Volume 3 (2020) Issue 2 No.2 Pages 22-31
Abstract
Currently, there is no standard therapy available for relapsed acute leukemia after allogeneic hematopoietic cell transplantation (allo-HCT). In this study, we evaluated the efficacy of cytoreduction with cytarabine followed by granulocyte colony-stimulating factor (G-CSF) -primed donor lymphocyte infusion (DLI) for patients with acute leukemia who relapsed after allo-HCT. We retrospectively reviewed 255 patients who had received allo-HCT for acute leukemia/myelodysplastic syndrome. Patients were divided into two groups based on the CD34+ cell dose they received during the initial transplantation; patients in the lower CD34+ group received a dose lower than 6×106 cells/kg and those in the higher CD34+ group received a dose higher than 6×106 cells/kg. No significant differences were noted between two groups with respect to overall survival, relapse-free survival, or graft-versushost disease (GVHD) -free/relapse-free survival. Patients who relapsed after allo-HCT (n=93) were assigned into early or late relapse groups using the median time to relapse as the threshold. Among the 93 patients with relapse, 39 received G-CSF-primed DLI. The median dose of CD3+ cells was 2.82×107 cells/kg (range: 0.05-10.1). In the late relapse group, one-year overall survival was significantly higher in patients receiving DLI than that in patients not receiving DLI (53.4% ±7.4% vs. 26.7% ±7.4%; P=0.039), whereas no DLI effect was detected within the early relapse group. In addition, the incidence of DLI-induced GVHD did not differ between the two groups. In conclusion, treatment with G-CSF-primed DLI after allo-HCT with a limited CD34+ cell dose constitutes a feasible and effective option, which could replace second HCT in treatment of late-relapse patients.
Introduction
Allogeneic hematopoietic cell transplantation (allo- HCT) is a potentially curative therapy for acute leukemia1. However, patients with acute leukemia who relapse after allo-HCT show poor prognosis with a median survival of 34 months2. A second allo-HCT results in a longterm survival rate of only 10-35% with a higher treatment- related mortality. Currently, no standard treatment approach is available for relapsed acute leukemia3,4.
Induction of graft-versus-leukemia (GVL) effects with donor lymphocyte infusions (DLIs) is an attractive option for patients with relapsed hematological malignancies. However, GVL efficacy depends on disease subtype and tumor burden at the time of DLI3,5. Schmid et al. demonstrated an overall survival (OS) benefit of DLI in patients with acute myelogenous leukemia (AML) who relapsed after allo-HCT (20% ±3% vs. 9% ±2%; P<0.001) 6. Several different strategies have been explored to improve patient outcomes, such as dose-escalation of DLIs, addition of immunosuppressive agents to prevent graft-versus- host disease (GVHD), and modified DLI treatment with granulocyte colony-stimulating factor (G-CSF) 7-10. A combination of chemotherapy and DLI showed promising results11. Here, we investigated the effect of cytoreduction with a high dose of cytarabine followed by DLI. A pilot study performed at our institution reported a beneficial effect of cytarabine combined with G-CSF-primed DLIs using cryopreserved cells on patients with hematological malignancies who relapsed after allo-HCT12. This strategy was cost-effective and convenient for donors. In the current study, we aimed to determine the effectiveness of cytarabine in combination with G-CSF-primed DLI in treatment of patients with acute leukemia who relapsed after allo-HCT.
Materials and Methods
Data Collection
We conducted a retrospective review of the medical records of 255 patients who received allo-HCT for AML, myelodysplastic syndrome (MDS), or acute lymphoblastic leukemia (ALL) between December 1998 and August 2013 at the Department of Hematology/Oncology, Kyungpook National University Hospital (KNUH). Clinical and laboratory data were collected from electronic medical records according to protocol approved by the KNUH institutional review board.
Definitions
The risk status at transplantation was determined based on previously published classification schemes13. Poorrisk cytogenetics were classified according to the revised Medical Research Council classification system for AML and the International Prognostic Scoring System for MDS14,15. Poor-risk cytogenetics for ALL were defined as MLL rearrangement, BCR/ABL1 translocation, hypoploidy, or complex karyotype. Graft failure was defined as the lack of myeloid engraftment in patients surviving in remission for at least 28 days after transplantation. The Keystone staging system was used to score acute GVHD (aGVHD) and chronic GVHD (cGVHD) 16,17. Relapse was defined as the reappearance of leukemic cells in the peripheral blood, bone marrow, or extramedullary lesions after allo-HCT.
A novel composite end-point of refined GVHD-free/ relapse-free survival (GRFS), where events included grade ?-? aGVHD, systemic therapy requiring cGVHD, relapse, or death, was also18. OS was calculated from the date of the first allo-HCT to the date of death, or to the last follow-up. Relapse-free survival (RFS) was calculated from the date of the first allo-HCT to the date of disease recurrence or to the date of death. Post-relapse survival (PRS) was defined as the time from relapse posttransplantation to death or to the last follow-up19.
