Volume 3 (2020) Issue 4 No.1 Pages 71-73
Abstract
Precursor B-cell acute lymphoblastic leukemia (pre-B ALL) relapse after allogeneic hematopoietic stem cell transplantation (HSCT) typically has poor outcomes. Both bispecific T-cell engager (BiTE, blinatumomab) and chimeric antigen receptor T-cell (CAR-T) are novel immuno-designed therapies for advanced acute lymphoblastic leukemia. However, the treatment and effects of their combination remain unclear. Two patients with pre-B ALL experienced overt leukemic relapse after allogeneic HSCT. Both patients received one standard cycle of blinatumomab treatment, and complete remission was achieved. Subsequently, during remission, both patients underwent treatment with CAR-T cells prepared from their own T-cells. One patient received CD22-CAR-T cell as a consolidative therapy, while the other underwent CD19-CAR-T cell therapy. No cytokine release syndrome occurred. After treatment, both patients are alive and do not have leukemia recurrence for more than one year. In conclusion, sequential combination of BiTE followed by CAR-T cell therapy may show promising results for relapsed and advanced B-cell leukemia. This novel combination is worthy of further investigation.
Introduction
Patients with advanced precursor B-cell acute lymphoblastic leukemia (pre-B ALL), especially those who relapse after allogeneic hematopoietic stem cell transplantation ( HSCT), inevitably have poor outcomes. Although recently designed immunotherapies such as bispecific T-cell engager (BiTE, blinatumomab) antibody and chimeric antigen receptor T-cell therapy (CAR-T) may provide some opportunity for disease control, it remains a challenging issue. We report two cases wherein these two treatments were combined, and we discuss the treatment outcomes, which may elicit new thoughts in this challenging field.
Case Presentation
Case 1
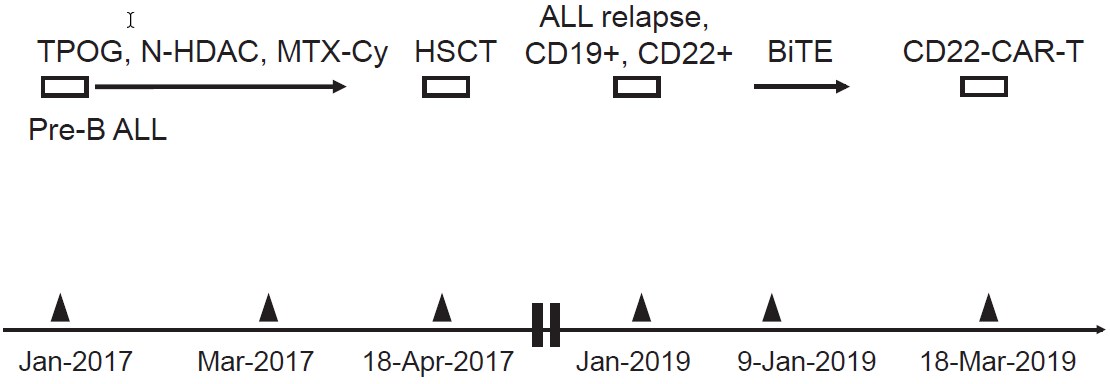
A 23-year-old woman was diagnosed with pre-B ALL with normal cytogenetics in January 2017. The initial response to induction chemotherapy of the TPOG-ALL protocol (Taiwan Pediatric Oncology Group) was poor. She was referred to National Taiwan University Hospital (Taipei, Taiwan). After salvage therapy with mitoxantrone ( Novantrone) plus high-dose cytosine arabinoside (Ara-C) in February, followed by high-dose methotrexate plus cyclophosphamide in March, leukemic cells reduced to a minimal residual level of 0.05% as measured using flow cytometry. She subsequently received a myeloablative allogeneic HSCT from her HLA-matched brother in April 2017. Acute grade ? graft-versus-host-disease (GvHD) occurred but was well controlled. All immunosuppressant agents were tapered off later. Unfortunately, overt relapse of pre-B ALL occurred in January 2019 with the same phenotype, CD19+ (100%), CD10+, and CD22+ (86.8%). She was then treated with a standard cycle of blinatumomab for 28 days (9μg/day for the first 7 days and 28μg/day thereafter by continuous intravenous infusion over 4 weeks) without significant adverse effects. She then regained complete remission (CR) and was back to full donor chimerism. In March 2019, she was referred to Beijing Boren Hospital (Beijing, China) to prepare her own CD22-CAR-T cells, which were smoothly re-infused after standard lymphodepleting chemotherapy with fludarabine plus cyclophosphamide. The infusion of CD22-CAR-T cells was uneventful and without development of cytokine release syndrome except for a short period of low-grade febrile reaction that later subsided spontaneously. There was no occurrence of any GvHD, and no immunosuppressant agent was given post CAR-T therapy. The patient returned to her usual life and has remained disease-free post-treatment. Figure 1 shows a summary of the whole treatment course in Case 1.
