Volume 3 (2020) Issue 3 No.4 Pages 59-70
Abstract
1. Introduction: COVID-19 (SARS-CoV-2 infection)
COVID-19, a disease caused by infection by a novel coronavirus called SARS-CoV-2, was declared an epidemic by WHO on March 11, 2020. The Government of India invoked the Epidemic Diseases Act in March and declared a nationwide lockdown from March 24. The impact on healthcare practice and hematopoietic stem cell transplantation activity has been significant, and this is likely to continue for some time. The scientific committee of the ISBMT reviewed the guidelines from several sources including the MoHFW-Government of India, ICMR, EBMT, ASTCT, WMDA, NICE, ECDC, CDC, and NCCN1-11, and invited comments from reputed academic centers in India, and finally arrived at the recommendations.
The time from exposure to symptom development is 2-14 days (median 5 days). Symptoms may vary from none or very mild symptoms of an upper respiratory infection to very severe indications that need intensive care to avert death from Acute Respiratory Distress Syndrome ( ARDS). Increasing age and the presence of comorbidities, such as pulmonary disease, cardiovascular disease, obesity, diabetes mellitus, hypertension, and immunocompromised conditions are reported risk factors for contracting the severe disease that often results in mortality2,4.
2. Scope of this Document
The recommendations in the ISBMT guidelines are meant for the conduct of hematopoietic stem cell transplantations during the ongoing global pandemic. They are intended primarily to support the decision-making processes of the clinical and administrative teams of transplant centers. It is not mandatory to apply the recommendations, and the guidelines do not override the responsibility of making decisions appropriate to the circumstances of the individual, in consultation with them, and their families and caretakers or guardian.
Individual centers should use these recommendations only as general guidance because circumstances will vary from center to center, and institutional procedures should be followed. In view of the rapidly changing circumstances, the provisions of this document are applicable only to the current situation. Advice may change as COVID-19 prevalence trends change. The current recommendations may be considered applicable until the WHO and/or government authorities announce that the epidemic phase of COVID-19 is over and the Epidemic Diseases Act is withdrawn. These guidelines are not based on concrete evidence regarding COVID-19 and SARSCoV- 2. The ISBMT will update this document on their website at www.isbmt.org when significant new information, tests, or treatment are available.
3. Prevention Policies and Procedures for Transplant Centers
National, local, and institutional guidelines should be followed1. Avoiding exposure by adhering to the recommended hygiene procedures, isolation of SARS-CoV-2 infected persons, and social distancing are the only prevention strategies (see The WHO recommendations, Section 13).
a. Minimize face-to-face contact by offering telephone or telemedicine consultations whenever possible, cutting non-essential one-on-one follow up, using alternative ways of delivering medicine (such as friends, family members, and volunteers), and coordinating access to blood tests for post-transplant investigations7
b. Ask patients to attend appointments with no more than one family member or caretaker, or alone if they can, to reduce the risk of contracting or spreading the infection7.
c. Minimize time in the waiting area by careful scheduling, encouraging patients not to arrive early, and using text messaging.
d. When patients with known or suspected COVID-19 symptoms are identified, follow appropriate government guidance on infection prevention and control. This includes recommendations on patient transfers, transport, and options for outpatient settings. This may also necessitate converting positive pressure rooms to negative pressure ones1,7.
e. All healthcare workers involved in receiving, assessing, and caring for patients with known or suspected COVID-19 history should follow the government guidance on infection prevention and control. This includes information on using the personal protective equipment (PPE). Training of staff in proper procedures is critical (donning, doffing, and sample collection) 1,7.
f. Visitors should be restricted, as much as possible, to transplant floors. One attendant can be allowed with the patient.
g. Staff with respiratory symptoms should stay at home. Testing of staff/attendants should be done according to national and local guidelines. (currently by RT PCR) 1.
h. Have intradepartmental discussions on how to modify usual care to reduce patient exposure to COVID-19 and make the best use of resources (workforce, facilities, intensive care, equipment) by minimizing in-patient and day-case admissions.
i. Work within local transplant programs to support stem cell processing and harvesting, specialized diagnostics, and cryopreservation.
j. Make decisions about modifications to usual care at an organizational level according to current quality management systems within the HSCT program. If a center cannot meet quality standards, temporary closure is an option7.
k. If a center is temporarily closed, work within local transplant programs to prioritize clinically urgent HSCT, and transfer patients as needed7.
l. For patients with allogeneic HSCT, identify a backup donor in case there are problems with harvesting or transport4,5,7.
