology. 2010; 115: 1873-9.
26. Sahebi F, Spielberger R, Kogut NM, Fung H, Falk PM, Parker P, et al. Maintenance thalidomide following single cycle autologous peripheral blood stem cell transplant in patients with multiple myeloma. Bone Marrow Transplant. 2006; 37: 825-9.
27. Spencer A, Prince HM, Roberts AW, Prosser IW, Bradstock KF, Coyle L, et al. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J Clin Oncol. 2009; 27: 1788-93.
28. McCarthy PL, Holstein SA, Petrucci MT, Richardson PG, Hulin C, Tosi P, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017; 35: 3279-89.
29. Elice F, Raimondi R, Tosetto A, D?Emilio A, Di Bona E, Piccin A, et al. Prolonged overall survival with second on-demand autologous transplant in multiple myeloma. Am J Hematol. 2006; 81: 426-31.
30. Bjorkstrand B, Iacobelli S, Hegenbart U, Gruber A, Greinix H, Volin L, et al. Tandem autologous/reduced-intensity conditioning allogeneic stem-cell transplantation versus autologous transplantation in myeloma: long-term follow-up. J Clin Oncol. 2011; 29: 3016-22.
Search
News



