Volume 3 (2020) Issue 1 No.2 Pages 6-10
Abstract
Adult T-cell leukemia-lymphoma (ATL) is a rare type of mature T cell lymphoma caused by human T-lymphotropic virus type 1 (HTLV-1) with dismal outcome with conventional chemotherapy. A humanized anti-CC chemokine receptor 4 (CCR4) monoclonal antibody, mogamulizumab (MOG), has been shown to be effective for CCR4-positive ATL. However, MOG administration before allogeneic hematopoietic stem cell transplantation (allo-HSCT) was reported to be associated with an increased risk of severe acute graft-versus-host disease (aGVHD) after allo-HSCT due to reduction of donor-derived regulatory T cells (Tregs). We herein present a patient with ATL who underwent allo-HSCT after MOG administration without developing severe GVHD while referring to serum MOG concentration. The clinical course of our case suggests that it might be possible to determine the appropriate timing of allo-HSCT with monitoring of residual MOG level while avoiding the detrimental effect of MOG on post-transplant clinical outcome without increasing the risk of relapse/progression.
Introduction
Adult T-cell leukemia-lymphoma (ATL) is a rare type of mature T cell lymphoma caused by human T-lymphotropic virus type 1 (HTLV-1) 1. Although patients with aggressive ATL are generally treated with multi-agent chemotherapeutic regimens or anti-viral therapy, longterm survival is unsatisfactory1-4. Therefore, allogeneic hematopoietic stem cell transplantation (allo-HSCT) is considered1.
Patients with chemo-refractory ATL are treated with mogamulizumab (MOG), a humanized anti-CC chemokine receptor 4 (CCR4) monoclonal antibody5,6. However, MOG administration before allo-HSCT reportedly increases the risk of developing acute graft-versus-host disease (aGVHD), possibly due to donor-derived regulatory T cell (Treg) depletion7. Thus, the decision to either directly proceed to allo-HSCT or administer MOG before allo-HSCT in patients with chemo-refractory ATL remains controversial.
Here, we present a patient with ATL who underwent allo-HSCT after MOG administration. We monitored the patient's MOG levels while deciding the timing of allo- HSCT.
Case Presentation
A 50-year-old Japanese woman, who had been an asymptomatic HTLV-1 carrier for approximately 14 years, presented with ATL progression. The Eastern Cooperative Oncology Group (ECOG) performance status upon admission was 0. The patient's laboratory results were as follows: white blood cell count, 9.9× 109/L with 19.5% lymphocytes and 36% abnormal lymphocytes; serum lactate dehydrogenase, 343 IU/L (normal range 124-222 IU/L) ; soluble interleukin-2 receptor, 1,356 IU/mL (normal range 122-496 IU/mL). Skin rash and papules were observed on her femora and abdomen, which were pathologically diagnosed as ATL. She was diagnosed with unfavorable chronic-type ATL according to the Shimoyama criteria8.
Using the HTLV-1 analysis system comprising flow cytometry (HAS-Flow) to analyze cells in the peripheral blood (PB), typical ATL cells with CCR4 expression were detected9 (Figure 1A). The patient's clinical course is shown in Figure 2A and 2B.