m. Be aware of the availability of any planned conditioning treatments, and arrange alternatives based on availability and clinical indication7.
n. If a donor tests positive for COVID-19, the cells to be used should be moved to a marker-positive tank unless they will be utilized within one month7. o. Ship and cryopreserve all donations before starting conditioning, unless exceptional circumstances mean this is not possible. Cryopreserve separate graded dose aliquots of lymphocytes for potential donor lymphocyte infusions from donor stem cell harvests, when possible. Work with local processing laboratories to caution them of each donation, and whether to cryopreserve or not4,5,7.
p. Use G-CSF mobilized peripheral blood stem cells as the primary choice of hematopoietic stem cells from adult donors, to reduce demand on operation theatres for bone marrow harvesting.
q. For children and young people under 16 years, use the most appropriate source of stem cells based on donor age, access to theatres for bone marrow harvesting, and urgency of HSCT.
r. For stem cell mobilization in adults having autologous HSCT, use granulocyte-colony stimulating factor (G-CSF) and Plerixafor (where required), to minimize the use of chemotherapy priming.
s. There has been an unprecedented cancellation of planned blood donations due to social distancing, travel restrictions, and other non-pharmacological exigencies. Transplant teams are well advised to coordinate with blood bank colleagues, volunteer groups, and family attendants to ensure adequate supply of blood products and prevent blood shortage.
4. Communicating with Patients and Counselling
a. Communicate with patients, their families, and caretakers periodically.
b. Anticipate logistic constraints in the care continuum of patients due to lockdown, transport restrictions, specialty care consultations, and investigative modalities. Inform patients and their caregivers of these challenges. Where appropriate, design consent forms and consult with patients and families, when deemed necessary.
c. Costs of care will be dynamic. Counsel the patient and caregivers on the possibility of escalation of patient care costs during this pandemic, and take appropriate permission from them if required.
5. Scheduling and Prioritizing Transplant Procedures
a. Due to the dynamic situation of the COVID-19 pandemic, access to a stem cell donor might be restricted either due to the donor becoming infected, or due to the logistical reasons at the harvest centers in the middle of a strained healthcare system, or even due to the travel restrictions across international borders.
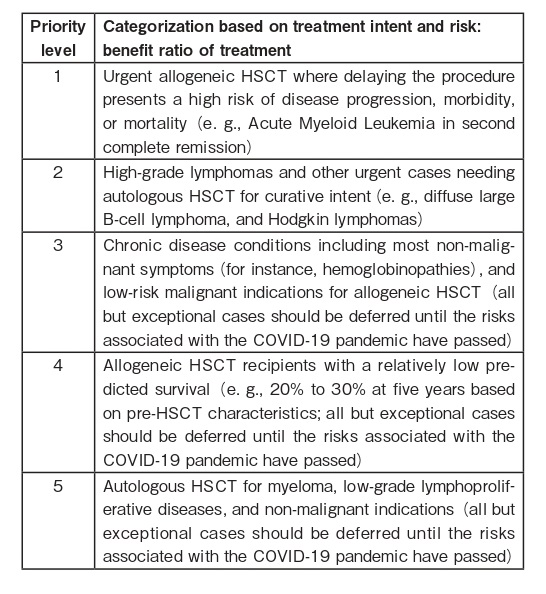
b. Adapted from the BSBMTCT group9 the three tables below provide a useful guide for prioritizing HSCT. The guidelines are based on the following principles:
?the balance of risks posed by their disease compared with the post-HSCT risks of becoming seriously ill from COVID-19.
?the risk of requiring critical care support and risk of disease relapse.
?center service capacity issues, such as limited resources (workforce, facilities, intensive care, equipment).
c. Use transplant- and disease-related prognostic tools where appropriate, and consider deferring HSCT in patients with predicted poor outcomes or if the risk from further treatment and immunosuppression would put them at more risk from COVID-197.
d. Discuss the risks, benefits, and possible outcomes of different treatment options with patients, families, and caretakers using decision support tools (where available) so that they can make informed decisions. Communicate decisions with written documentation.
e. Make treatment decisions as part of a multidisciplinary team, and ensure each patient is considered on an individual basis. Ensure that the reasoning behind each decision is recorded.
f. It is strongly recommended to have secured stem cell product access by freezing the product before start of conditioning; if this is not possible, an alternative donor should be identified as a back-up4.