Transplantation procedures
Preparative regimens for allogeneic peripheral blood stem cell transplantation (PBSCT) included busulfan (Bu, 4 mg/kg PO or 0.8 mg/kg IV for 4 days) and cytoxan (Cy, 60 mg/kg for 2 days) administered to 100 patients; Bu (3.2 mg/kg for 2-4 days) and fludarabine (Flu, 30 mg/m2 for 6 days) administered to 135 patients; and total body irradiation and Cy (60 mg/kg for 2 days) administered to 20 patients. PBSCs were mobilized with 10μg/kg per day G-CSF[filgrastim (Leukokine®; CJ, Co., Korea) or lenograstim (Neutrogin®; Chugai Co. Ltd, Tokyo, Japan) ]alone (n=183, 71.8%) or in combination with a concurrent regimen of 5μg/kg per day G-CSF and 5μg/kg per day granulocyte macrophage colony-stimulating factor (GM-CSF) (n=72, 28.2%) from the donor. Administration of G-CSF with or without GM-CSF was continued, and apheresis was repeated every morning until the targeted cell dose (6×106 CD34+ cells/kg) was reached. GVHD prophylaxis consisted of methotrexate ( MTX) and cyclosporine A (CyA) or MTX and tacrolimus (Tac).
Collection and infusion of donor lymphocytes
Collecting the targeted amount of PBSCs (more than 6×106 CD34+ cells/kg) allowed us to cryopreserve some PBSCs, including several CD3+ cells at the time of harvest for transplantation. The extra cells were cryopreserved with dimethyl sulfoxide in a nitrogen tank. DLI with cryopreserved cells was available only for patients who were able to receive more than 6×106 CD34+ cells/kg from donors. Other patients would receive salvage chemotherapy or second transplantation when they relapse.