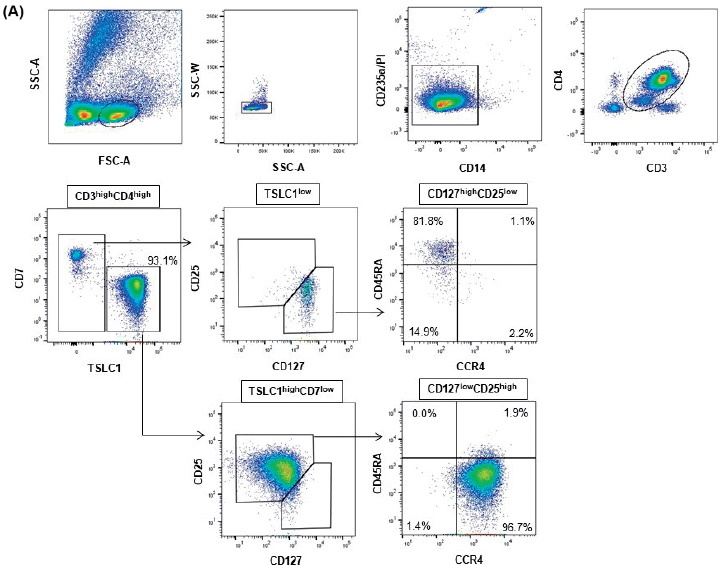
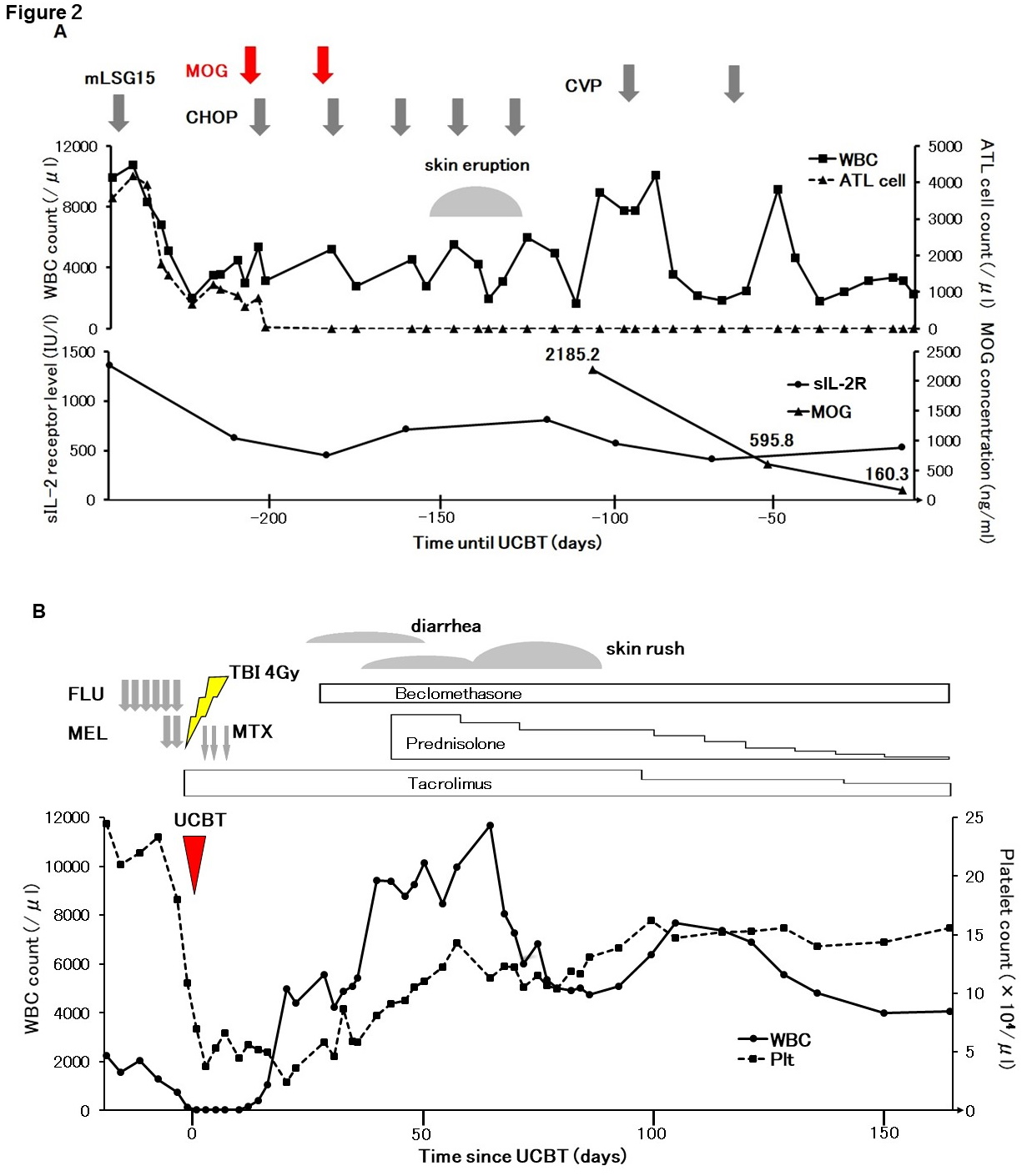
The patient received mLSG15 regimen1,2. After one cycle, the population of ATL cells remained same in the PB. As the number of ATL cells increased with neutrophil recovery, ATL was expected to progress. Additionally, grade 3 fatigue and other complications, such as mucositis and nausea with grade 2 or less, were observed (Common Terminology Criteria for Adverse Events[CTCAE] v5.0). Since the patient was reluctant to continue conventional chemotherapy, we offered an alternative regimen, i. e., MOG combined with the cyclophosphamide, doxorubicin, vincristine, prednisolone (CHOP) regimen. This regimen caused the ATL cells to disappear immediately.
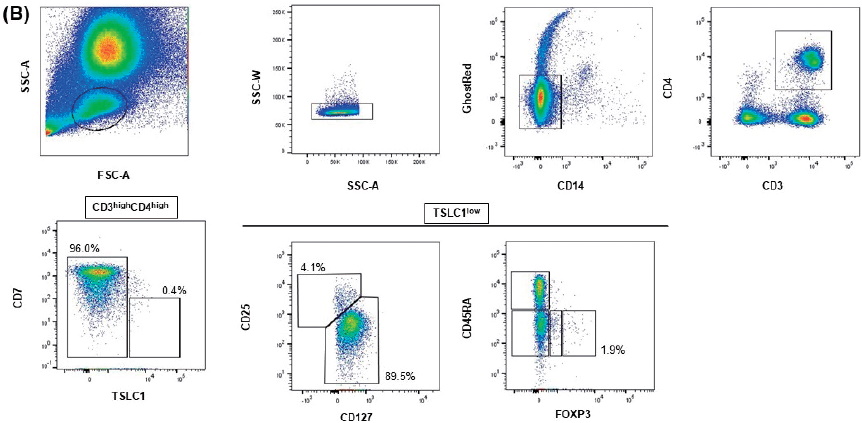
After two doses of MOG, her general condition improved. Subsequently, MOG administration was terminated considering the risk of developing GVHD after allo-HSCT. The proportion of effector-type Tregs at 41 days after the final MOG dose was low (Figure 1B).
Although we scheduled the allo-HSCT after the CHOP regimen, the serum MOG concentration at the end of the fourth CHOP regimen, 76 days after the final MOG administration, was 2,185.2 ng/mL (detected by enzymelinked immunoassay[ELISA]). Due to the high concentration, we postponed the planned allo-HSCT. Gradually, 127 days and 166 days after the last MOG dose, MOG concentration decreased to 595.8 ng/mL and 160.3 ng/ mL, respectively.
After the patient achieved complete remission, she underwent umbilical cord blood transplantation (UCBT) with HLA 2-allele mismatched (HLA-B and HLADRB1) cord blood following a regimen comprising reduced-intensity conditioning with fludarabine (25 mg/m2 daily for 6 days), melphalan (40 mg/m2 daily for 2 days), and total body irradiation (4 Gy), 181 days after the last MOG dose. Tacrolimus and short-term methotrexate were used for GVHD prophylaxis.