6. Recommendation for Proposed Transplant Recipients
a. Patients planned to be admitted for a transplant should try to minimize the risk by home isolation for 14 days before the start of the transplant conditioning, if possible. Non-necessary clinic visits and non-essential travel should be avoided1,4,5,7.
b. All patients should be tested for SARS-CoV-2 by RT PCR, and the test results should be negative at least 72 hours before the start of the conditioning regardless of whether upper respiratory symptoms are present4,7. In the case of autologous transplant being proceeded with, SARS-CoV-2 testing may be done prior to the start of mobilization (and conditioning, if collection and start of conditioning are more than one week apart). If a patient is found negative, have intrateam discussions regarding a repeat test within one week considering the asymptomatic period of the disease9. If a family attendant stays with the patient throughout the transplant, consider screening the attendant for COVID-19.
c. If a transplant candidate/patient is diagnosed with COVID-19 by RT PCR, transplant ought to be deferred for at least three months (ECDC recommendations). However, this is not always possible due to the risk from the underlying disease. Therefore, in patients with high-risk disease (AML, high risk MDS, aplastic anemia, immunodeficiency), HCT should be deferred until the patient is asymptomatic and presents two consecutive virus PCR negativities at least one week apart (deferral of 14 days minimum). In patients with nonhigh- risk disease, a three-month HCT deferral is recommended4,5,7,9.
d. In case of close contact with a person diagnosed with COVID-19 by RT PCR, any transplant procedures (PBSC mobilization, BM harvest, conditioning) shall not be performed within 21 days from the last contact. Patient should be closely monitored for the presence of COVID-19, with confirmed PCR negativity before any transplant procedure is undertaken5,7.
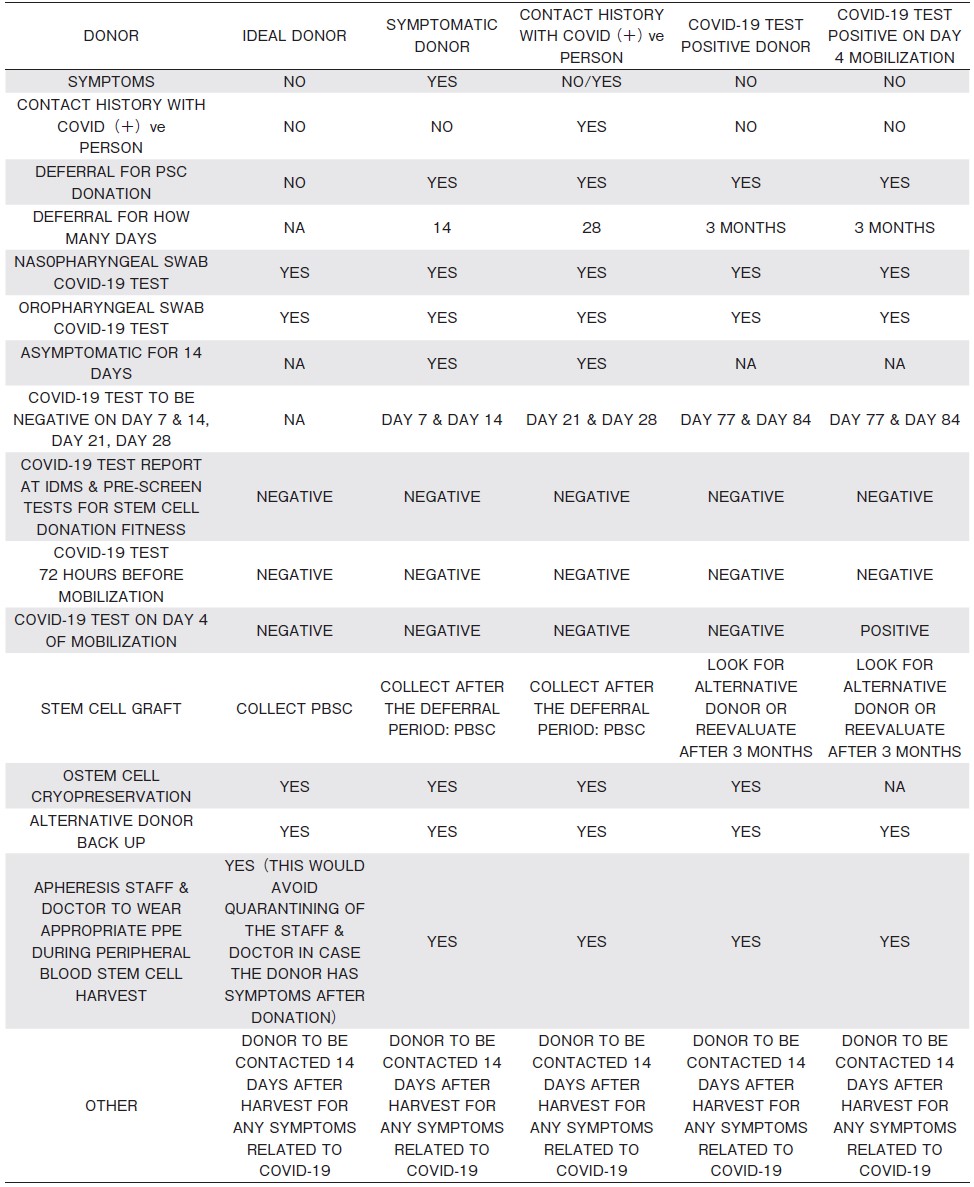
7. Recommendations for Transplant Donors:Table 1
SARS-CoV and MERS-CoV have been detected in blood, although there have not been any reports of transmission from donor to recipient either in transfusion of blood products or in cellular therapies4,5.