For those patients who relapsed after allo-HCT, DLI was promptly performed using the cryopreserved cells. The CD3+ cell-count was determined by flow cytometry and used to calculate the DLI dose. Before DLI, immunosuppressive agents were discontinued and patients received pre-DLI chemotherapy with high-dose cytarabine ( 1 g/m2; twice a day on days 1, 3, and 5). No patient received prophylactic immunosuppressive therapy after DLI.
The chimerism status, which was assessed by the number of tandem repeats or short tandem repeats, was compared before and after DLI. All patients underwent a bone marrow examination within 60 days after DLI or sooner (if clinically indicated) for the assessment of the response.
Statistical Analysis
Categorical data were analyzed using a chi-square test. Survival analysis was conducted using the Kaplan-Meier method, and groups were compared using a log-rank test. The cumulative incidence of GVHD was calculated using the Gray method considering treatment-related mortality and relapse as competing risks. The Cox proportional regression model was used to analyze potential risk factors affecting survival. Statistical analyses were per- formed using the SPSS software version 18 (SPSS Inc., Chicago, IL, USA) and EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan) 20.
Results
Patient and transplant characteristics
A total of 255 patients were analyzed. The median infused cell doses were as follows: mononuclear cells, 7.94×108 cells/kg (range: 0.36-25.12) ; CD34+ cells, 5.13×106 cells/kg (range: 0.46-20.6) ; and CD3+ cells, 2.82×108 cells/kg (range: 0.05-10.0). Patients were reclassified into two groups according to the targeted CD34+ cell dose (6×106 cells/kg) according to the KNUH protocol. The lower CD34+ group (n=165; 64.7%) included patients who underwent allo-HCT with a CD34+ cell dose of lower than 6×106 cells/kg, and the higher CD34+ group (n=90; 35.3%) included patients who underwent allo-HCT with a CD34+ cell dose of at least 6×106 cells/kg. Patient characteristics are summarized in Table 1. No statistically significant differences in the transplantation outcomes, such as the incidence of aGVHD, cGVHD, and relapse rate, were found between the two groups (Table 2).
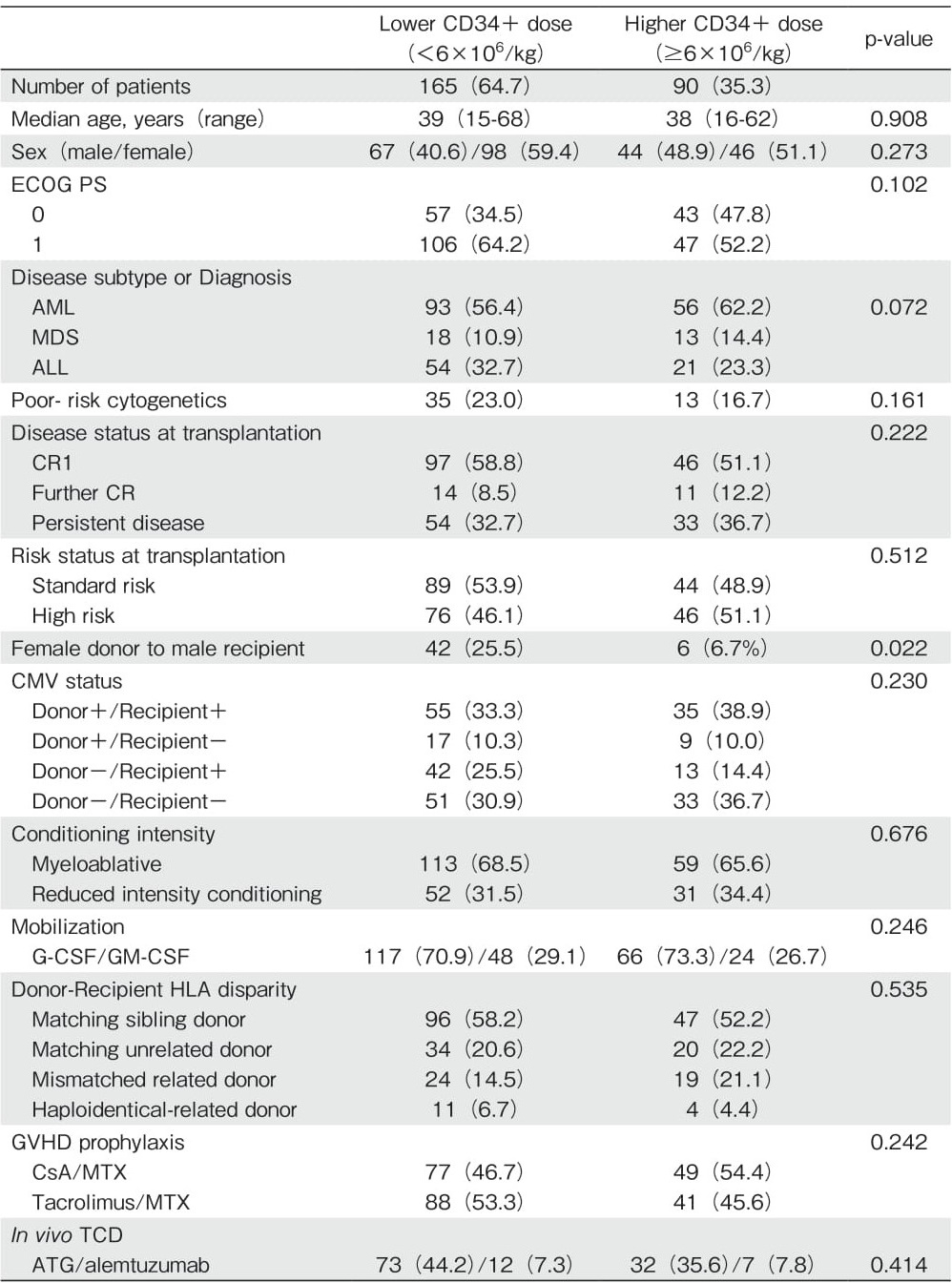
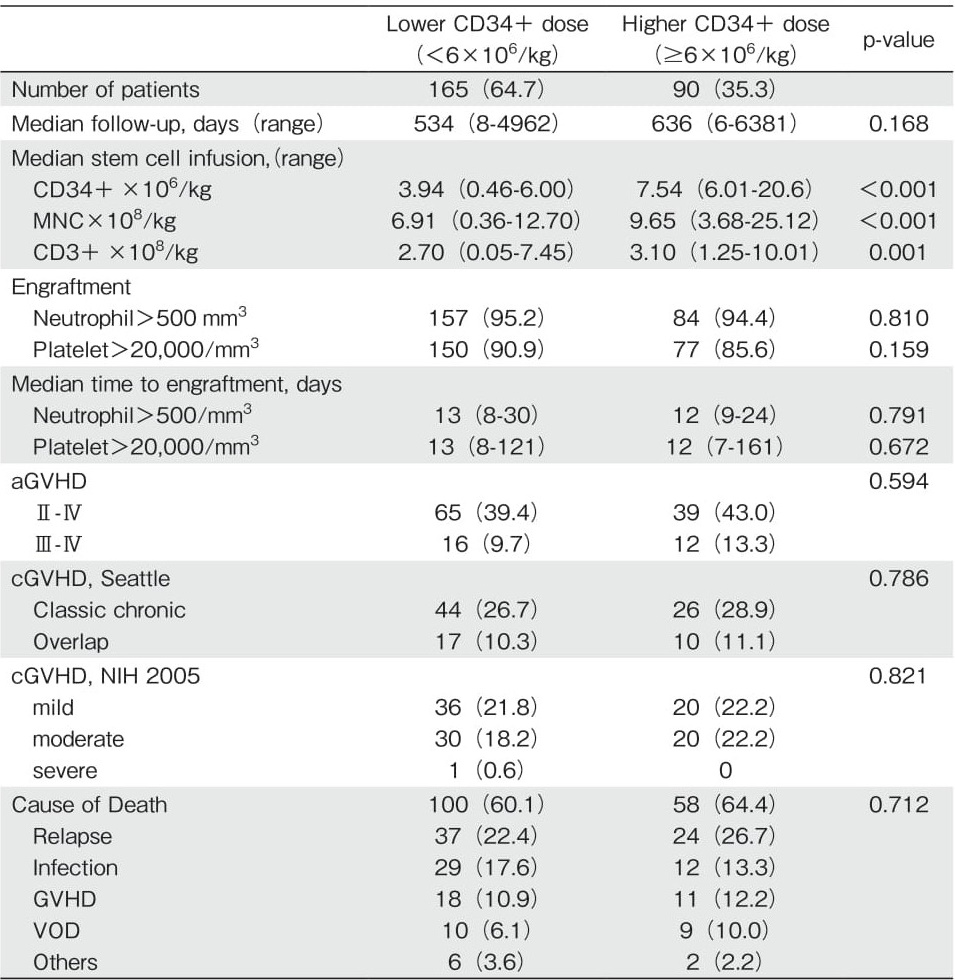
Impact of CD34+ cell dose on GRFS
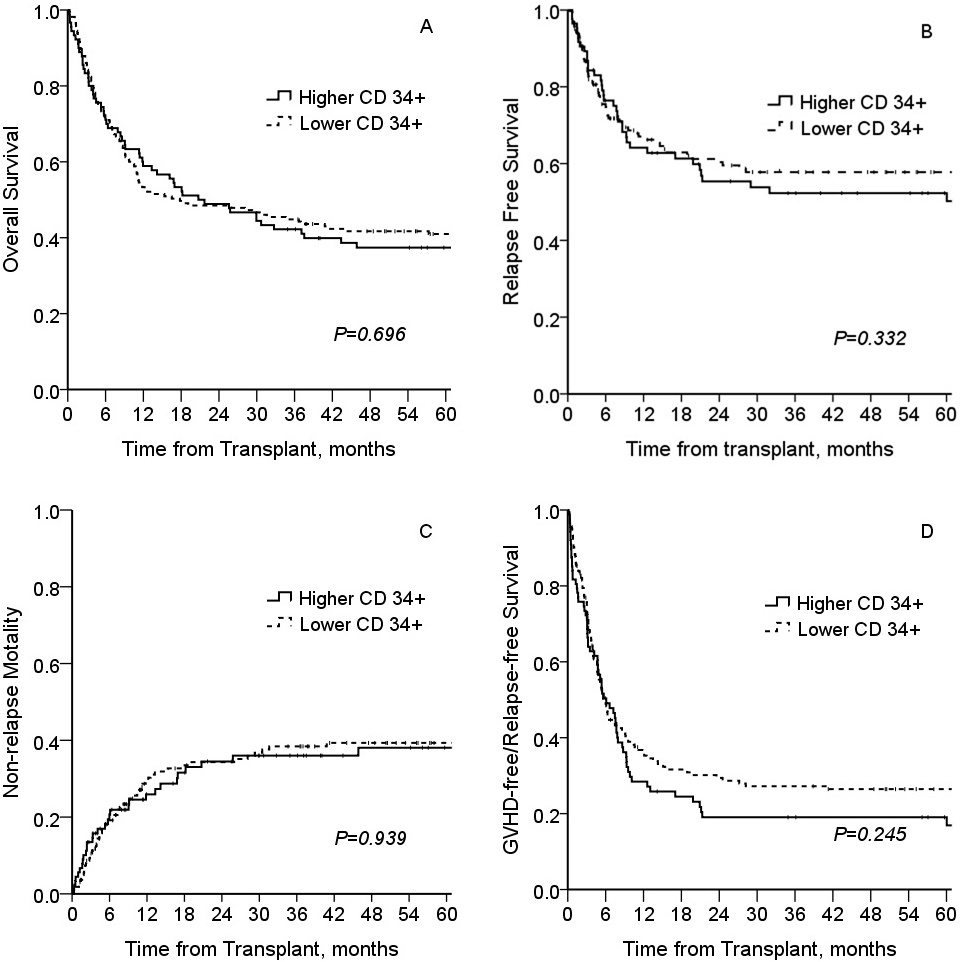
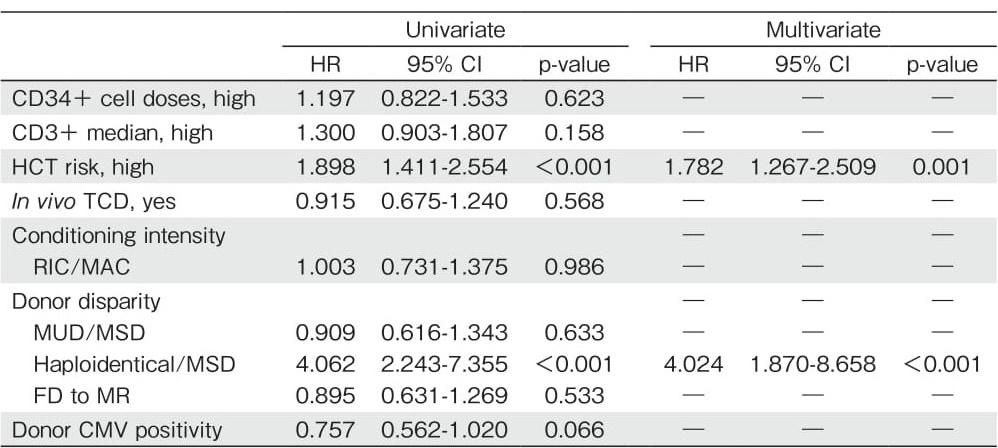
The median follow-up duration was 18.1 months with a range of 0.2 to 209.7 months. The 1-year OS, RFS, and non-relapse mortality (NRM) were 55.3% ±3.1%, 66.0% ±3.2%, and 28.2% ±0.3%, respectively. The cumulative incidences of aGVHD and cGVHD were 40.7% ±0.3% and 41.6% ±0.3%, respectively. The unadjusted Kaplan-Meier estimate of 1-year GRFS was 32.9% ±3.1%. No significant difference was found in OS, RFS, NRM, or GRFS between the two groups classified according to the CD34+ cell dose (Figure 1). Moreover, there was no significant correlation between the number of infused CD3+ and CD34+ cells (Spearman correlation coefficient, P=0.307). However, a trend of more CD3+ cells (more than 3.1×108 cells/kg) was noted in the higher CD34+ group (P=0.001; Table 2). In the univariate analysis, patients transplanted with higher CD34+ and CD3+ cell doses did not show an improved GRFS (Table 3, P=0.623 and P=0.158, respectively). The risk status at transplantation was an independent factor associated with worse GRFS (hazard ratio (HR) =1.782, 95% confidence interval (CI) : 1.267-2.509, P=0.001; Table 3).