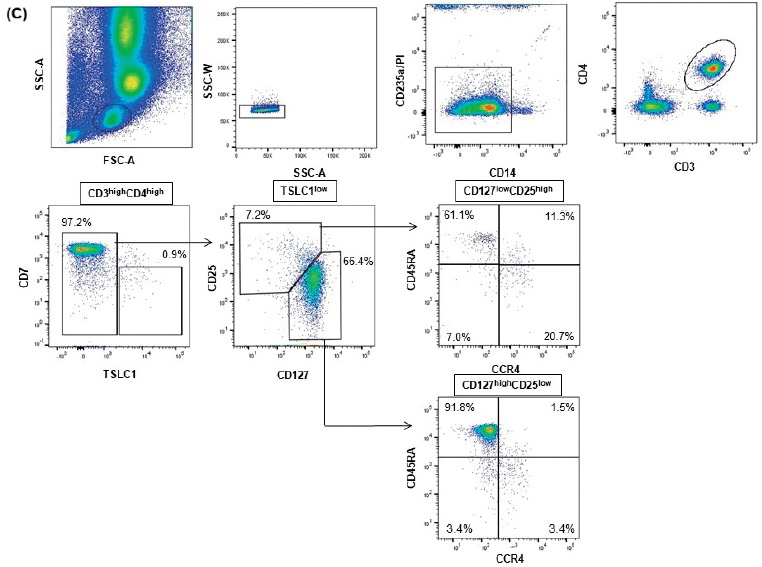
The patient achieved neutrophil engraftment with complete donor chimerism. HAS-flow analysis of PB at 35 days after UCBT showed that ATL cells were undetectable. The proportion of Tregs (CD25highCD27low fraction) among the total population of CD4+ cells was 7.2%, and comprised 32.0% of CCR4+ cells (Figure 1C). She developed diarrhea at 21 days after UCBT, which was diagnosed as aGVHD (overall grade?, gut stage 1), and she received oral beclomethasone dipropionate alone for treatment. Subsequently, she developed skin aGVHD (overall grade?, skin stage 2) at 32 days after UCBT, which was managed by topical steroid treatment. However, once the skin rash worsened (, overall grade?, skin stage 3), prednisolone (0.5 mg/kg/day) was additionally administered and the skin GVHD subsequently resolved. Approximately 6 months after UCBT, the HTLV-1 proviral load in ATL cells from the PB was undetectable. Consequently, we stopped all immunosuppressant treatments.
Discussion
The use of MOG in transplant-eligible patients considering the increased risk of severe aGVHD after allo- HSCT is controversial7. However, insufficient disease control before allo-HSCT is a poor prognostic factor later7. Thus, whether MOG should be used to improve chemotherapy or if it is better to directly proceed to allo- HSCT in chemo-refractory ATL remains controversial. A factor in the decision to administer MOG is the disease site of ATL, since MOG is highly effective in the PB.
Another important issue that occurs when a patient receives MOG before allo-HSCT is the timing of allo- HSCT after MOG. A previous large retrospective study showed that patients that began allo-HSCT>50 days after MOG treatment had significantly worse outcomes7. However, presently, the concentration of MOG was 2,185.2 ng/mL, 76 days after the last dose of MOG. Although the half-life of MOG is reportedly~16-18 days10, we calculated the half-life of MOG to be approximately 27 days. Our data suggest the importance of monitoring the level of MOG before allo-HSCT to prevent severe aGVHD.
As a limitation, we must emphasize that this study is just a case report, and further clinical trials are needed to confirm the effectiveness of this treatment strategy. Another important limitation is that no established threshold exists to avoid the risk of severe aGVHD. We expect that the minimal dose of MOG is less than 300 ng/mL, since CR was achieved in Cohort 1 of the phase ? trial (dose level 0.01 mg/kg) for ATL with a Cmax of~ 300 ng/mL. Thus, one possibility is to use a cut-off dose of 300 ng/mL, which should be prospectively assessed in the future.