a. Explain to donors about the clinical signs and symptoms of COVID-19, transmission risks and related donation restrictions, because this will inform any decision to self-defer from donating.
b. COVID-19 testing should be done by RT-PCR, and if positive, the donor must be excluded from donation. With paucity of evidence, it is not possible to give recommendations when such an individual can be cleared for donation, but at least three months deferral can be considered. Advise donors not to donate blood products for at least three months from the time their symptoms have been resolved4,7.
If the patient's need for transplant is urgent, the donor is completely well, the RT PCR test is negative for SARS-CoV-2, and there are no suitable alternative donors, earlier collection may be considered if local quarantine requirements permit.
c. In case of close contact with a person diagnosed with SARS-CoV-2, the donor shall be excluded from donation for at least 28 days. Donor should be closely monitored in home quarantine for the presence of COVID-19 by RT PCR4.
d. In case of travel to high-risk COVID-19 areas (as defined by health authorities) or being in close contact with a person travelling from such an area, the donor shall be excluded from donation for at least 28 days from the date of travel/contact, if feasible5.
e. Donors should practice good hygiene (as per WHO recommendations, Section 13) and avoid non-essential travel, crowded places, and large group gatherings in the 28 days before donation.
f. For cryopreservation donations, test for COVID-19 at the assessment level, and again at the stage of harvest of stem cells or donor lymphocytes7.
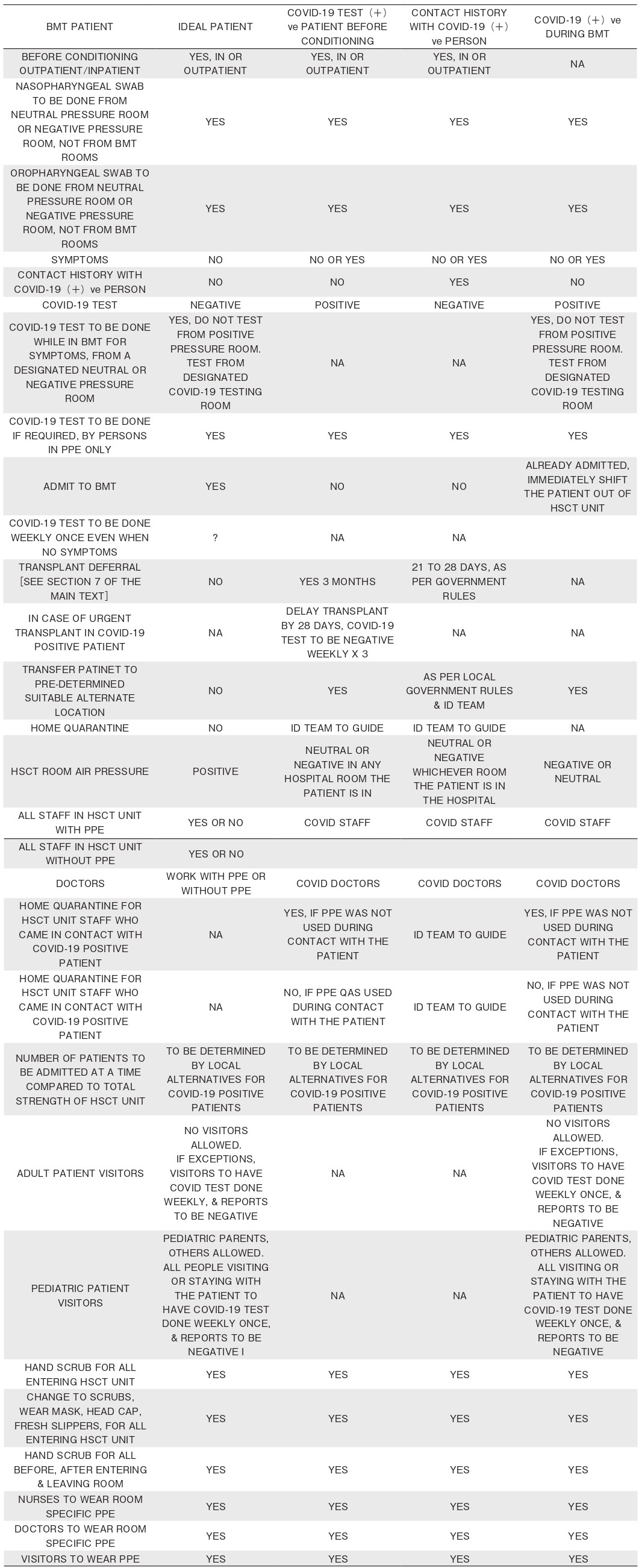
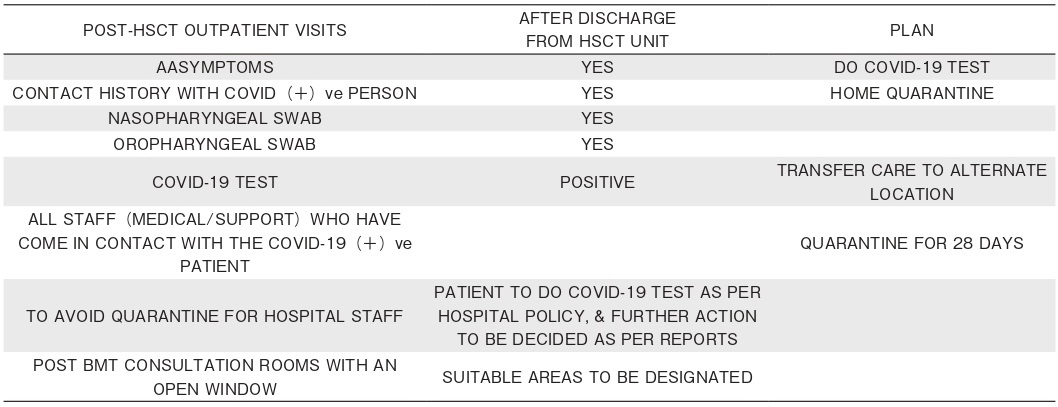
8. Recommendations for Recipients Post-transplant:Tables 2 and 3
a. Counsel patients who have had hematopoietic stem cell transplantation (HSCT) to follow the Government and regional guidance1 on shielding and protecting people considered as medically extremely vulnerable to COVID-19:
?if they had an autologous HSCT within the last year; and
?if they had an allogeneic HSCT within the last two years, are having continuous immunosuppressive therapy, or have chronic graft versus host disease (GvHD), or there is evidence of ongoing immunodeficiency ( or belonging to other extremely vulnerable groups based on clinical assessment) 7.