Post-Relapse Survival
Among the 255 patients, 93 (36.4%) relapsed after allo-HCT. The median time from allo-HCT to relapse was 4.6 months (range: 1.5-59.1). After relapse, 45 patients (48.4%) were treated with salvage chemotherapy with a regimen based on fludarabine or mitoxantrone (FLAG or MEC), 9 (9.7%) with a second allo-HCT because of unavailability of reserved cells for DLI, and 39 (41.9%) with G-CSF-primed DLI (4 patients received DLI only because of their refusal on the use of cytarabine). Thereafter, 13 patients (30.0%) achieved DLIinduced complete remission, 24 progressed, and 2 were not evaluable for response. DLI-induced aGVHD was observed in 24 patients (61.5%) with a median of 20 days after DLI (range: 3-98 days) ; ten patients with grade ?, six with grade ?, five with grade ?, and three with grade ?. As shown in Table 4, univariate analysis revealed that poor-risk cytogenetics (HR=2.512, P= 0.015), risk status at transplantation (HR=4.406, P< 0.001), myeloablative conditioning regimen (HR=0.567, P=0.007), cGVHD (HR=0.525, P=0.006), and longer post-transplantation remission duration (HR=0.297, P< 0.001) were significantly associated with PRS. A longer post-transplantation remission duration was the only independent factor correlated with PRS (HR=0.297, 95% CI: 0.193-0.457, P<0.001).
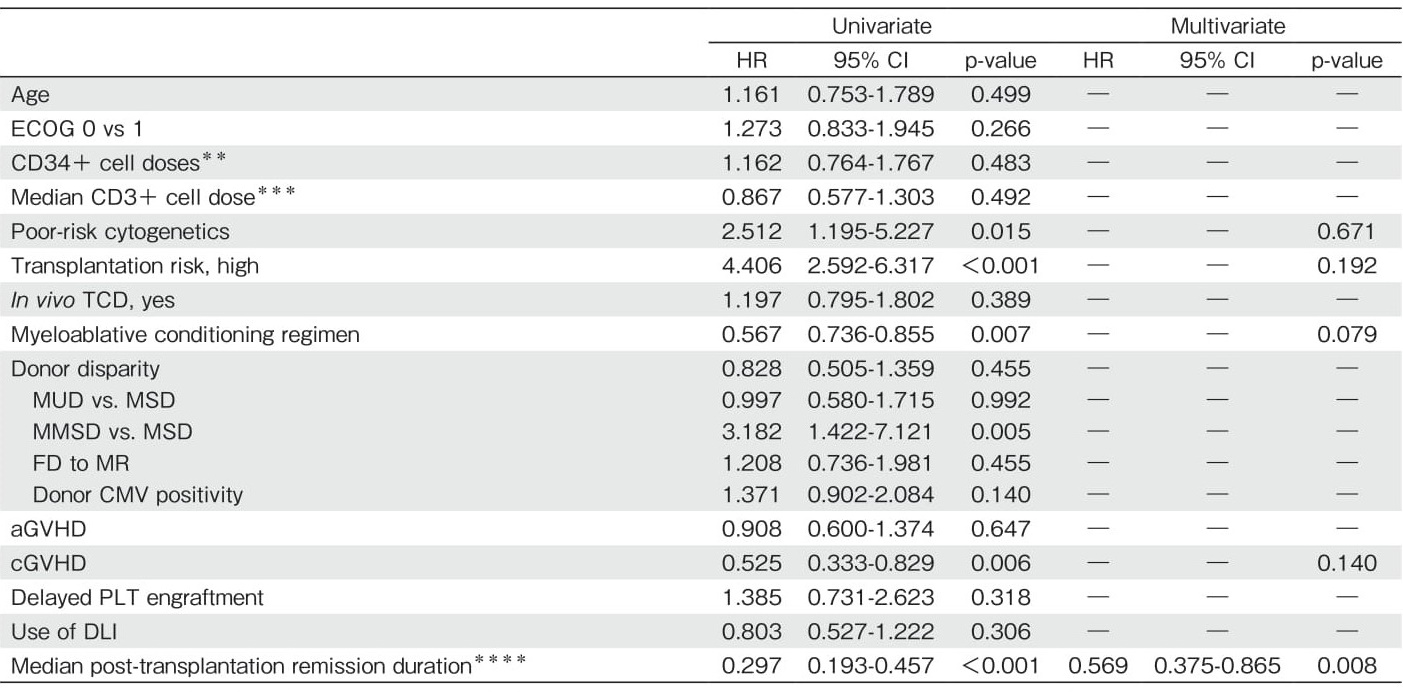
G-CSF-primed DLI effect on PRS
Among the 39 patients (41.9%) who received DLIs, 34 received one infusion and 5 received two infusions. The median amount of CD3+ cells was 2.82×107 cells/ kg (range: 0.05-10.1). The patient and transplant characteristics according to the post-transplantation remission duration are described in Table 5. Patients were classified into early and late relapse groups based on post-transplantation remission duration relative to the median RFS of 4.6 months (range: 1.5-59.1). For patients with early relapse (remission duration<4.6 months), one-year PRS rates were 7.7% ±5.3% and 5.3% ±4.3% in the DLI and non-DLI groups, respectively (Figure 2A; P=0.667). For patients with late relapse (remission duration?4.6 months), one-year PRS rates were 40.0% ±7.4% and 17.9% ±7.2% in the DLI and non-DLI groups, respectively ( Figure 2B; P=0.039). The number of second allo-HCT cases was small (n=9), they were, therefore, excluded from comparison analysis.