In conclusion, we reported a successful case of CBT without severe aGVHD for chemo-refractory ATL after combination chemotherapy and MOG. Monitoring the PB concentration of MOG is important to avoid the risk of severe aGVHD, and this should be explored in future studies.
Acknowledgements
This research was partially supported by the Practical Research for Innovative Cancer Control program of the Japan Agency for Medical Research and Development (19ck0106342h0003) and a National Cancer Center Research and Development Fund (29-A-14).
Author's contribution
K. N. and S. F. wrote the manuscript. K. N., S. F., M. K., Y. T., H. M., H. Y., and J. I. collected the data. E. W., S. K., A. T., and K. U. performed the flow cytometric analysis. All authors read and approved the final manuscript.
Conflict of interest
S. F. received honoraria from Kyowa Hakko Kirin Co., Ltd.Disclosure forms provided by the authors are available here.
References
1. Cook LB, Fuji S, Hermine O, Bazarbachi A, Ramos JC, Ratner L, et al. Revised Adult T-Cell Leukemia-Lymphoma International Consensus Meeting Report. J Clin Oncol. 2019; 37: 677-87.
2. Fuji S, Yamaguchi T, Inoue Y, Utsunomiya A, Moriuchi Y, Owatari S, et al. VCAP-AMP-VECP as a preferable induction chemotherapy in transplant-eligible patients with aggressive adult T-cell leukemia-lymphoma: a propensity score analysis. Bone Marrow Transplant. 2019; 54: 1399-1405.
3. Bazarbachi A, Plumelle Y, Carlos Ramos J, Tortevoye P, Otrock Z, Taylor G, et al. Meta-analysis on the use of zidovudine and interferon-alfa in adult T-cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010; 28: 4177-83.
4. Malpica L, Pimentel A, Reis IM, Gotuzzo E, Lekakis L, Komanduri K, et al. Epidemiology, clinical features, and outcome of HTLV-1-related ATLL in an area of prevalence in the United States. Blood advances. 2018; 2: 607-20.
5. Phillips AA, Fields PA, Hermine O, Ramos JC, Beltran BE, Pereira J, et al. Mogamulizumab versus investigator's choice of chemotherapy regimen in relapsed/refractory adult T-cell leukemia/ lymphoma. Haematologica. 2019; 104: 993-1003.
6. Fuji S, Utsunomiya A, Inoue Y, Miyagi T, Owatari S, Sawayama Y, et al. Outcomes of patients with relapsed aggressive adult T-cell leukemia-lymphoma: clinical effectiveness of anti-CCR4 antibody and allogeneic hematopoietic stem cell transplantation. Haematologica. 2018; 103: e211-4.
7. Fuji S, Inoue Y, Utsunomiya A, Moriuchi Y, Uchimaru K, Choi I, et al. Pretransplantation Anti-CCR4 Antibody Mogamulizumab Against Adult T-Cell Leukemia/Lymphoma Is Associated With Significantly Increased Risks of Severe and Corticosteroid- Refractory Graft-Versus-Host Disease, Nonrelapse Mortality, and Overall Mortality. J Clin Oncol. 2016; 34: 3426-33.
8. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984-87). Br J Haematol. 1991; 79: 428-37.
9. Kobayashi S, Nakano K, Watanabe E, Ishigaki T, Ohno N, Yuji K, et al. CADM1 expression and stepwise downregulation of CD7 are closely associated with clonal expansion of HTLV-Iinfected cells in adult T-cell leukemia/lymphoma. Clin Cancer Res. 2014; 20: 2851-61.
10. Yamamoto K, Utsunomiya A, Tobinai K, Tsukasaki K, Uike N, Uozumi K, et al. Phase? study of KW-0761, a defucosylated humanized anti-CCR4 antibody, in relapsed patients with adult T-cell leukemia-lymphoma and peripheral T-cell lymphoma. J Clin Oncol. 2010; 28: 1591-8.
News