b. Stem cell transplant patients should restrict their risk of exposure to infected individuals as much as possible and conduct hygiene routines including hand washing and use of alcohol-containing hand sanitizers with care. Assess the need for any procedures outside of isolation against the risk of exposure to COVID- 191,2,7.
c. Stem cell transplant patients should refrain from traveling out of their city, according to national guidelines;
if travel is necessary, journey by private car instead of train, bus, or plane is recommended, if feasible1,7.
d. Diagnostic procedures should follow national guidelines1. Patients who reside in an area with high risk of transmission (community hotspots) of SARSCoV- 2, or who have been in close contact with a person living in or having traveled to such areas, should be tested for the virus. It is important to note that test results for SARS-CoV-2 can be a false negative, and thus needs to be repeated after 72 hours if there is a strong suspicion of COVID-19, such as in the case of pneumonia or severe illness. It is also important to test for other respiratory viral pathogens including influenza and RSV preferably by multiplex PCR.
e. All patients testing positive for SARS-CoV-2 in an upper respiratory tract sample should undergo chest imaging preferably by CT and evaluation of oxygenation impairment5.
f. Routine bronchoalveolar lavage (BAL) is not recommended if patient is tested positive with SARSCoV- 2, given the risk of transmission amongst healthcare workers, unless a co-infection is suspected. If chest imaging is abnormal in patients for whom it is clinically indicated (e.g., those receiving invasive mechanical ventilation), a lower respiratory tract aspirate or BAL sample should be collected and tested for SARS-CoV-2. Co-pathogens should be evaluated and treated4,5.
g. Supportive Care Considerations:
?Consider withholding infusions that are used for supportive purposes (e.g., intravenous immunoglobulin
[IVIG], elemental iron, and bisphosphonates) to minimize exposure of patients and caregivers within healthcare facilities. Skipping doses of some infusions may not be harmful for certain patients. There may also be oral alternatives that can be prescribed7.