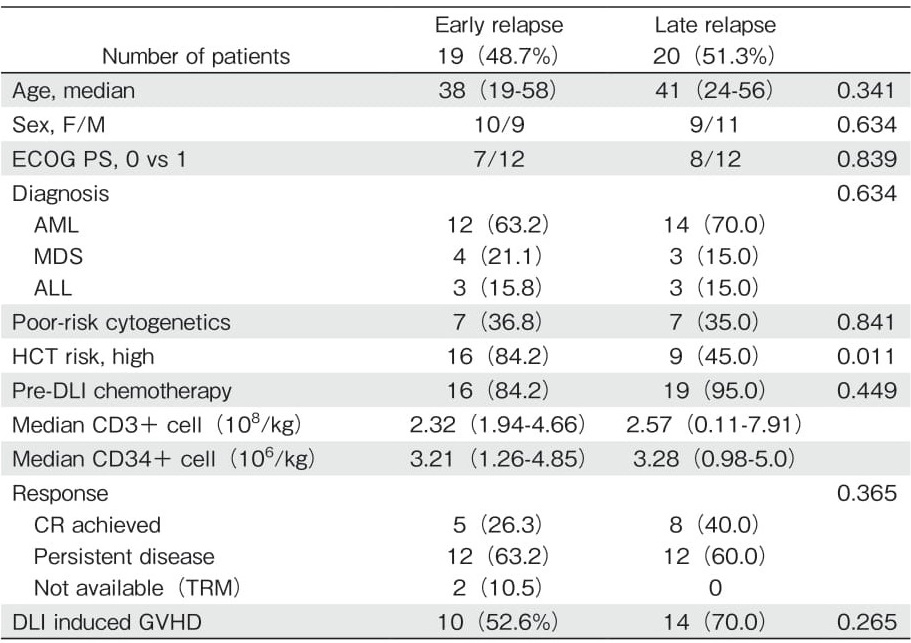
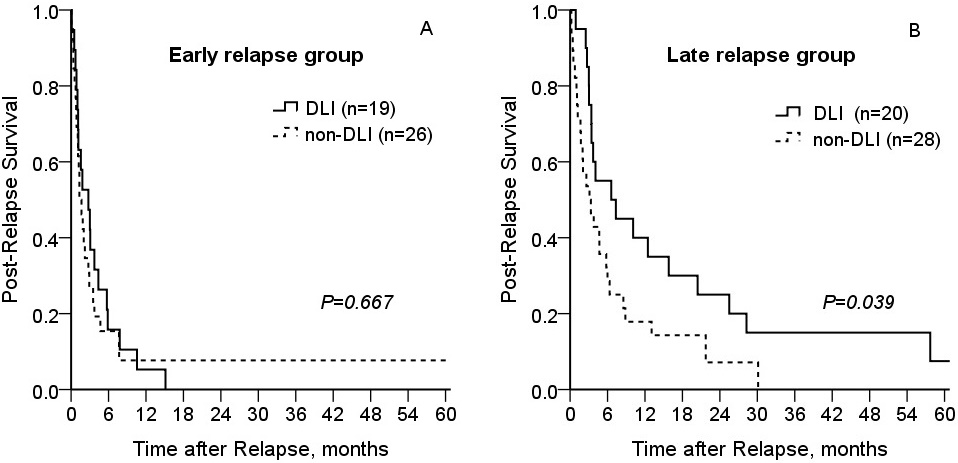
Discussion
The current study investigated the efficacy of cytarabine- based chemotherapy in combination with G-CSFprimed DLI in patients with acute leukemia who relapsed after allo-HCT. G-CSF-primed DLI treatment after allo- HCT with a limited CD34+ cell dose (lower than 6×106 cells/kg) constituted a feasible and effective option in terms of GRFS, donor convenience, and cost. Moreover, this treatment option may replace a second HCT for laterelapse patients. Although stem cell dose has already been explored in relation to the incidence of GVHD, relapse, and survival, no consensus has been reached21-23.
Regarding CD 34+ cell dose for allogeneic PBSCT, higher CD34+ dose (higher than 8×106 cells/kg) resulted in better neutrophil and platelet recovery, and was associated with development of chronic GVHD, but was not correlated with improved survival. Optimal CD34+ dose seemed to be dependent on donor type and stem cell source24.
Preliminary results from our institution demonstrated that CD34+ cell transplantation with a dose of higher than 6×106 cells/kg did not improve refined GRFS (median survival 5.5 months vs. 6 months, P=0.245; Figure 1D). Moreover, a higher CD34+ cell dose did not increase the neutrophil or platelet engraftment rate. As the current study found no correlation between the CD3+ and CD34+ cell numbers in the harvested cells (Spearman correlation coefficient, P=0.307), it is planned to limit the CD34+ cell dose (6×106 CD34+ cells/kg) for transplantation and cryopreserve the rest of the harvested cells for relapse or prophylactic use.