Case 2
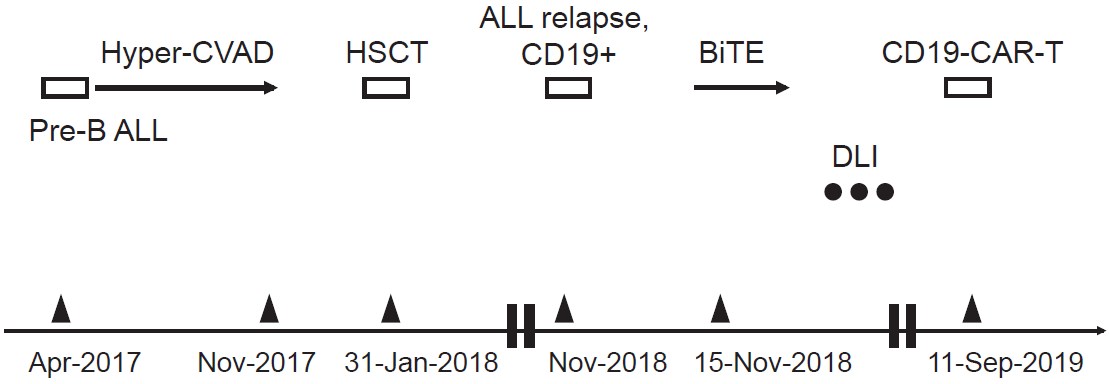
A 52-year-old man was diagnosed with pre-B ALL in April 2017 at a local hospital with no specific chromosomal abnormality. He received treatment with a standard M. D. Anderson-based hyper-CVAD protocol and maintained continuous CR until November 2017. He was then referred to Hualien Tzu Chi Hospital (Hualien, Taiwan) to undergo an HLA-matched myeloablative unrelated allogeneic HSCT in January 2018. He showed a progressive increase in recipient chimerism proven to be a T-cell population after sorting analysis post-HSCT. Donor lymphocyte infusion (DLI) was administered twice to induce hepatic GvHD and 100% donor chimerism was regained. However, he was confirmed to have ALL relapse in November 2018 with the same phenotype, CD19+ (100%), CD10+, CD20+ (100%), and CD22-. Fortunately, after one treatment cycle with blinatumomab plus rituximab, a second CR was achieved. After that, three DLIs with escalating doses of T-cells were given, but no GvHD was induced. In September 2019, he was referred to Beijing Boren Hospital to prepare his own CD19- CAR-T cells, which were smoothly re-infused after standard lymphodepleting chemotherapy. The infusion of CD19-CAR-T cells was uneventful. There was no development of cytokine release syndrome except for a lowgrade febrile reaction that subsided spontaneously. There was no flare-up of the prior GvHD, and no immunosuppressant agents were given post CAR-T therapy. The patient returned to his usual life and has remained disease- free post-treatment. Figure 2 shows a summary of the whole treatment course for Case 2.
Discussion
We report two patients with advanced pre-B ALL who attained leukemia-free status and had normal life for more than one year after sequential immuno-designed novel treatments of BiTE followed by consolidative CAR-T cell therapy. Notably, in the past, the 5-year overall survival rate in adult ALL after relapse was very low (generally less than 10%), not to say of relapse after allogeneic HSCT or failed multiple lines of therapy, with a reported median overall survival of approximately 3-6 months1,2.