?Consider lower thresholds for blood and platelet transfusions for some patients in whom it would be deemed safe, to reduce infusion clinic visits, and conserve limited blood supply7.
?To limit healthcare exposures, central lines may be flushed as infrequently as every (e.g., up to 12 weeks for chemoport), thus reducing face-to-face interactions with healthcare personnel. Other strategies for catheter management should be explored to this end as well7.
?Vaccinations: Delivery of some clinical preventive services, such as immunization, requires face-toface encounters. In areas with community transmission of COVID-19, these visits should be postponed except when:
・an in-person visit must be scheduled for some other purpose and the clinical preventive service can be delivered during that visit with no additional risk; or
・an individual patient and their clinician believe that there is a compelling need to receive the service based on an assessment that the potential benefit outweighs the risk of exposure to the virus that causes COVID-19.
?2020 CDC USA Vaccination guidance for Immunocompromised11.
9. Role of Matched Unrelated Donor Transplantation (MUD)
The WMDA International Emergency Task Force is collaborating with registries, governments, and courier companies to ensure continuity of care. However, in addition to transportation difficulties, possible exposure of the donor may affect the safety of the stem cell product. It is currently uncertain whether COVID-19 is transmissible through parenteral means. Hence, it is prudent to defer donors from endemic areas until suitable testing and observation time can ensure product safety. Timely communication to donors and patients, with strategies to assist in the provision of alternative products and procedures to optimize cell collection, are required. Pre-emptive cryopreservation of products that are likely to be required when transport may be further restricted is one option4,6.
Currently, there are restrictions in air travel to and from India. All registries are closely monitoring travel restrictions and are in contact with transport managements in different regions. Hence, all MUD transplants are recommended to be deferred, and should be considered only in special situations.
10. Healthcare Workers Including Those Who are in Self-isolation
Centers are advised to follow institutional guidelines. The following guidance is meant to aid in decision making:
a. All clinical care and support staff should always wear level 1 PPE (i.e., surgical mask and gloves), or better, if advised by institutional guidelines. Any patient contact requires an N95 mask, gown, cap, gloves, and shoe covers (as appropriate). A full complement of PPE must be considered if there is aerosol generating procedure involved.
b. Routine screening of asymptomatic healthcare staff is not recommended at present; however, institutional guidelines must be followed, especially in community hotspots.
c. If a healthcare professional needs to self-isolate, ensure that they can continue to help by:
?enabling telephone or video consultations and attendance at multidisciplinary team meetings.
?identifying patients who are suitable for remote monitoring and follow up, and those who are vulnerable and need support; and
?carrying out tasks that can be done remotely, such as entering data7.
d. Staff who test positive for or have symptoms of COVID-19 should self-isolate, and not return to work directly with hematopoietic stem cell transplantation patients until they:
?show no symptoms for two weeks; and
?test negative for COVID-19 after this period7.
e. Staff can resume work in other clinical areas after self-isolating in line with government advice on households with possible coronavirus (COVID-19) infection.
f. To facilitate mental wellbeing, encourage staff to communicate their problems related to transport or home. They can be debriefed daily, and any assistance from the hospital can be provided, if feasible. To reduce risk of infection to all staff, avoid congregating at meal or break times.
g. Provide all staff with visible leadership and supportive messaging, to maintain morale.
h. All healthcare workers, including cleaning staff, must be trained in donning and doffing of PPE, and all waste disposal as per the guidelines for COVID-19.