Cryopreservation for the purpose of future DLI was not performed in patient group who received a dose of lower than 6×106 CD34+ cells/kg in the initial allo-HCT.
This retrospective study has several limitations, including the heterogeneity of patients and transplant characteristics. Furthermore, DLI treatment has a minimal effect in the case of a rapidly advancing disease, as evidenced in patients with early relapse who experienced no benefits from DLIs. However, patients with longer post-transplantation remission duration showed better PRS in the DLI group (Figure 2B; 1-year OS: 46.7% ±12.9% vs. 21.7% ±8.9%, P=0.039).
A second allo-HCT is regarded as the optimal option for patients who relapse after the first transplantation. Yet, it is only available for a limited number of patients due to concerns of high mortality and unavailability of donors. Thus, for the late relapse group, DLI treatment may replace second HCT. A faster recovery can also be expected in the case of chemotherapy followed by G-CSF-primed DLI treatment with a sufficient number of CD34+ cells. Regarding GRFS, allo-HCT with a limited CD34+ cell dose (lower than 6×106 cells/kg) is not inferior to allo-HCT with a higher CD34+ cell dose. Moreover, the surplus cells from the harvest can be cryopreserved at the time of the first transplantation. Then, DLI treatment using these cryopreserved cells can be promptly performed at the time of relapse without a need for a new harvest. From the perspective of donor convenience and cost-effectiveness, this strategy constitutes an attractive option for patients with dismal prognosis after post-transplantation relapse.
Well-designed prospective clinical trials are required to answer such DLI-related questions as to when, how, and to whom. Previous studies have shown multiple biological effects of G-CSF on peripheral blood stem cells, including the ability to polarize T cells from Th1 to Th2, promotion of regulatory T cells, and tolerogenic dendritic cell differentiation25,26. In addition, this study indicated that G-CSF-primed DLI, rather than unstimulated DLI, included more CD34+ cells and led to early recovery. Moreover, interestingly, low mortality was associated with DLI-induced GVHD, and most of the mortality resulted from disease relapse or refractory disease rather than GVHD.
According to a study from Japan, in the case of nonprimed DLI27, complete remission (CR) rate was 38% and the probability of remaining in CR at 3 years was 7% in AML. Acute GVHD (grade 2 or higher) developed in 31 out of 89 (35%) patients with HLA-identical related donors and was fatal for seven (8%). The incidence of aGVHD (cumulative incidence of aGVHD; 40%) was similar to that in the current report. Chronic GVHD developed in 24 of 73 (33%) patients who received DLI from HLA-identical relatives.
Recently, Claiborne et al.28 reported that treatment of relapsed AML/MDS (n=28) after allo-HCT with acombination of azacytidine and DLI resulted in a two-year OS rate of 35%, and noted a trend towards higher absolute CD4+ cell count in the patient group achieving remission. Hypomethylating agents may induce synergistic effect by promoting cytotoxic effects against leukemic cells when combined with DLI28. Schroeder et al. also reported complete and partial remission rates of 27% and 6%, respectively, which correspond to an overall response rate of 33%, following the treatment of relapsed AML/ MDS after allo-HCT with a combination of azacytidine and DLI. Two-year OS rate was higher in MDS patients and correlated with disease burden in patients with AML. Overall incidence of aGVHD after treatment with Aza and DLI was 23% among all patients included in the study (n=154) and 31% among patients who had received DLI (n=105). The authors concluded that the combination of azacytidine and DLI was an effective and well-tolerated treatment option for patients with relapse after allo-HCT, in particular for those with MDS or AML with low disease burden29. Another group, which tested a combination of decitabine and DLI, reported that the effectiveness of this regimen was not restricted to patients with low leukemic burden30.
In conclusion, our results indicate that G-CSF-primed DLI treatment after allo-HCT with a limited CD34+ cell dose (lower than 6×106 cells/kg) is a feasible and effective option in terms of GRFS, donor convenience, and cost. Therefore, it has potential to replace the second HCT in treatment of late relapse patients.
Author's Contribution
As the first author, Y. J. L. collected and interpreted the data, drafted the article, and critically revised the important intellectual content; Y. J. L., D. W. B., H. J. J., J. H. M. and S. K. S. performed the treatment, supplied the acquisition of data, and revised the manuscript; S. K. S. provided the conception and design of the study, critically revised the article for important intellectual content, and gave the final approval of the version to be submitted.
Conflict of Interest
The authors declar no conflict of interest. Disclosure forms provided by the authors are available here.
Financial Support
This research was supported by Kyungpook National University Research Fund, 2018-2020.
References
1. Copelan EA. Hematopoietic Stem-Cell Transplantation. NEJM. 2006; 354: 1813-26.
2. Savani BN, Mielke S, Reddy N, Goodman S, Jagasia M, Rezvani K. Management of relapse after allo-SCT for AML and the role of second transplantation. Bone Marrow Transplant. 2009; 44: 769-77.
3. Deol A, Lum LG. Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev. 2010; 36: 528-38.
4. Barrett AJ, Battiwalla M. Relapse after allogeneic stem cell transplantation. Expert Rev Hematol. 2010; 3: 429-41.
5. Chang YJ, Huang XJ. Donor lymphocyte infusions for relapse after allogeneic transplantation. When, if and for whom? Blood Rev. 2013; 27: 55-62.