Blinatumomab is a BiTE antibody construct with dual specificity for CD19 and CD3. It can bind to CD19+ B cells and CD3+ cytotoxic T-cells simultaneously, causing T-cell-mediated lysis of malignant B tumor cells3. In a multicenter phase 2 study, 43% of 189 enrolled relapsed or refractory adult pre-B ALL patients achieved CR or CRh (partial hematological recovery) after two cycles of blinatumomab treatment4. However, median relapse-free survival was only 5.9 months (95% confidence interval 4.8-8.3 months) for patients in CR or CRh. This strongly suggested that although single-agent blinatumomab shows reasonable anti-leukemia activity, it does not have a durable effect; thus, combination treatment with other approaches is needed.
CAR-T cell therapy targeting CD19 provides excellent rescue of advanced pre-B ALL with a CR rate of up to 70-90%5,6. However, reports showed that a substantial proportion (43-55%) of CR patients ultimately relapsed within one year7,8, especially if there was no bridge to allogeneic HSCT. The situation was extremely difficult in our two patients who already displayed failed allogeneic HSCT with prior GvHD. Tong and Wu et al., who led the cell therapy team in Beijing Boren Hospital, have demonstrated that CD22-CAR-T cell therapy was as effective as CD19-CAR-T cell therapy, and sequential CD19- followed by CD22-CAR-T cell therapies may prevent immune escape, resulting in prolonged response9,10, which was applied in Case 1 because higher expression of CD22 on leukemic cells was found. However, because of the lack of CD22 in Case 2, only CD19-CAR-T could be reasonably prepared. Our choices for these two patients entering into CR after BiTE were to offer CAR-T cell therapy as “consolidation” to achieve the best response without trying a second HSCT.
To the best of our knowledge, this is the first report to use BiTE followed by CAR-T cell therapy in the salvage treatment for advanced pre-B ALL. Further follow-up is needed. This novel treatment modality warrants further exploration.
Acknowledgements
This work was performed under the cooperation of the following three hospitals: Hualien Tzu Chi Hospital, Taiwan, National Taiwan University Hospital, Taiwan, and Beijing Boren Hospital, China.
Author Contributions
WHH, YCL and CCL arranged BiTE treatments for these two patients. CRT and TW prepared CAR-T cell therapy for these two patients. WHH and CCL wrote the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.Disclosure forms provided by the authors are available here.
References
1. Gökbuget N, Stanze D, Beck J, Diedrich H, Horst HA, Hüttmann A, et al. Outcome of relapsed adult lymphoblastic leukemia depends on response to salvage chemotherapy, prognostic factors, and performance of stem cell transplantation. Blood. 2012; 120: 2032-41.
2. O'Brien S, Thomas D, Ravandi F, Faderl S, Cortes J, Borthakur G, et al. Outcome of adults with acute lymphocytic leukemia after second salvage therapy. Cancer. 2008; 113: 3186-91.
3. Hoffmann P, Hofmeister R, Brischwein K, Brandl C, Crommer S, Bargou R, et al. Serial killing of tumor cells by cytotoxic T cells redirected with a CD19?/CD3?bispecific single-chain antibody construct. Int J Cancer. 2005; 115: 98-104.
4. Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015; 16: 57-66.
5. Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014; 371: 1507-17.
6. Pan J, Yang JF, Deng BP, Zhao XJ, Zhang X, Lin YH, et al. High efficacy and safety of low dose CD19 directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017; 31: 2587-93.
7. Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the CART before the horse. Biol Blood Marrow Transpl. 2017; 23: 235-46.
8. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015; 385: 517-28.
9. Pan J, Niu Q, Deng B, Liu S, Wu T, Gao Z, et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia. 2019; 33: 2854-66.
10. Pan J, Zuo S, Deng B, Xu X, Li C, Zheng Q, et al. Sequential CD19-22 CAR T therapy induces sustained remission in children with r/r B-ALL. Blood. 2020; 135: 387-91.
Search
News