11. Approach to Patients with New Onset of Flulike Symptoms
a. Advise outpatient candidates, if they feel unwell, to contact the transplant center to ensure their symptoms are assessed appropriately.
b. Hematopoietic stem cell transplantation patients are immunocompromised and may have atypical presentations of COVID-19. Furthermore, symptoms of COVID-19, neutropenic sepsis, and viral pneumonitis may be difficult to differentiate at initial presentation4,5,7.
c. If patients have fever (with or without respiratory symptoms), suspect neutropenic sepsis because this can be rapid and life-threatening, and follow institutional guidelines. Consider COVID-19 as an additional differential diagnosis and evaluate and treat appropriately.
d. If COVID-19 is later diagnosed in a patient not isolated from admission or presentation, follow government guidance for health professionals.
e. If a patient not previously known or suspected to have COVID-19 shows new suggestive symptoms, the general advice is to follow the government guidance on investigation and initial clinical management of possible cases. This includes information on testing and isolating patients.
f. If a patient turns COVID-19 positive, discuss with the hospital infection control team and consider isolating or shifting the patient (preferably in a negative pressure room), and avoid retaining them in positive pressure or HEPA filtered rooms, unless the ventilation can be safely turned off.
12. Guidance on Treatment
Treatment guidelines have been issued by various government authorities and WHO. Transplant centers are advised to be well-versed with their regional guidelines and practices.
a. Currently there is no approved treatment options in India, and there is no available vaccine.
b. As appropriate, neutropenic fever and sepsis protocol should be initiated as per institutional guidelines.
c. In case of diagnosis of COVID-19 infection:
?It is unclear if patients with no/mild upper respiratory symptoms benefit from therapy; only supportive care measures may suffice.
?No specific antivirals have been proven to be effective as per currently available data.
?In patients with progressing symptoms or lower respiratory tract indications, the possibility of applying therapy should be investigated through clinical trials, if possible. At the time of manuscript preparation, reasonable data exists for chloroquine/ hydroxychloroquine with increasing experience of use from several centers. Combination therapy with azithromycin have also been used.
?Anti-inflammatory therapy is left to the physician's discretion and can be considered in more severe cases. Steroids should be avoided, unless necessary.
?Treatment for viral, bacterial, and fungal copathogens should be optimized. Drug interactions must be factored in during optimization of polypharmacy.
?Based on the available information (uncontrolled clinical trials), the following drugs may be considered as an off-label indication in patients with COVID-19 infection, and any respiratory compromise ( raised respiratory rate or lowered oxygen saturation) :
・Hydroxychloroquine (Dose 400 mg BD-for one day followed by 200 mg BD for four days).
・In combination with Azithromycin (500 mg OD for five days).
・These drugs should be administered under close medical supervision, with monitoring for side effects including QTc interval.
・If critically ill, and if ABO-compatible convalescent plasma is available, consider enrolling in a clinical trial (ICMR or institutional or local network group study), until approved by CDSCO/ DCGI1,4.
?Emerging evidence suggests that the microvascular thrombosis could be the early pathology in the lung, and it is best monitored by serial d-dimer levels. High (>two-three fold above normal) d-dimer
levels have been associated with much higher incidence of respiratory failure. Adequate anticoagulation should, therefore, be considered if not contraindicated due to other complications of HSCT12.
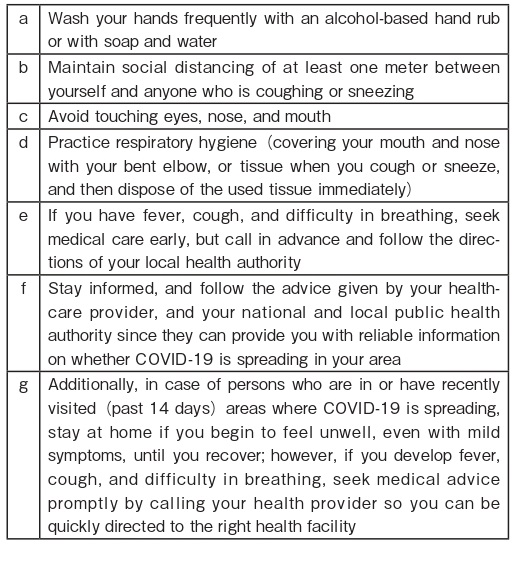
13. WHO Recommendations on How to Protect Yourself and Others from COVID-19 Box 12
14. COVID-19 Positive Scenario in the HSCT Unit
a. If a patient tests COVID-19 positive during BMT, adhere to the following:
?Clinical management should be based on clinical severity, contact tracing, testing, and quarantine, is necessary, based on risk categorization of exposure.
?Specific antiviral therapy including use of convalescent plasma, if available, should be considered under specific research protocols, if active, or other institutional processes on“compassionate”or named patient basis.
?Shifting to a negative pressure room is highly desirable and recommended, but if this is not possible, shifting the patient to a room without shared AHU, should be considered.