6. Schmid C, Labopin M, Nagler A, Bornhauser M, Finke J, Volin L, et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk ractors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol. 2007; 25: 4938-45.
7. Huang XJ, Wang Y, Liu DH, Xu LP, Liu KY, Chen H, et al. Administration of short-term immunosuppressive agents after DLI reduces the incidence of DLI-associated acute GVHD without influencing the GVL effect. Bone Marrow Transplant. 2009; 44: 309-16.
8. Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and donor lymphocyte infusions as first salvage therapy for relapse of AML or MDS after allogeneic stem cell transplantation. Leukemia. 2013; 27: 1229-35.
9. Chen SH, Li X, Huang XJ. Effect of Recombinant Human Granulocyte Colony-Stimulating Factor on T-Lymphocyte Function and the Mechanism of This Effect. Int J Hematol. 2004; 79: 178-84.
10. Huang X, Guo N, Ren H, Zhang Y, Gao Z, Lu D. An improved anti-leukemic effect achieved with donor progenitor cell infusion for relapse patients after allogeneic bone marrow transplantation. Chin Med J (Engl). 2003; 116: 736-41.
11. Sun W, Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Chemotherapy plus DLI for relapse after haploidentical HSCT: the biological characteristics of relapse influences clinical outcomes of acute leukemia patients. Bone Marrow Transplant. 2019; 54: 1198-207.
12. Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS, et al. A pilot study of cytoreductive chemotherapy combined with infusion of additional peripheral blood stem cells reserved at time of harvest for transplantation in case of relapsed hematologic malignancies after allogeneic peripheral blood stem cell transplant. Bone Marrow Transplant. 2004; 33: 231-6.
13. Armand P, Kim HT, Logan BR. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014; 123: 3664-71.
14. Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities amongst 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010; 116: 354-65.
15. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997; 89: 2079-88.
16. Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995; 15: 825-8.
17. Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus- host disease: ?. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005; 11: 945-56.
18. Ruggeri A, Labopin M, Ciceri F, Mohty M, Nagler A. Definition of GvHD-free, relapse-free survival for registry-based studies: an ALWP-EBMT analysis on patients with AML in remission. Bone Marrow Transplant. 2016; 51: 610-1.
19. Solh M, Zhang X, Connor K, Brown S, Solomon SR, Morris LE, et al. Post-relapse survival after haploidentical transplantation vs matched-related or matched-unrelated hematopoietic cell transplantation. Bone Marrow Transplant. 2016; 51: 949-54.
20. Kanda Y. Investigation of the freely available easy-to-use software"EZR"for medical statistics. Bone Marrow Transplant. 2013; 48: 452-8.
21. Baron F, Maris MB, Storer BE, Sandmaier BM, Panse JP, Chauncey TR, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005; 19: 822-8.
22. Dhédin N, Prébet T, De Latour RP, Katsahian S, Kuentz M, Piard N, et al. Extensive chronic GVHD is associated with donor blood CD34+ cell count after G-CSF mobilization in non-myeloablative allogeneic PBSC transplantation. Bone Marrow Transplant. 2012; 47: 1564-8.
23. Czerw T, Labopin M, Schmid C, Cornelissen JJ, Chevallier P, et al. High CD3+ and CD34+ peripheral blood stem cell grafts content is associated with increased risk of graft-versus-host disease without beneficial effect on disease control after reduced-intensity conditioning allogeneic transplantation from matched unrelated donors for acute myeloid leukemia-an analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Oncotarget. 2016; 7: 27255-66.
24. Heimfeld S. HLA-identical stem cell transplantation: is there an optimal CD34 cell dose? Bone Marrow Transplant. 2003; 31: 839-45.
25. Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol. 2005; 175: 7085-91.
26. Anderlini P, Champlin RE. Biologic and molecular effects of granulocyte colony stimulating factor in healthy individuals: recent findings and current challenges. Blood. 2008; 111: 1767-72.
27. Shiobara S, Nakao S, Ueda M, Yamazaki H, Takahashi S, Asano S, et al. Donor leukocyte infusion for Japanese patients with relapsed leukemia after allogeneic bone marrow transplantation: lower incidence of acute graft-versus-host disease and improved outcome. Bone Marrow Transplant. 2000; 26: 769-74.
28. Claiborne J, Bandyopathyay D, Roberts C, Hawks K, Aziz M, Simmons G, et al. Managing post allograft relapse of myeloid neoplasms: azacitidine and donor lymphocyte infusions as salvage therapy. Leuk Lymphoma. 2019; 60: 2733-43.
29. Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK. Treatment of acute myeloid leukemia or myelodysplastic syndrome relapse after allogeneic stem cell transplantation with azacitidine and donor lymphocyte infusions–a retrospective multicenter analysis from the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant. 2015; 21: 653-60.
30. Sommer S, Cruijsen M, Claus R, Bertz H, Wäsch R, Marks R, et al. Decitabine in combination with donor lymphocyte infusions can induce remissions in relapsed myeloid malignancies with higher leukemic burden after allogeneic hematopoietic cell transplantation. Leuk Res. 2018; 72: 20-6.
Search
News