?Access to family/visitors is best reduced to tele/ video links unless rules about self-quarantine under these circumstances change in the country. Mothers/ other caretakers of very young children undergoing HSCT should have the option of staying in the ward with the patient for the duration of the HSCT.
b. If a BMT nurse tests COVID-19 positive, the following guidance will help:
?Clinical management based on clinical severity, contact tracing, testing, and quarantine is a must founded on risk categorization of exposure. Institutional guidelines should be followed keeping in view the national requirements also.
?Irrespective of staff strength, if>50% of the assigned nursing staff ends up in quarantine, depending on availability of other suitable nurses and the planned/acceptable nurse patient ratio in the HSCT unit, the options will include the following:
・an alternate isolation facility within the hospital with active consideration of re-purposing suitable staff familiar with management of hematological patients from other unaffected areas of the hospital.
・temporary closure of the facility, and shifting patients to alternative transplant facility in the town/city.
c. If the BMT physician tests COVID-19 positive a) when he is the only physician or b) when two or more physicians are available, note the following:
?Clinical management based on clinical severity, contact tracing, testing, and quarantine, based on local guidelines, is a must founded on risk categorization of exposure.
?If the she/he is the only BMT physician:
・temporary closure and shift of patients to nearest transplant center, if possible, may be considered.
・if not feasible and if the BMT physician is asymptomatic/with mild symptoms in quarantine, hand over to the critical care team/internal medicine/ pediatrics division (as appropriate) and use technology for teleconsultation for supervised care of patients.
・if not a practical option and if BMT physician is SICK, hand over to the critical care team/internal medicine/pediatrics division (as appropriate), and use technology for teleconsultation for supervised care of patient by a distant BMT physician (for teleconsultation) assigned by the primary BMT consultant or Institution.
These are unusual circumstances, and it is difficult to define the right or best option, but the aforementioned possibilities are intended to help and guide decision making. It will be crucial to inform HSCT patients and discuss with them these potential challenges during their management under the current circumstances, as well as modify existing consent forms to include some of the aforementioned considerations, as appropriate.
15. Acronyms
HSCT: Haematopoietic Stem Cell Transplantation MoHFW: Ministry of Health and Family Welfare, Government of India ICMR: Indian Council of Medical Research EBMT: European Society for Blood and Marrow Transplantation ASTCT: American Society for Transplantation and Cellular Therapy WMDA: World Marrow Donor Association NICE: National Institute for health and Care Excellence, United Kingdom ECDC: European Centre for Disease prevention and Control WHO: World Health Organization RT PCR: Reverse Transcription Polymerase Chain Reaction BSBMTCT: British Society of Blood and Marrow Transplantation and Cellular Therapy SARS-CoV: Severe Acute Respiratory Syndrome Corona Virus MERS: Middle East Respiratory Syndrome Corona Virus CDC: Centers for Disease Control and Prevention
16. Conflicts of Interest
The authors declare no conflict of interest.Disclosure forms provided by the authors are available here.
17. References
1. Government of India, Ministry of health and family welfare advisories
a. https://www.mohfw.gov.in/pdf/RevisedNationalClinicalManagementGuidelineforCOVID1931032020.pdf
b. https://www.mohfw.gov.in/pdf/FinalGuidanceonMangaementofCovidcasesversion2.pdf
2. WHO Resources COVID-19
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
3. ICMR Resources COVID-19
https://www.icmr.gov.in/ctechdocad.html
4. CORONAVIRUS DISEASE COVID-19: EBMTRECOMMENDATIONS,
updated April 7, 2020
https://www.ebmt.org/sites/default/files/2020-04/EBMT-COVID-19-guidelines_v.6.1%282020-04-07%29.pdf
5. ASTCT COVID-19 Interim Patient Guidelines
6. WMDA GUIDELINES 2020
https://share.wmda.info/pages/viewpage.action?pageId=344866320#/
7. NICE 2020 Rapid guideline COVID-19
8. ECDC
9. BSBMTCT 2020 Recommendations
10. NCCN COVID19 Resources
https://www.nccn.org/covid-19/default.aspx
11. CDC 2020 Vaccination Guidance
a. https://www.cdc.gov/vaccines/schedules/downloads/adult/adult-combined-schedule-bw.pdf
b. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf
12. JeckoThachil, Mary Cushman, Alok Srivastava. A Proposal for Staging COVID-19 Coagulopathy. Res Pract Thromb Hemost First published: 11 May 2020
https://onlinelibrary.wiley.com/doi/abs/10.1002/rth2.12372
All references were accessed on May 23, 2020.
Search
News



